Abstract
Objectives:
The aim of this study was to evaluate the cytotoxic effects of fiber reinforced composite bonded retainers in comparison with flexible spiral wires (FSWs) under high and low cariogenic-simulated environments using human oral fibroblasts.
Materials and Methods:
Four types of bonded retainers were evaluated: (1) reinforced with glass fibers: Interlig (Angelus), (2) reinforced with polyethylene fibers: Connect (Kerr), (3) reinforced with quartz fibers: Quartz Splint UD (RTD), and (4) FSW. Twenty specimens of each sample group were prepared with the same surface area and halved. Next, half of them were placed in a high cariogenic environment 60 min in 10% lactic acid 3 times a day and remained in Fusayama Meyer artificial saliva for the rest of the day) and the other half were placed in a low cariogenic environment 20 min in 10% lactic acid 3 times a day and remained in Fusayama Meyer artificial saliva for the rest of the day) for 1, 7 and 30 days. Cell viability was assessed by MTT assay. Data were analyzed using SPSS software (α =0.05).
Results:
During the 1st month, cytotoxicity reduced gradually. In the low cariogenic-simulated environment, the cytotoxicity of all of the groups were reported to be mild at day 30 and the difference between them was significant (P = 0.016). In the same period in the high cariogenic-simulated environment, the cytotoxicity of Connect and Quartz Splint was mild, and they had lower cytotoxicity than the other groups. Meanwhile, Interlig had moderate (52%) and FSW had severe cytotoxicity (22%) and the difference between the groups was also significant (P = 0.000).
Conclusions:
FSW retainers are not recommended in those at high-risk for dental caries. However, in those at low-risk, there is no difference from the standpoint of cytotoxicity.
Keywords: Cell viability, cytotoxicity, fiber reinforced composite, retainer
INTRODUCTION
Many appliances have been introduced to prevent relapse after orthodontic treatment such as the fixed bonded retainer which not depend on patient cooperation. Two conventional fixed retainers widely used are the flexible spiral wire (FSW) and fiber reinforced composite (FRC) retainers.[1] FRC is composed of silanized fibers that are embedded in a composite matrix.[2] The most important advantage of FRC retainers compared with FSW retainers is high transparency, which makes these retainers almost invisible. Therefore, it can be bonded closer to the incisal edges of the teeth, which is a benefit from the biological and biomechanical aspects.[2] One drawback of FRC retainers is the need to cover FRC bars with composite resins, especially in interproximal spaces, making oral hygiene more difficult.[2,3] It should be noted that dental material should be biocompatible in addition to strong, esthetic and have ease of application.[4,5] Polymerized resins may be cytotoxic due to the release of remaining monomers, initiators, and activators. Moreover, these agents could be released as a result of physical and chemical degradation.[5,6] After 1 time eating sucrose the pH of the tooth surface falls under 5.5 for 20 to50 min. However, in individuals eating sweet snacks between meals the pH falls under 5.5 for a longer time.[7] This reduction in pH may release the composition of FRC retainers and cause cytotoxicity. Therefore, it seems necessary to evaluate cytotoxicity of the retainers under a high cariogenic-simulated environment. Another factor that may cause cytotoxicity is when FRC bars are exposed to the oral cavity after finishing and polishing in interproximal regions.
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide) assay is a colorimetric test for assessing cell viability. NAD(P) H – dependent cellular oxidoreductase enzymes may, under defined conditions, reflect the number of viable cells. These enzymes are capable of reducing the tetrazolium dye MTT to its insoluble formazan, which has a purple color. MTT assay is a quantitative method, which is its main advantage.[8]
The aim of this study was to evaluate the cytotoxicity of different FRC retainers in comparison with FSW retainers under a high and low cariogenic-simulated environment using human gingival fibroblasts (HGFs).
MATERIALS AND METHODS
Preparation of Specimens
This was an in vitro study. Four types of bonded retainers were selected: (1) Reinforced with glass fibers: Interlig (Angelus, Londrina, PR, Brazil), (2) reinforced with polyethylene fibers: Connect (Kerr, 3M Co, St Paul, MN, USA), (3) reinforced with quartz fibers: Quartz Splint UD (RTD, France), and (4) FSW (19.5 mil multi-strand wire) (Dentaurum, Inspringen, Germany). To compare their cytotoxicity the surface area should be the same. For this purpose, the width, length, and thickness of the retainers were measured by a digital caliper with an accuracy of 0.01 mm. Therefore, all of the FRCs had the same surface area.
Simulating the Environments
For simulating a high cariogenic environment, samples were placed in 10% lactic acid (pH: 5.3) for 60 min, 3 times a day, and then remained in Fusayama Meyer artificial saliva for the duration of the day. Furthermore, for simulating a low cariogenic environment, samples were placed in 10% lactic acid for 20 min, 3 times a day, and then remained in Fusayama Meyer artificial saliva for the duration of the day. The saliva and acid were replaced every 3 days. Each group was divided into seven subgroups, in which 10 samples were placed in each subgroup; three groups for evaluating the low-cariogenic environment and the other three groups for the high-cariogenic and one group as a control. The duration of the test for the first, second, and third group was 1, 7, and 30 days respectively. All the specimens, except for the control group were subsequently thermal cycled (5000 cycles, 5°C –55°C).
MTT Assay
The MTT test was carried out based on Edmondson et al.[9] HGFs (Pasteur Institute, Tehran, Iran) were cultured in DMEM medium, supplemented with penicillin (100 u/ml), streptomycin (100 mg/ml), 4 mM L-glutamine and 5% fetal bovine serum (DAA Laboratories, GmbH, Pasching, Austria). The samples of the groups, from the first to the sixth, were rinsed with artificial saliva and then they were sterilized by ultraviolet-irradiation for 45 min. Each sample was placed in the wells of a 96-well flask and 330 lambda (0.001 ml) DMEM medium was added to each well and then incubated for 24 h. HGFs (8000 cells per well) were cultured in 96-well cell culture clusters containing DMDEM medium and were incubated for 24 h. All wells were aspirated, and 200 lambda extract of FSW or FRCs were added and all of them were then incubated for 24 h. Twenty lambda of MTT solution was then added to each well, followed by 4 h of incubation in darkness. The spectrophotometric absorbance at 570 nm was then measured using the Elisa reader device (VMax, PA, USA). Cytotoxicity was measured based on cell viability as follows:[10] noncytotoxic >90% cell viability, slightly cytotoxic = 60–90% cell viability, moderately cytotoxic = 30–50% cell viability, and severely cytotoxic ≤30%.
The test was repeated using the same extracts. The Kolmogorov-Smirnov test was used for evaluating data normality. Data were analyzed by the one-way ANOVA and Tukey's test and independent t-test (P < 0.05).
RESULTS
The result of the study showed that cell viability of all experimental groups were not statistically significant different on the day 0 (P = 0.18) as is shown in Table 1.
Table 1.
Cell viability percentage at day 0

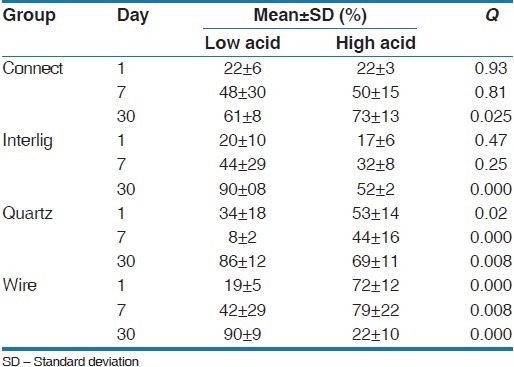
Cell viability percentages of all experimental groups at days 1, 7, and 30 are presented in Table 2. At first, on the seventh and 13th days the one-way ANOVA test showed significant differences between all of the groups and the Tukey's test was used for measuring inter-group differences [Table 3]. On the 1st day in the low-cariogenic environment, there was a significant difference in cell viability between the groups (P = 0.016) and the cell viability percentage was greater in the Quartz Splint group. Furthermore, in the high-cariogenic environment the difference between the groups was significant (P = 0.000) and the cell viability percentage was greater in the FSW group. On the 7th day in the low-cariogenic environment there was a significant difference between the groups and those differences were a result of the Quartz Splint group, in which the cell viability percentage was significantly less than the other groups and the other groups did not have a significant difference with each other. In the high-cariogenic environment, the difference between the groups was significant (P = 0.000) and FSW showed significantly high cell viability compared with the other groups.
Table 2.
Cell viability percentage at day 1, 7, and 30 in the low and high acidic environment
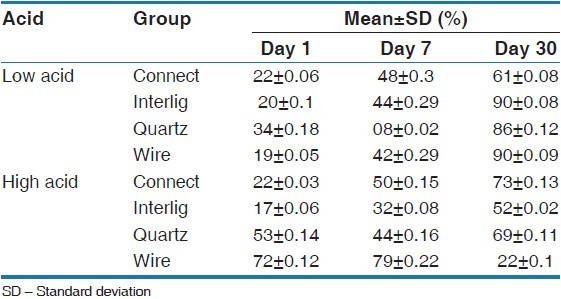
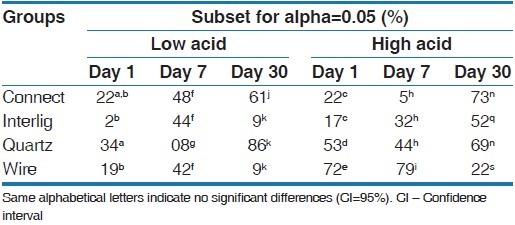
Table 3.
The comparison of cell viability between groups
After 1-month in the low-cariogenic situation there was a significant difference between the groups (P = 0.000). Connect showed significantly less cell viability percentage compared with the other groups. Also, in the high-cariogenic environment the difference was statistically significant between the groups and FSW had the least cell viability. In the Connect group, the mean of cell viability on the 7th day was significantly greater than 0 and the 1st days, although there was no significant difference with the 3rd day. In the Interlig group, the mean of cell viability at the 7th day increased significantly in comparison with 0 and the 1st day. The difference between the seventh and 13th day was also significant, which indicates improvement in cell viability. In the Quartz splint group, the mean of cell viability showed a descending trend on the 7th day, although the cell viability was significantly improved on the 13th day. In the wire group in the low cariogenic environments, the mean of cell viability on the 7th day was significantly greater than 0 and the 1st day and on the 13th day only negligible cytotoxicity was observed. In the high cariogenic environment ton the first and 7th days the amount of cell viability was higher, but after 1-month it reduced significantly [Table 4] [Figures 1–4].
Table 4.
One-way ANOVA test for evaluating cell viability in the groups at low and high acidic situations
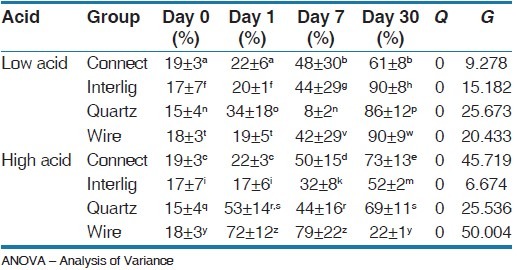
Figure 1.
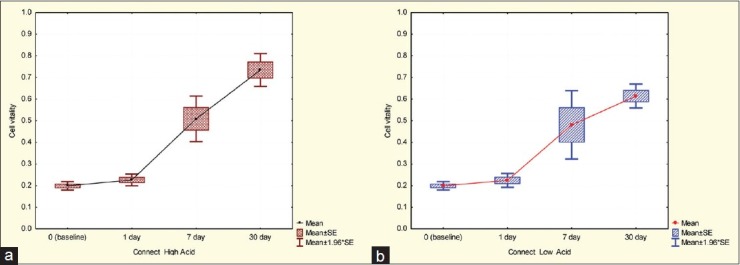
Cell viability in the Connect group,(a) High acidic environment,(b) Low acidic environment
Figure 4.
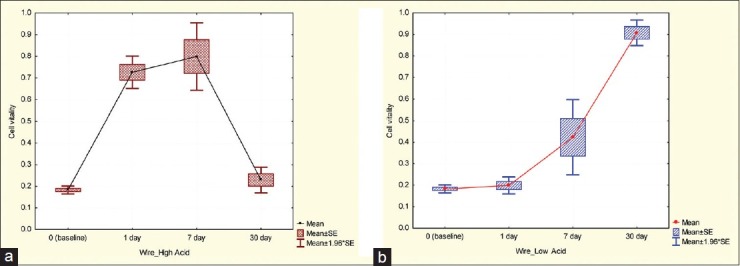
Cell viability in the Wire group, (a) High acidic environment, (b) Low acidic environment
Figure 2.
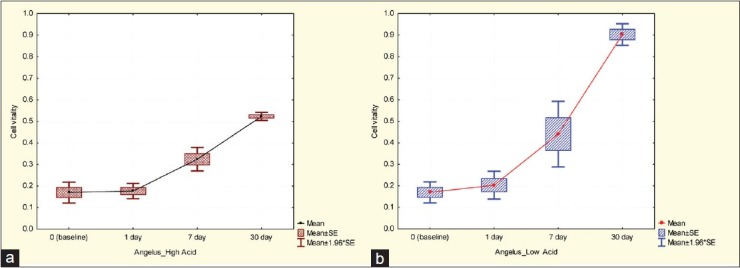
Cell viability in the Interlig group, (a) High acidic environment, (b) Low acidic environment
An independent t-test was used to evaluate the effect of low or high cariogenic environments in the groups [Table 5]. There was no significant difference between the two environments (low or high cariogenic) after the 1st and 7th days in the Connect group, although after 1-month and the more acidic the environment, cell viability was higher. In the Interlig group after day 1 and 7 there was no significant difference between the two environments; however, after 1-month in more acidic environments the cell viability significantly reduced in the FSW and Quartz Splint groups. Moreover, after days 1 and 7 in the more acidic environments cell viability was more, although after 1-month the results become inverted.
Table 5.
Student's u-test for evaluating cell viability in a low and high acidic environment in the experimental groups
DISCUSSION
The biologic characteristics and the toxicity of dental materials are important in clinical dentistry. In vitro assessment of cytotoxicity is a crucial step before applying new material in vivo.[11] It has been reported that the amount of cytotoxicity varies for different cells.[12] Therefore, in our study HGFs were used to simulate gingival tissue.
The fully polymerized resins have no harmful biological effect.[13] However, complete polymerization of orthodontic adhesive resins seems unlikely.[14] The remnant unpolymerized monomers in the composite are the primary cause of its cytotoxicity.[15] Ferracane showed that 5–10% of remnant monomers in cured composites were released into the solvent.[16] Thompson reported that the amount of leaked material from cured orthodontic adhesive during 48 h was 14% of bulk material.[17] Bis GMA is the main released monomers from dental composites,[18] which has the most cytotoxic potential among dimethacrylate derivations.[19,20]
The cytotoxic features of dental materials are related to their surface area.[21] In our study, all of the samples had a similar surface area and the volume of medium was added in proportion to the samples' surface area to minimize errors. In this current study, the cytotoxicity of glass fibers, polyethylene, and Quartz Splint, which were used in FRC retainers were evaluated and compared to conventional stainless steel (SS) multi-strand wires. All the groups on 0 day and 1-day after that, in both low and high cariogenic environments, showed moderate to high cytotoxicity [Tables 1 and 2].
Lebfevre et al. examined the effects of released materials from the denture based on Hamster's oral epithelial cells and observed that the released materials caused inhibition of cell proliferation in the first 24 h.[22] In addition, it has been reported that most of the unpolymerized resins were released in the first 24 h.[11] Sheridan[23] and Ozen[24] reported that the cytotoxicity of acrylic resins was at its maximum 24 h after polymerization and then was reduced. This finding was in accordance with our study groups, with the exception of two of them. The first exception was the Quartz Splint group, in which the cytotoxicity increased significantly after 1-week in both environments and then after 1-month the amount of cytotoxicity decreased significantly [Figure 3].
Figure 3.
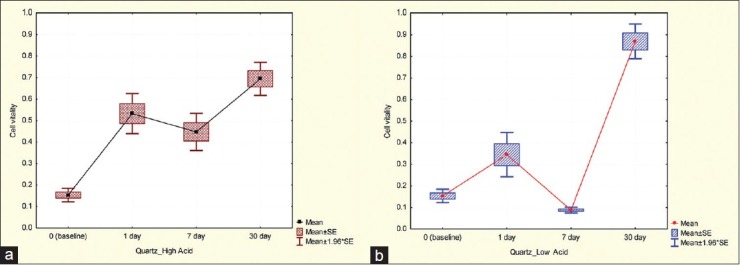
Cell viability in the Quartz Splint group, (a) High acidic environment, (b) Low acidic environment
In one study the effects of quartz on the lung of mice was evaluated and it was determined that the Quartz Splint could cause cell membrane degradation, and this phenomenon occurred from the fourth until the 8th day.[25] Similar to this study, we found a comparable increase in cytotoxicity on the 7th day, which then decreased gradually. The second exception was in the FSW group, in which the high cariogenic environment showed an increase in cytotoxicity after 1-week and the amount of cytotoxicity was reported high on the 13th day [Figure 4a].
Saliva, the environmental factor, and microbial flora of the mouth may stimulate a corrosion process. Wataha et al. showed that after much initial release, the amount of released ions from material reduced gradually. This study also indicated that most of the time, ion released from multiphase alloys such as SS occurs after the 1st week.[26] The results of our study were similar to this study, because SS wire showed higher cytotoxicity after the 1st week. Our results showed that in individuals with high caries risk, it would be better not to use FSW retainers and use the alternatives such as FRC retainers, in particular Connect, which had the least cytotoxicity among the groups in the high cariogenic environment [Figure 5].
Figure 5.
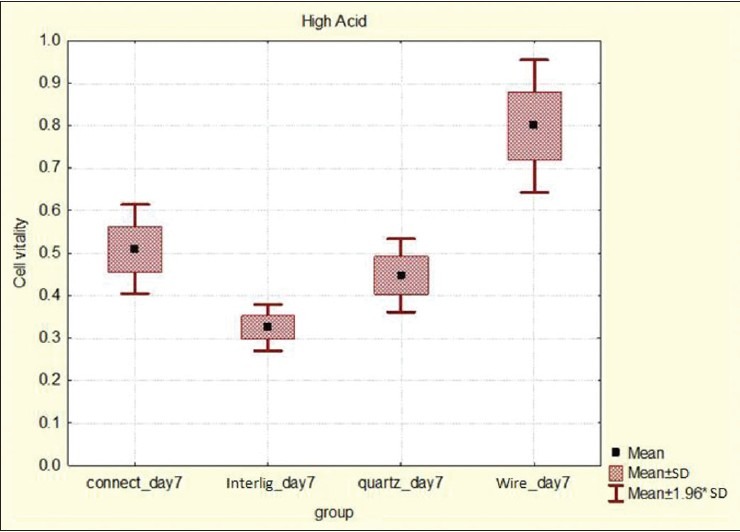
Box plot for evaluating cell viability in the high acidic environment on day 7
Bishara et al. suggested that thermocycling should be a part of new adhesive test protocols;[27] therefore, in accordance with the suggestion, the samples in our study were thermocycled. Vakiparta et al. examined the cytotoxicity effect of glass fibers on HGFs. They showed that after 11 days, growth of HGFs returned to normal status; therefore, it was concluded that glass fibers were not cytotoxic.[28]
Andrade et al.'s study, showed cytotoxicity of glass fibers after 5 and 10 days, were similar to each others (61%).[29] It has been reported that this amount of cytotoxicity is low, and it is comparable with international standards.[30,31] These studies are in accordance with our study, in which the amount of cytotoxicity in the high cariogenic environment on the seventh and 13th days was 48% and 68% respectively, and in the low cariogenic environment on the seventh and 13th days was 56% and 10%, respectively. In a study by Meric et al. it was observed that thermal cycled silica glass fibers showed 10% cytotoxicity. Therefore, they concluded polymerized silica glass FRCs did not show toxicity and thermal cycling did not affect this feature.[32] However, in our study the cytotoxicity of silica glass FRC (Interlig group) after 30 days reached 10%. The cause of lower reported cytotoxicity in Meric et al.'s study may be due to more thermal cycles (9000 cycles) and performing the MTT test on the mouse's lung fibroblast L929. In our study 5000 thermal cycles had been performed, and HGFs were applied, which were more sensitive. Also, we simulated the oral environment by using artificial saliva but in Meric et al.'s study the samples were stored in water. It should be noted that only the cytotoxicity of FRC retainers were evaluated in our study, and the cytotoxicity of adhesive resins around the retainer is another issue. An in vitro study showed no-mix adhesives had moderate cytotoxicity on the 1st day; whereas, light cure adhesive and flowable composite showed excellent biocompatibility.[33] Moreover, the primers are a potent source of cytotoxicity. Investigations on the cytotoxicity of conventional orthodontic primers showed that all of the primers were cytotoxic.[34] In another study, self-etch primers showed a similar amount of cytotoxicity with conventional primers.[35]
CONCLUSION
Flexible spiral wire retainers are not recommended in those at high-risk for dental caries. In this population, it is better to use acid-resistant retainers like Connect and Quartz Splint, due to their low cytotoxicity, but in the low-risk population, there is no difference from the standpoint of cytotoxicity.
ACKNOWLEDGMENT
The authors would like to thank the Vice-Chancellor for Research of the Mashhad University of Medical Sciences for the financial support of this project. The results described in this paper were part of a M.Sc. student thesis proposal.
Footnotes
Source of Support: This research was financially supported by research vice chancellor of Mashhad University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Graber TM, Vanarsdall V. Orthodontics: Current Principles and Techniques. 4th ed. St. Louis: Elsevier Inc; 2005. p. 1144. [Google Scholar]
- 2.Brauchli L, Pintus S, Steineck M, Lüthy H, Wichelhaus A. Shear modulus of 5 flowable composites to the everstick ortho fiber-reinforced composite retainer: An in-vitro study. Am J Orthod Dentofacial Orthop. 2009;135:54–8. doi: 10.1016/j.ajodo.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Schwarze J, Bourauel C, Drescher D. Tooth mobility of lowerincisors after direct bonding of lingual retainers. J Orofac Orthop. 1995;56:25–33. doi: 10.1007/BF02265836. [DOI] [PubMed] [Google Scholar]
- 4.Hanks CT, Wataha JC, Sun Z. In vitro models of biocompatibility: A review. Dent Mater. 1996;12:186–93. doi: 10.1016/s0109-5641(96)80020-0. [DOI] [PubMed] [Google Scholar]
- 5.Tunçel A, Ozdemir AK, Sümer Z, Hürmüzlü F, Polat Z. Cytotoxicity evaluation of two different composites with/without fibers and one nanohybrid composite. Dent Mater J. 2006;25:267–71. doi: 10.4012/dmj.25.267. [DOI] [PubMed] [Google Scholar]
- 6.Issa Y, Watts DC, Brunton PA, Waters CM, Duxbury AJ. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater. 2004;20:12–20. doi: 10.1016/s0109-5641(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 7.Roberson TM, Heymann H, Swift EM. Sturdevant's Art and Science of Operative Dentistry. 4th ed. St. Louis: Mosby; 2002. [Google Scholar]
- 8.Riss TL, Moravec RA. Use of multiple assay endpoints to investigate the effects of incubation time, dose of toxin, and plating density in cell-based cytotoxicity assays. Assay Drug Dev Technol. 2004;2:51–62. doi: 10.1089/154065804322966315. [DOI] [PubMed] [Google Scholar]
- 9.Edmondson JM, Armstrong LS, Martinez AO. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J Tissue Cult Methods. 1988;11:15–7. [Google Scholar]
- 10.Sjögren G, Sletten G, Dahl JE. Cytotoxicity of dental alloys, metals, and ceramics assessed by millipore filter, agar overlay, and MTT tests. J Prosthet Dent. 2000;84:229–36. doi: 10.1067/mpr.2000.107227. [DOI] [PubMed] [Google Scholar]
- 11.Huang FM, Tai KW, Hu CC, Chang YC. Cytotoxic effects of denture base materials on a permanent human oral epithelial cell line and on primary human oral fibroblasts in vitro. Int J Prosthodont. 2001;14:439–43. [PubMed] [Google Scholar]
- 12.Danilewicz-Stysiak Z. Experimental investigations on the cytotoxic nature of methyl methacrylate. J Prosthet Dent. 1980;44:13–6. doi: 10.1016/0022-3913(80)90038-4. [DOI] [PubMed] [Google Scholar]
- 13.Williams DF, editor. Systemic Aspects of Biocompatibility. Ch. 4. Boca Raton, Florida: CRC Press; 1981. Introduction to the toxicology of polymer-based materials; pp. 50–7. [Google Scholar]
- 14.Thompson LR, Miller EG, Bowles WH. Leaching of unpolymerized materials from orthodontic bonding resin. J Dent Res. 1982;61:989–92. doi: 10.1177/00220345820610081501. [DOI] [PubMed] [Google Scholar]
- 15.Caughman WF, Caughman GB, Shiflett RA, Rueggeberg F, Schuster GS. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials. 1991;12:737–40. doi: 10.1016/0142-9612(91)90022-3. [DOI] [PubMed] [Google Scholar]
- 16.Ferracane JL. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441–52. doi: 10.1111/j.1365-2842.1994.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson LR, Miller EG, Bowles WH. Leaching of unpolymerized materials from orthodontic bonding resin. J Dent Res. 1982;61:989–92. doi: 10.1177/00220345820610081501. [DOI] [PubMed] [Google Scholar]
- 18.Rathbun MA, Craig RG, Hanks CT, Filisko FE. Cytotoxicity of a BIS-GMA dental composite before and after leaching in organic solvents. J Biomed Mater Res. 1991;25:443–57. doi: 10.1002/jbm.820250403. [DOI] [PubMed] [Google Scholar]
- 19.Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res. 1995;74:1602–6. doi: 10.1177/00220345950740091601. [DOI] [PubMed] [Google Scholar]
- 20.Thonemann B, Schmalz G, Hiller KA, Schweikl H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent Mater. 2002;18:318–23. doi: 10.1016/s0109-5641(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 21.Meryon SD. The influence of surface area on the in vitro cytotoxicity of a range of dental materials. J Biomed Mater Res. 1987;21:1179–86. doi: 10.1002/jbm.820211002. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre CA, Schuster GS, Marr JC, Knoernschild KL. The effect of pH on the cytotoxicity of eluates from denture base resins. Int J Prosthodont. 1995;8:122–8. [PubMed] [Google Scholar]
- 23.Sheridan PJ, Koka S, Ewoldsen NO, Lefebvre CA, Lavin MT. Cytotoxicity of denture base resins. Int J Prosthodont. 1997;10:73–7. [PubMed] [Google Scholar]
- 24.Ozen J, Cumhur S, Alper AR, Dalkiz M. In vitro cytotoxicity of glass and carbon fiber-reinforced heat-polymerized acrylic resin denture base material. Turk J Med Sci. 2006;36:121–6. [Google Scholar]
- 25.Donaldson K, Bolton RE, Jones A, Brown GM, Robertson MD, Slight J, et al. Characterization and cytotoxicity of ions released from stainless steel and nickel titanium orthodontic alloys. Am J Orthod Dentofac Orthop. 2004;125:24–9. doi: 10.1016/j.ajodo.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Wataha JC, Lockwood PE, Nelson SK. Initial versus subsequent release of elements from dental casting alloys. J Oral Rehabil. 1999;26:798–803. doi: 10.1046/j.1365-2842.1999.00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Bishara SE, Ajlouni R, Laffoon JF. Effect of thermocycling on the shear bond strength of a cyanoacrylate orthodontic adhesive. Am J Orthod Dentofacial Orthop. 2003;123:21–4. doi: 10.1067/mod.2003.1. [DOI] [PubMed] [Google Scholar]
- 28.Väkiparta M, Koskinen MK, Vallittu P, Närhi T, Yli-Urpo A. In vitro cytotoxicity of E-glass fiber weave preimpregnated with novel biopolymer. J Mater Sci Mater Med. 2004;15:69–72. doi: 10.1023/b:jmsm.0000010099.58361.1b. [DOI] [PubMed] [Google Scholar]
- 29.Andrade AL, Turchetti-Maia R, Lopes M, Salas C, Domingues R. In vitro bioactivity and cytotoxicity of chemically treated glass fibers. Mater Res. 2004;7:635–8. [Google Scholar]
- 30.Annual Book ASTM Standard. Philadelphia: Medical Devices and Services; 1995. Standard A.S.T.M. Standard Practice for Direct Contact Cell Evaluation of Materials for Medical Devices; pp. 233–6. [Google Scholar]
- 31.United States Pharmacopeia, USP-XXIII. Rockville, MD: United States Pharmacopeial Convention Inc; 1995. US Pharmacopeia. Biological reactivity tests, in vitro; pp. 1697–9. [Google Scholar]
- 32.Meriç G, Dahl JE, Ruyter IE. Cytotoxicity of silica-glass fiber reinforced composites. Dent Mater. 2008;24:1201–6. doi: 10.1016/j.dental.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Ahrari F, Tavakkol Afshari J, Poosti M, Brook A. Cytotoxicity of orthodontic bonding adhesive resins on human oral fibroblasts. Eur J Orthod. 2010;32:688–92. doi: 10.1093/ejo/cjq019. [DOI] [PubMed] [Google Scholar]
- 34.D'Antò V, Spagnuolo G, Polito I, Paduano S, Ambrosio L, Valletta R. In vitro cytotoxicity of orthodontic primers. Prog Orthod. 2009;10:4–11. [PubMed] [Google Scholar]
- 35.Rhee CH, Kim IR, Kim GC, Kim SS, Son WS. In vitro cytotoxicity of self-etching primers. Korean J Orthod. 2006;36:422–33. [Google Scholar]


