Abstract
Osteoarthritis (OA) is a major public concern as it is one of the leading causes of morbidity and lays a huge medical and economic burden on health resources. Early detection of OA has been a clinical challenge as early signs of joint inflammation are often not evidently identifiable on routine radiographic images. This presents a dire unmet medical need for a biomarker, which could detect early signs of joint inflammation much before irreversible joint damage and radiographic changes set in. Besides, the treatment of OA has remained mainly symptomatic. A disease modifying OA drug (DMOAD), which can act as targeted anti-OA therapy has not been able to receive regulatory approval yet. The clinical development of a DMAOD too warrants the need of a biomarker; which can act as a surrogate clinical endpoint used to monitor therapeutic efficacy and to validate a clinically meaningful change within the restricted time frame of a clinical study. In this regard, the current review focuses on cartilage oligomeric matrix protein (COMP), a potential OA biomarker which has shown significant clinical promise as a tool for early detection, therapeutic monitoring, prognostication and drug development for OA. This brief review is pivoted around the findings of selected relevant publications from PubMed indexed journals.
Keywords: Biomarker, cartilage oligomeric matrix protein, disease modifying osteoarthritis drug, drug development, monitoring, osteoarthritis, prognosis
INTRODUCTION
Osteoarthritis: Making the healthcare fraternity go “weak in its knees”
Clinicians, patients, healthcare authorities, and researchers all over the world are watching the growing prevalence of osteoarthritis (OA) and its associated debility, with deep seated concern and apprehension. These concerns can be traced to robust epidemiological evidence elucidating the huge burden of OA in developing nations and its rapidly growing prevalence.[1]
As advancing age is one of the foremost factors in the etiology of OA, an ageing population increases the disease burden of such age-related degenerative disorders. In this regard, epidemiological figures state that the percentage of the geriatric populace in Asia (defined as those ≥65 years of age) is expected to double from 6.8% in 2008 to 16.2% in 2040. Speaking specifically of India, the proportion of geriatrics in India is expected to increase by a whopping 274% by 2040. Dating back to 2008, in terms of absolute number of geriatric individuals, India ranked third in the world with a total head count of 60 million geriatric individuals.[1]
Let us consider this postulate: The total Asian population is estimated to touch the 4.3 billion mark in the next 20 years. Of these, approximately 20% would be people above 60 years of age. If 15% of these elderly individuals experience symptomatic OA (which is a rather conservative figure), would imply that Asia alone would have 130 million cases of symptomatic OA in the next 20 years. Even if one-third of these symptomatic cases experienced long standing aggravation of disease, Asia would be grappling with an exponential 40 million cases of OA with severe debility.
Besides ageing, strenuous occupational physical labor, which is rampant in rural Asia and rising obesity reported in the urban pockets further compound the problem. Lack of accessibility to surgical measures like joint replacement therapy is an added concern.[1] What makes matters worse is the fact that in developing countries like India, <15% of the total population is covered under medical insurance. Hence, OA as a disease lays a great socioeconomic burden on healthcare resources.[2]
The overall appraisal of this epidemiological evidence sends across a loud and clear message: “Early diagnosis of OA can be the best method to arrest its growing prevalence. Better clinical monitoring markers are needed to assess the efficacy of ongoing OA therapy and the severity of the disease. The situation also presents an unmet medical need to identify and develop markers for prognostication of OA cases to facilitate effective treatment decisions and to facilitate clinical trials of targeted therapies”.
A clinical quest for biomarkers in osteoarthritis
In early stages of OA, degenerative articular changes are not evident on radiological images. Joint space narrowing and presence of osteophytes on radiological scans are abnormalities, which can be seen at a later stage. Besides, it is known that routine radiographic techniques show poor sensitivity and specificity toward detection of OA. Though advanced radiographic methods like magnetic resonance imaging overcome the limitations of plain film radiography, these methods are very expensive and not used as frequently as routine techniques.[3]
Osteoarthritis is an inflammatory degeneration of the joint accompanied by specific molecular and pathophysiological changes. These molecular changes obviously cannot be captured on a radiological image. Besides, these molecular changes often precede radiological degeneration. These shortcomings of routine radiography collectively present an unmet medical need of potential biomarkers for timely identification of patients at a risk of developing OA or those with early disease.[3]
A few other impediments have also been cited inaccurate early diagnosis of OA. The pathophysiology of OA as a disorder per se is very complex, dynamic and has not yet been fully understood to a granular level. To add to this, the multifactorial etiology of OA and its phase wise and site specific pathological changes; further complicate the understanding of the disease process. Besides, OA has a very slow course of progression. As a result of all these factors, developing targeted therapies and newer drug molecules for OA poses a great scientific and technical challenge. Deciphering the possible mechanism of action of a new molecule, which could be a therapeutic candidate for OA, poses a complex clinical question. So also, given the slow progressing nature of OA, any new therapy would require a relatively longer period of time to manifest tangible clinical benefits. For example, even though a disease modifying OA drug (DMOAD) would interrupt disease progression and slow down joint space narrowing, its benefit would not become evident on radiographic images in a short period of time. Due to such factors, clinical trials of DMOADs or of new targeted therapies for OA need to be extended for a sufficiently longer duration. This makes their conduct complex and expensive. In such situations, a dire need is felt for a biomarker, which can serve as an early surrogate clinical endpoint; to predict the efficacy of a DMAOD or of any other targeted therapy, within the restricted time frame of a clinical trial.[3,4]
In this context, the case of biomarkers in OA is very interesting as one technical difficulty or loophole leads to another. Consider this sequence of facts: The proposition of biomarkers in OA has been known to the research fraternity for decades together, is evolving since then but has not yet been able to reach a point wherein it could be used as a routine screening tool for all OA patients to catch them early!! On one hand, clinicians all over the world are waiting for a DMOAD for more definite management of OA. However, hitherto a DMAOD has not been developed or approved for routine use as anti-OA therapy. On the other hand, the very development of a DMAOD requires a biomarker to monitor its efficacy during clinical trials. This forms a vicious circle.[4]
Therefore, several cartilage derived, synovium based, blood derived, or bone derived biomarkers are being studied for their utility in the monitoring and prognosis of OA to be employed in routine practice as well as in clinical trials of DMOADs. Cartilage oligomeric matrix protein (COMP), matrix metalloproteinases, markers derived from type I collagen (NTx), derived from type II collagen (N-terminal propeptide and C-telopeptide 2 [CTX-II]), aggrecanases, calcitonin, and hyaluronic acid are some of the biomarkers, which have been extensively investigated.[3]
In lieu of this growing interest for biomarkers of OA, the “OA Biomarkers Network” of the National Institute of Health, put forth a widely accepted classification of OA biomarkers. As per this classification, biomarkers of OA have been divided into six broad categories as biomarkers which predict “burden of disease”, “investigative”, “prognostic,” those mirroring “efficacy of intervention,” “diagnostic,” and “safety” (BIPEDS) related biomarkers.[5] These six categories have been encapsulated into a single acronym as BIPEDS. Table 1 summarizes the utility of BIPED biomarkers of OA (safety not included) and highlights the most suitable clinical trial design recommended for evaluating each of these, based on inputs and inferences from previous research.[5]
Table 1.
Clinical utility and recommended trial designs for OA biomarkers as per BIPED classification
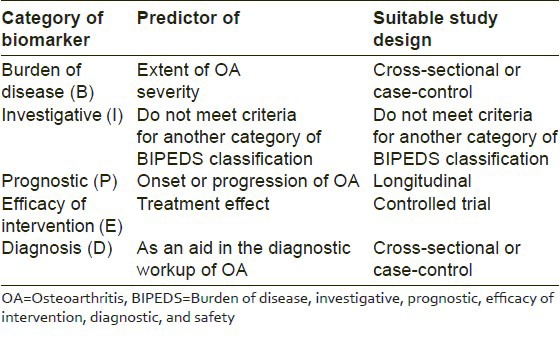
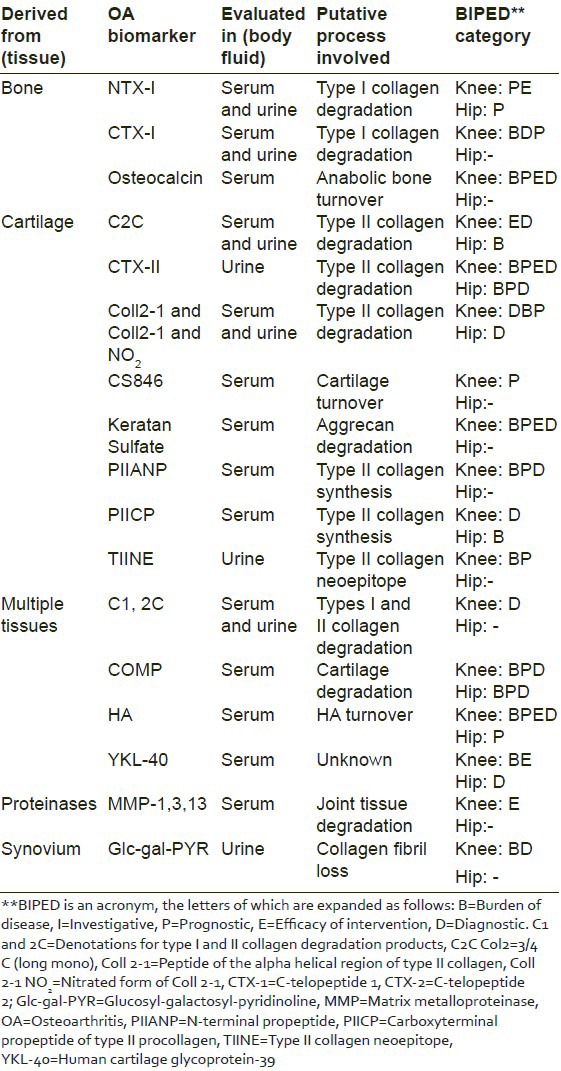
Given the context, it would be interesting and pertinent to highlight the clinical utility of a few prominent biochemical biomarkers of the knee and hip OA, in the light of the BIPEDS classification. A summary has been presented in Table 2.[5]
Table 2.
Clinical utility of tissue derived OA biomarkers as per BIPED classification
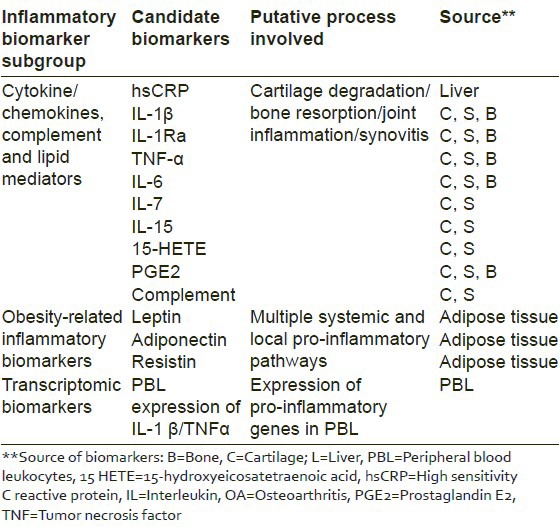
Past studies have laid special emphasis on the inflammatory biomarkers of OA. These can be mainly categorized as cytokines/chemokines/lipid mediators, obesity related and transcriptomic biomarkers [Table 3].[6]
Table 3.
Inflammatory biomarkers of OA
Clinical evidence suggests that among the several biomarkers researched for their potential utility in OA, COMP has shown the best clinical promise. It is thus a frontrunner among biomarkers of OA, which could foray into routine clinical practice and drug development activities for OA.[7] This review focuses on the utility of COMP as a biomarker for monitoring disease progression and therapeutic efficacy in OA and for prognostication of this disease.
For the purpose of this review, an extensive search of PubMed indexed journals was conducted. The results generated by the search were analyzed by the authors for direct relevance to the research area of COMP, as a monitoring and prognostic biomarker in OA and its implications in drug development. A total of 21 such publications were identified for inclusion in the review. These were selected based on the authors’ subjective analysis of the robustness and pertinence of the clinical information put forth by each of these publications, toward the topic of interest. In addition to these, the publication by Fransen et al. and a World Health Organization bulletin were also included for presenting qualitative epidemiological data pertaining to OA in Asia.
Cartilage oligomeric matrix protein as a biomarker in osteoarthritis: The pathophysiological rationale
Cartilage oligomeric matrix protein is an essential structural and functional component of the extracellular matrix of the cartilage. COMP has a definite role to play in the cellular proliferation and apoptosis and in the regulation of cell movement and attachment in the cartilaginous tissue. OA is a typical inflammatory condition characterized by degradation of cartilage and its extracellular matrix. Using COMP as a biomarker of cartilage metabolism can proficiently mirror these degenerative inflammatory changes in the joint. Serum COMP measurements can quantitatively predict variations in joint remodeling, cartilage loss, and depletion of extracellular matrix. As cartilage degradation commences, serum COMP levels begin to escalate. As a result, serum COMP levels can be detected quite early during the inflammatory process, in the synovial fluid and subsequently in the bloodstream, much before radiographic joint degeneration and irreversible damage set in. Therefore, patients at risk and those with early inflammation can be identified effectively with serum COMP assessments. This cascades a series of favorable events. Early detection aids early therapeutic intervention and interrupts the process of disease progression, thereby preventing irreversible radiographic changes. Serological identification of OA patients thus presents a promising, noninvasive, and cost-effective method in the clinical management of OA.[8]
Clinical evidence speaks
Mounting clinical evidence supports the use of COMP as a marker of monitoring and prognostication of OA.
Data from the Johnson County OA Project's clinical study revealed that serum COMP levels correlate closely with disease severity. COMP levels tend to escalate with an increase in the number of arthritic joints in an individual. Hence, patients with bilateral knee or hip OA would have higher COMP levels as compared to those with unilateral involvement.[9] Clinical evidence has also established a strong correlation of COMP levels with bone scan abnormalities seen in OA patients. Higher COMP levels have been reported in the subgroup of OA patients with radiographically evident articular damage.[10] This also gives an idea about the positive predictive potential of serum COMP measurements. Past studies have confirmed that COMP levels increase as OA progresses radiographically on the Kellgren Lawrence scale.[8] Elevated COMP levels have also been reported in familial OA associated with genetic mutations in collagen metabolism.[11]
Past clinical investigations conducted over a period of 1-3 years have demonstrated serum COMP elevations in early as well as chronic OA cases.[12,13] Baseline levels of COMP are known to be predictive of progression. Evidence has revealed that patients with higher baseline COMP levels were more vulnerable to develop progressive advanced OA, which was later confirmed radiographically through joint space narrowing seen over a 1-year period.[14] In another similar study, increase in OA severity expressed as an increase in Kellgren Lawrence scales; correlated positively with a corresponding elevation of COMP levels.[15] Logistic regression analysis figures from past clinical trials reveal that a 1-unit increase in serum COMP levels is associated with a possibility of 15% radiographic progression.[16] Data from past studies has confirmed elevations in serum COMP levels post joint replacement therapy and in knee injury leading to posttraumatic arthritis.[16,17]
Data cited here is perhaps just the tip of the iceberg. Mounting volumes of clinical evidence speak in favor of the use of COMP as a biomarker for monitoring and prognostication of OA. We thus infer that COMP is a promising biomarker for preradiographic prediction of OA based on clinical signs and symptoms, risk stratification of vulnerable groups as well as for use in conjunction with imaging techniques in radiographically established OA. COMP has also shown promise in monitoring OA progression, determining OA severity, treatment planning, in predicting the overall prognosis, and future course of the disease.
Do cartilage oligomeric matrix protein levels correlate with Western Ontario and Mcmaster Universities Arthritis Index scores? Divergent evidence
The Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a widely employed and well validated scale to assess pain, stiffness, and the status of physical functioning; in patients with knee and hip OA. An improvement in WOMAC scores is a measure of symptomatic relief and improved quality-of-life in OA patients. It is thus essential to understand whether changes in the level of an OA biomarker are reflected in the WOMAC scores of the patient. In other words, the correlation between levels of OA biomarkers and WOMAC scores comprises a crucial clinical end point.
In this regard, it is noteworthy to consider the results of past studies wherein the correlation between COMP levels and WOMAC scores was analyzed as one of the variables. Serum COMP levels were assessed in a total of 150 patients of knee OA in a controlled study at Lucknow, India and the COMP values obtained thereof were subsequently correlated with WOMAC scores.[18] The study showed a strong positive correlation between COMP values and WOMAC scores. Higher COMP values among OA patients resulted in poor WOMAC scores as compared with controls. Furthermore, an improvement was seen in WOMAC scores with a reduction in COMP values among OA patients posttreatment.[18] Darweesh et al. studied the correlation between COMP levels and clinical parameters among OA patients. The study showed a statistically significantly positive correlation between synovial and serum COMP levels and the WOMAC index.[19] A similar finding was put forth by Azab et al. who reported a statistically significantly positive correlation between COMP values and WOMAC scores among 40 patients with symptomatic knee OA.[20]
In contrast to these studies, which showed a positive correlation between COMP levels and all parameters of the WOMAC scale; Sowers et al. have reported that serum COMP levels correlated with knee pain scores, but not with knee function scores of the WOMAC index; in a study among OA affected women.[21] Similarly, Wislowska and Jablonska too reported a statistically significant correlation between COMP levels and only the pain component of the WOMAC index; among 30 OA patients.[22]
With regards to COMP levels and their impact on WOMAC scores, a few other studies have put forth completely contradictory views. In a study conducted on 57 serum samples of knee OA patients, to assess the prognostic potential of COMP; Lai et al. did not find any correlation between COMP values and WOMAC scores.[23] El-Arman et al. conducted a study among 66 patients to study the diagnostic utility of aggrecan and COMP in knee OA. In this study too, WOMAC scores correlated neither with COMP nor with aggrecan levels.[24] Garnero et al. evaluated a wide panel of biochemical markers of OA and studied their correlation with clinical parameters in 67 patients of knee OA. In this evaluation, urinary CTX-II, Glc-Gal-PYD, S-total OC, and S-PIIINP were the only biomarkers which showed a significant correlation with WOMAC scores. None of the other biomarkers evaluated in this study, including COMP, were found to be predictive of WOMAC scores.[25]
The presence or absence of a correlation between the levels of an OA biomarker and WOMAC scores, leads us to medically divergent explanations. The presence of such a correlation logically stems from the fact that reduced serum levels of cartilage degradation products like COMP are directly indicative of reduced cartilage loss, reduced inflammation. These changes can get mirrored as symptomatic improvement documented on the WOMAC scales. On the other hand, the absence of any correlation between COMP levels and the WOMAC index is perhaps attributable to the understanding that WOMAC scores are merely symptomatic in nature. These scores may not necessarily correlate with the actual degree of cartilage loss. Symptomatic relief as depicted by the WOMAC index may not necessarily imply cessation or significant reduction in tissue damage.[23,24]
Hence, it can be inferred that currently, clinical evidence regarding the correlation of COMP with WOMAC scores is divergent in nature. Though WOMAC scores have been a frequent variable of past studies evaluating COMP as a biomarker of OA; there is a serious dearth of studies assessing this correlation as the primary endpoint. Due to this lack of robust data, it seems difficult to build a common medical consensus regarding this correlation. Hence, this represents a need to conduct large, adequately powered, multicentric, and controlled clinical trials primarily to determine the correlation between COMP values and the WOMAC index among OA patients.
Can biomarkers in osteoarthritis change the paradigm of treatment?
The US-Food and Drug Administration mandates that for marketing approval, a DMOAD needs to show clinically meaningful change within a period of 1 year. Hitherto, none of the DMOAD candidates that entered clinical trials have been able to fulfill this criterion. As explained earlier in this paper, this is due to the slowly progressive nature of OA due to which any therapeutic intervention requires a long time to show its efficacy. This is particularly true when the efficacy of a DMOAD is measured through radiographic improvement seen vis-à-vis reduction in joint space narrowing. This is a very slow change and it could take years together to ascertain interruption of joint space narrowing by a DMAOD on a radiograph. If that's the case, how do we gather evidence in favor of DMOADs? Should we run clinical trials for several years together until joint space narrowing occurs or gets interrupted? Is this practical or diligent enough an approach given the exponentially high costs and logistic complexities involved in conducting clinical trials? Nevertheless, the idea of clinical development of DMOADs has remained stagnated by these technical glitches. Besides, the mainstay of OA treatment has remained restricted to symptomatic treatment without addressing the root cause at a structural and pathological level.[3,4]
This conundrum can be resolved if biomarkers of OA can be introduced as surrogate endpoints in clinical trials and subsequently as surrogate markers in clinical practice. Therapeutic benefit caused by a DMOAD can be expressed with the aid of a biomarker like COMP which can mirror and flag this therapeutic effect at a much earlier stage than radiography. This approach can assist in establishing a “clinically meaningful change” within a year which can fulfill the regulatory prerequisite for approving and marketing a DMOAD. Furthermore, whenever DMOADs come into routine clinical practice, a biomarker like COMP can be employed for assessing disease severity pre- and post-DMOAD therapy and to plan future therapy more effectively.
If we succeed in adopting this surrogate approach toward drug development and clinical practice, perhaps we shall also succeed in shifting the treatment paradigm of OA from merely symptomatic to robustly pathophysiological and molecular. In this regard, a biomarker like COMP can certainly act as a “strong joint”.[3,4]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis. 2011;14:113–21. doi: 10.1111/j.1756-185X.2011.01608.x. [DOI] [PubMed] [Google Scholar]
- 2. [Last accessed on 2013 Dec 15]. Available from: http://www.who.int/bulletin/volumes/88/7/10. 0207 10/en/
- 3.Abramson S, Krasnokutsky S. Biomarkers in osteoarthritis. Bull NYU Hosp Jt Dis. 2006;64:77–81. [PubMed] [Google Scholar]
- 4.Patra D, Sandella LJ. Recent advances in biomarkers in osteoarthritis. Curr Opin Rheumatol. 2011;23:465–70. doi: 10.1097/BOR.0b013e328349a32b. [DOI] [PubMed] [Google Scholar]
- 5.Wildi L, Tamborrini G. Biomarkers in osteoarthritis. In: Johanne Martel-Pelletier, Jean-Pierre Pelletier., editors. Text book on “Understanding Osteoarthritis from Bench to Bedside”. 1st edition. Kerala: Research Signpost Publishers; 2011. pp. 103–25. [Google Scholar]
- 6.Attur M, Krasnokutsky-Samuels S, Samuels J, Abramson SB. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25:136–44. doi: 10.1097/BOR.0b013e32835a9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams FM, Spector TD. Biomarkers in osteoarthritis. Arthritis Res Ther. 2008;10:101. doi: 10.1186/ar2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng S, Reddi AH, Di Cesare PE. Cartilage oligomeric matrix protein (COMP): A biomarker of arthritis. Biomark Insights. 2009;4:33–44. doi: 10.4137/bmi.s645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: The Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42:2356–64. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Petersson IF, Boegård T, Dahlström J, Svensson B, Heinegård D, Saxne T. Bone scan and serum markers of bone and cartilage in patients with knee pain and osteoarthritis. Osteoarthritis Cartilage. 1998;6:33–9. doi: 10.1053/joca.1997.0090. [DOI] [PubMed] [Google Scholar]
- 11.Bleasel JF, Poole AR, Heinegård D, Saxne T, Holderbaum D, Ionescu M, et al. Changes in serum cartilage marker levels indicate altered cartilage metabolism in families with the osteoarthritis-related type II collagen gene COL2A1 mutation. Arthritis Rheum. 1999;42:39–45. doi: 10.1002/1529-0131(199901)42:1<39::AID-ANR5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegård D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34:306–10. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 13.Petersson IF, Boegård T, Svensson B, Heinegård D, Saxne T. Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br J Rheumatol. 1998;37:46–50. doi: 10.1093/rheumatology/37.1.46. [DOI] [PubMed] [Google Scholar]
- 14.Conrozier T, Saxne T, Fan CS, Mathieu P, Tron AM, Heinegård D, et al. Serum concentrations of cartilage oligomeric matrix protein and bone sialoprotein in hip osteoarthritis: A one year prospective study. Ann Rheum Dis. 1998;57:527–32. doi: 10.1136/ard.57.9.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilím V, Olejárová M, Machácek S, Gatterová J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:707–13. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 16.Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479–88. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 17.Kühne SA, Neidhart M, Everson MP, Häntzschel H, Fine PR, Gay S, et al. Persistent high serum levels of cartilage oligomeric matrix protein in a subgroup of patients with traumatic knee injury. Rheumatol Int. 1998;18:21–5. doi: 10.1007/s002960050049. [DOI] [PubMed] [Google Scholar]
- 18.Shahi U, Shahi NT, Khanna V, Gupta A, Singh S, Bajpayi J. Cartilage oligomeric matrix protein: A potential diagnostic, prognostic and therapeutic biomarker of knee osteoarthritis. Scientific poster at Orthopaedic Research Society Congress. 2013 [Google Scholar]
- 19.Darweesh H, Abbass D, Kadah R, Rashad A, El Basel M, Nasr A. Serum and synovial cartilage oligomeric matrix protein in patients with rheumatoid arthritis and osteoarthritis. Indian J Rheumatol. 2010;5:112–7. [Google Scholar]
- 20.Naglaa IA, Taher AA, Ibrahim EM. Evaluation of the role of cartilage oligomeric matrix protein and ykl-40 as biomarkers in knee osteoarthritic patients. Nat Sci. 2012;10:43. [Google Scholar]
- 21.Sowers MF, Karvonen-Gutierrez CA, Yosef M, Jannausch M, Jiang Y, Garnero P, et al. Longitudinal changes of serum COMP and urinary CTX-II predict X-ray defined knee osteoarthritis severity and stiffness in women. Osteoarthritis Cartilage. 2009;17:1609–14. doi: 10.1016/j.joca.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisłowska M, Jabłońska B. Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin Rheumatol. 2005;24:278–84. doi: 10.1007/s10067-004-1000-x. [DOI] [PubMed] [Google Scholar]
- 23.Lai Y, Yu XP, Zhang Y, Tian Q, Song H, Mucignat MT, et al. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthritis Cartilage. 2012;20:854–62. doi: 10.1016/j.joca.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Arman MM, El-Fayoumi G, El-Shal E, El-Boghdady I, El-Ghaweet A. Aggrecan and cartilage oligomeric matrix protein in serum and synovial fluid of patients with knee osteoarthritis. HSS J. 2010;6:171–6. doi: 10.1007/s11420-010-9157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: Relations with disease activity and joint damage. Ann Rheum Dis. 2001;60:619–26. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]


