Abstract
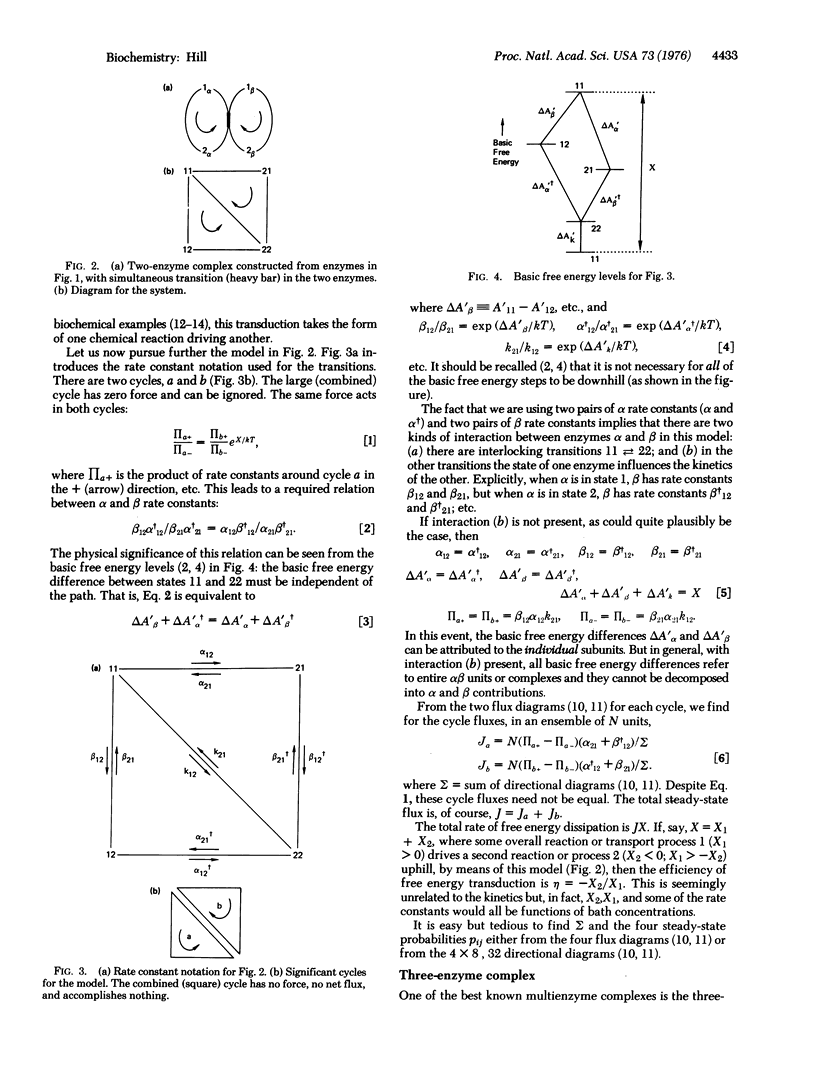
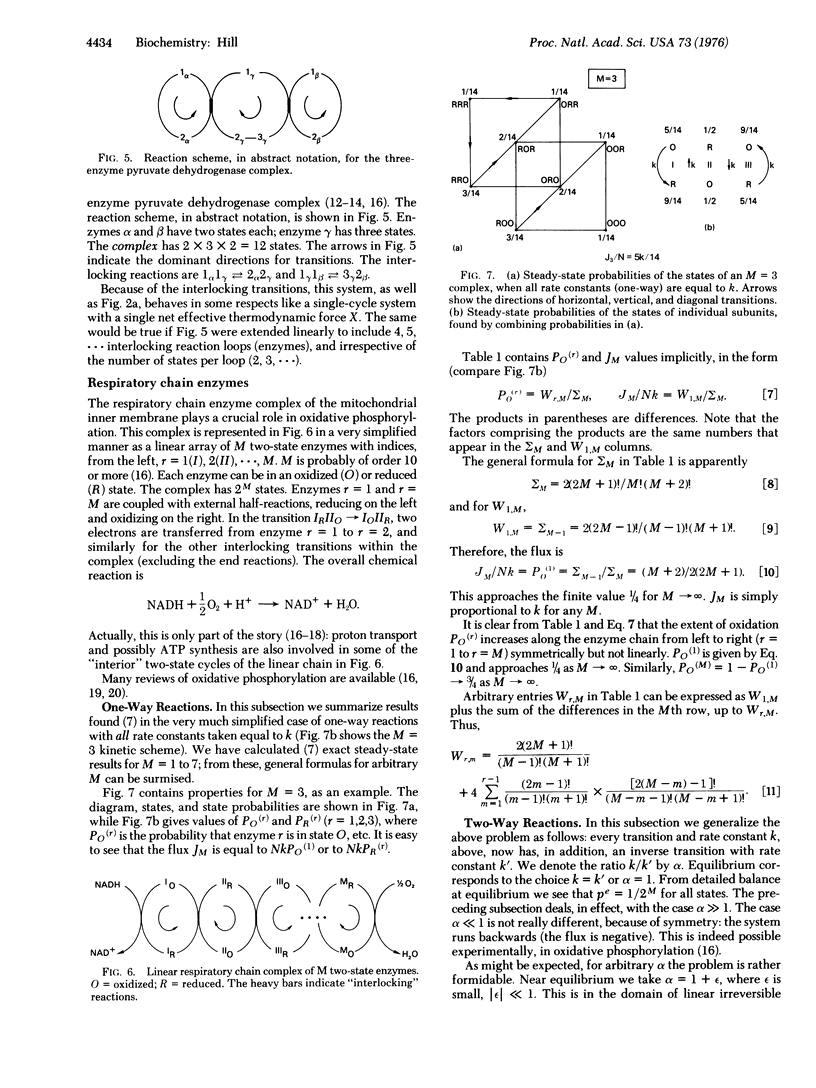
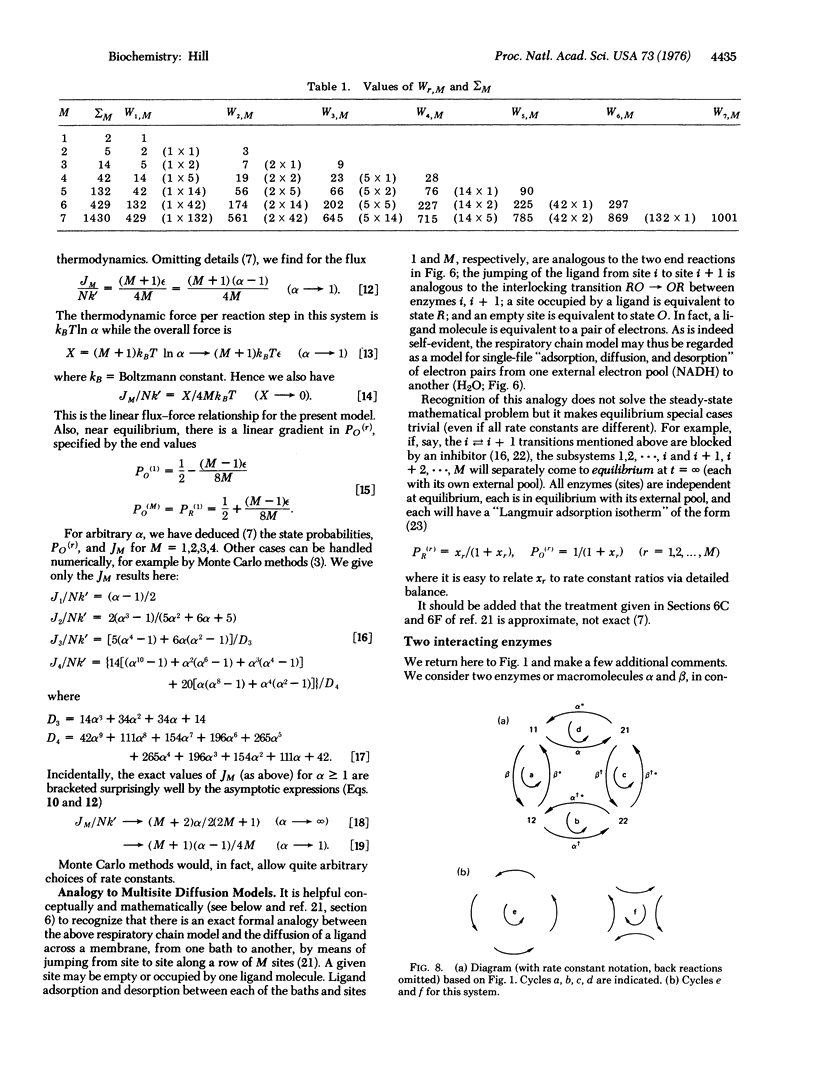
A kinetic formalism, quite generally valid for free energy transducing, steady-state, macromolecular systems in biology, is applied here to multienzyme complexes, oxidative phosphorylation, and interacting enzymes. Systems of this type, comprising several interacting subunits, each with its own discrete set of states, present no new features in principle. Hence, they may be handled by the earlier kinetic formalism without modification. However, the kinetic diagram can become quite complicated because the state of each subunit (enzyme) must be specified in order to specify any one state of the system (complex) as a whole. Cycles, forces, fluxes, free energy levels, and state probabilities are considered.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Ginsburg A., Stadtman E. R. Multienzyme systems. Annu Rev Biochem. 1970;39:429–472. doi: 10.1146/annurev.bi.39.070170.002241. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. Cooperative effects in models of steady-state transport across membranes. IV. One-site, two-site, and multisite models. Biophys J. 1971 Sep;11(9):685–710. doi: 10.1016/S0006-3495(71)86248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. Stochastics of cycle completions (fluxes) in biochemical kinetic diagrams. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1291–1295. doi: 10.1073/pnas.72.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Free energy and the kinetics of biochemical diagrams, including active transport. Biochemistry. 1975 May 20;14(10):2127–2137. doi: 10.1021/bi00681a014. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Simmons R. M. Free energy levels and entropy production associated with biochemical kinetic diagrams. Proc Natl Acad Sci U S A. 1976 Jan;73(1):95–99. doi: 10.1073/pnas.73.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Simmons R. M. Free energy levels and entropy production in muscle contraction and in related solution systems. Proc Natl Acad Sci U S A. 1976 Feb;73(2):336–340. doi: 10.1073/pnas.73.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Studies in irreversible thermodynamics. IV. Diagrammatic representation of steady state fluxes for unimolecular systems. J Theor Biol. 1966 Apr;10(3):442–459. doi: 10.1016/0022-5193(66)90137-8. [DOI] [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in energy transduction: a logical development of biochemical knowledge. J Bioenerg. 1972 May;3(1):5–24. doi: 10.1007/BF01515993. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Simmons R. M., Hill T. L. Definitions of free energy levels in biochemical reactions. Nature. 1976 Oct 14;263(5578):615–618. doi: 10.1038/263615b0. [DOI] [PubMed] [Google Scholar]
- Stone A. J. A proposed model for the Na+ pump. Biochim Biophys Acta. 1968 Jun 11;150(4):578–586. doi: 10.1016/0005-2736(68)90047-3. [DOI] [PubMed] [Google Scholar]


