Abstract
Prunus armeniaca L. (Apricot) is a tree cultivated in different parts of the world. Apricot kernel as a good dietary supplement has shown antioxidant, anti-inflammatory and other pharmacologic properties which suggest that it may be functional as an anticolitis agent. In this study we evaluated the effects of apricot kernel extract and oil on ulcerative colitis in rats. Rats were fasted for 36 h before the experiment. Colitis was induced by intra-rectal instillation of 50 mg/kg trinitrobenzene sulfonic acid in male Wistar rats. Treatments were started 6 h after colitis induction and continued every 24 h for 5 days. Apricot kernel extract (100, 200, 400 mg/kg p.o. and 100, 400 mg/kg i.p.) and apricot kernel extract/oil (100, 200, 400 mg/kg p.o.) were used as experimental treatments and prednisolone (4 mg/kg p.o. or i.p.) was used as reference drug. On the day 6, colon tissue was removed and macroscopic and pathologic parameters were evaluated. Ulcer index and total colitis index as representative of macroscopic and histologic parameters respectively showed ameliorating effects in experimental groups especially those treated by intraperitoneal administration route. Results also demonstrated that oil fraction was not able to potentiate the effects of extract. These data suggest that apricot kernel extracts (with or without oil) can be introduced for further mechanistic and clinical studies as a complementary medicine for inflammatory bowel disorders.
Keywords: Inflammation, Ulcerative colitis, Plant extract, Prunus armeniaca L., TNBS, Rats
INTRODUCTION
Inflammatory bowel diseases (IBDs) including ulcerative colitis (UC) and Crohn's disease are chronic inflammatory disorders of the gastrointestinal tract (1). They are defined by macroscopic and histopathologic features including mucosal inflammation that extends proximally from the rectum to a varying degree, severe inflammation and the coin-cident production of a complex mixture of inflammatory mediators, extensive superficial mucosal ulceration and presence of a significant number of neutrophils within the lamina propria and the crypt abscesses. Studies have demonstrated a recent slow increase in the incidence of UC in the world (2,3). Pharmacologic treatments of IBDs include drugs of different classes and mechanisms due to the unknown etiology of diseases including aminosalicylates, gluco-corticoids, immunomodulators and anti-tumor necrosis factors (4). Evidence exists that complementary medicine as a substantial part of health-care and a growing behavior in many parts of the world has been widely used by IBD patients and high levels of expenditures over these treatments have been reported (5,6).
Prunus armeniaca L. (Apricot) is a tree classified under Rosaceae family (7) and is mostly cultivated in Korea, China, India, Japan, Iran, North Africa and United States of America (8).
Apricot kernel is a good dietary source of protein, oil, fibre, phenolic and cyanogenic compounds (9,10,11). Oleic acid and linoleic acid are the main components of the oil fraction and pectin polysaccharides, cellulose and hemicellulose are the main polysaccharides (12). Apricot kernel has been traditionally used in gastric inflammations, dermatitis and also as a carminative agent. The oil has also been used as laxative and remedy for otitis and tinnitus in drop dosage form (8,13).
Pharmacologic studies have also shown antioxidant and radical scavenging properties (9,14), antimicrobial activity (14) and antitussive effects (15) for apricot kernel. Considering above mentioned activities, this study was carried out to determine whether two main fractions of apricot kernel, including extract and extract/oil, could exert anticolitis effects in rat model of experimental colitis.
MATERIALS AND METHODS
Extract preparation
Sweet apricot kernels were purchased from Maragheh (East Azarbaijan, Iran) in November 2009 and authenticated in Kermanshah Natural Sources Bureau.. Apricot kernels (500 g) were finely powdered and wetted with 500 ml of ethanol for 2 h and 500 ml ethanol was added. After 48 h 500 ml ethanol was added again. Twenty four h later, the extract was filtered through Buchner funnel. The obtained extract consisted of 2 phases (aqueous phase and oil phase). The solvents were evaporated and studies were carried out on the concentrated aqueous phase (extract) and aqueous phase mixed with oil (extract/oil: 50/50 v/v) separately.
Total phenol content of the fruit
Phenolic compounds were determined using the Folin-Ciocalteu method described by Waterhouse and coworkers (16). Gallic acid, as the standard for phenolic compound (0.5 g) was dissolved in 10% hydroalcoholic solution and was diluted with water to obtain 0, 50, 100, 150, 250 and 500 mg/L concentrations as the reference solutions. Extract fraction was used as samples solution. Absorbance of blank, reference and sample solutions (Memmert, France) were determined at 585 nm and absorbance-concentration plot was constructed. Phenol concentrations in unknown samples were determined by calibration curve. The phenolic content was expressed as gallic acid equivalent (GAE) of test samples.
Pectin determination
Sweet kernel powder (10 g) was boiled with 40 ml of ethanol 96% for 10 min, the solvent was decanted and 100 ml water was added and boiled again. It was filtered using Buchner funnel. The pH of the solution was adjusted to 6.5 with ammonia solution. It was then concentrated in rotary evaporator and ethanol was added (4 times of the remained residual) and pectin precipitated. Pectin was separated using Buchner funnel and dried at room temperature for 24 h (17).
Animals
Male Wistar rats (200 ± 25g) were bred in the animal house of Isfahan University of Medical Sciences and housed singly in wire-bottomed cages under normal temperature, humidity and light/dark cycles conditions where fasted for 36 h before induction of colitis. During the fast time they had free access to water. All experiments were conducted according to the Local Ethics Guidelines for Research on Animals and approved by the Research Committee of Isfahan University of Medical Sciences.
Chemicals
Prednisolone was provided by Iran Hormone Pharmaceutical Co. (Tehran, Iran). Trinitrobenzene sulfonic acid (TNBS) was purchased from Sigma-Aldrich (Buchs, Switzerland). Folin-Ciocalteu reagent and all other organic solvents which were of analytical grade were procured from Merck (Darmstadt, Germany).
Animal groups
Animals were divided randomly into 13 groups of 6 animals each including: sham group; without colitis induction and treated with extract vehicle (normal saline), control group: treated with vehicle 6 h after colitis induction which continued for 5 consecutive days, extract groups treated with kernel extract orally (p.o.) (100, 200, 400 mg/kg) and intraperitoneally (i.p.) (100, 400 mg/kg), extract/oil groups treated with kernel extract/oil (100, 200, 400 mg/kg) only by oral route. Reference groups treated with prednisolone 5 mg/kg (p.o. or i.p.) separately. All treatments were made the same as control group.
Induction of experimental colitis
Fasted rats were lightly anesthetized with diethyl ether inhalation. Colitis was induced by instillation of 0.3 ml TNBS (50 mg/kg) intra-rectally. Rats were held in a vertical position for 30 s to ensure that solution doesn’t leak out and were returned to their cages. After colitis induction, rats had free access to water and pelleted chow (18,19). Six h after colitis induction treatments were started and repeated daily for 5 consecutive days (19,20).
Scoring of lesions and pathological evaluation
At the day 6 (24 h after the last treatment), rats were euthanized by ether overdose inhalation. Abdomen was opened and 8 cm of colon and 3 cm proximal to the anus was excised, incised longitudinally and washed with normal saline solution. Then the tissues were weighed and weight/length ratio was calculated for each specimen. Ulcer area was measured using 3M® (USA) scaled tape and ulcers were scored (0-4) by the criteria described by Morris and coworkers (21). Macroscopic scores were: 0=no ulcer, 1=mucosal erythema only, 2=mild mucosal edema, slight bleeding or slight erosion, 3=moderate edema, bleeding ulcers or erosions, 4=severe ulceration, erosions, edema and tissue necrosis and perforation. Ulcer index was the result of summing ulcer severity and ulcer area which can be a good indicative of macroscopic lesions (20,22).
The colon tissues were also fixed in 10% formalin solution and then were embedded in paraffin, processed and sectioned into 4 μm-thick slices and samples were stained with hematoxyllin and eosin. Microscopic studies were carried out using a Zeiss® microscope equipped with a Sony® color video camera for digital imaging. Inflammation score was graded from 0 to 3 depending on the severity of the inflammation and infiltration of immune cells. Inflammation extent was graded from 0 to 3 with regard to the width of colon membrane affected by colitis including mucosa, sub-mucosa and transmural layers. Crypt damage was graded from 0 to 4 considering the damage of crypt and epithelial cells (23,24,25). Total colitis index (TCI) was calculated by summing inflammation severity, inflammation extent and crypt damage (19).
Data analysis
Data were processed by SPSS statistical software (version 15). Non-parametric data were analyzed by Mann-Whitney U test. Results are expressed as mean ± standard error of mean (SEM). Differences between groups were determined using one-way analysis of variance (ANOVA) with Scheffe multiple comparison test. Statistical significance was set at P<0.05.
RESULTS
Phytochemical analysis
Polyphenol compounds determined three times by Folin-Ciocalteu method yielded 3.91 ± 0.1%. Pectin assay yielded 3.71 ± 0.2% after three times measurements. Ethanolic extract yielded 14.0% dry matter after condensation and drying processes. Oil fraction achieved 15.0% of total extract following extraction processing.
Macroscopic assessment
Macroscopic damages of the colon revealed significant edema, mucosal hyperemia, erosion and ulceration in normal saline treated (control) groups. Sham group showed no changes suggesting that handling and surgical procedure had no interference with experimental outputs. Treatment with prednisolone as reference drug reduced damage scores including ulcer score, ulcer area, ulcer index and weight/length ratio (at least p<0.01) after both the oral or i.p. administrations. The groups treated with apricot kernel fractions showed a significant decrease (at least p<0.05) in macroscopic parameters excluding groups treated with extract and/or extract/oil orally at 100 mg/kg dose (Table 1, Fig. 1). There were no significant differences between effective doses (200, 400 mg/kg) of apricot kernel fractions with reference drug, prednisolone administered by the same route.
Table 1.
Macroscopic parameters of TNBS-induced colitis in rats treated with different doses of Apricot kernel extract and extract/oil fractions.
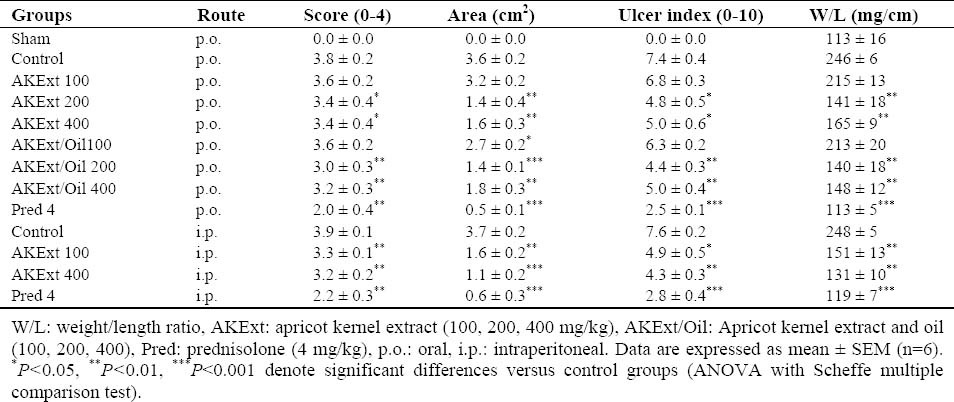
Fig. 1.
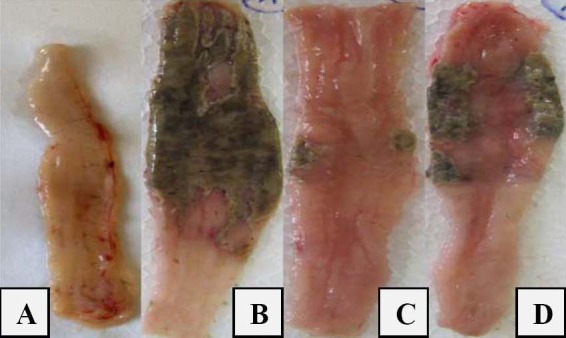
Macroscopic presentation of TNBS-induced colitis in rats. A; Normal colon treated with normal saline, B; Normal saline treated (control) colitis, C; Prednisolone (4 mg/kg, i.p.) treated colitis, D; Apricot kernel extract treated colitis (400 mg/kg p.o.).
Histological assessment
Microscopic evaluations yielded highest level of immune cells infiltration, epithelium and crypt damage in normal saline treated (control) groups supporting the results from macroscopic data. No histological damage was observed in sham (normal) group. Reference drug, prednisolone reduced inflammation severity and inflammation extent, crypt damage, and total colitis index (at least p<0.05) regardless of the route of the administration. Apricot kernel fractions were also effective to reduce the histological parameters as shown in Table 2 and Fig. 2. This was comparable with prednisolone efficacy after similar route of administration. Similar to macroscopic findings, fractions of extract and oil/extract at the dose of 100 mg/kg by oral route were not effective to alleviate tissue damages histopathologically.
Table 2.
Microscopic parameters of colitis induced by TNBS in rats treated with Apricot kernel extract and extract/oil fractions.
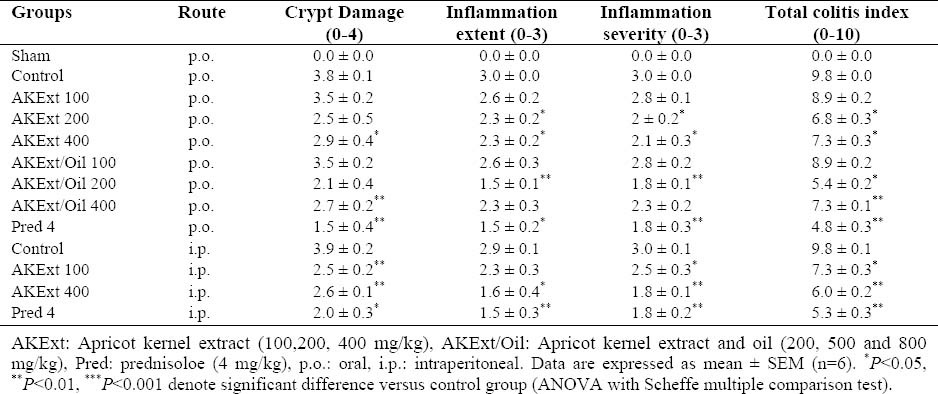
Fig. 2.
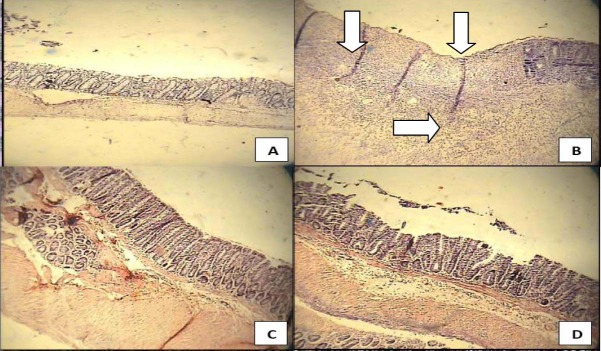
Histological sections of colons in selected groups of TNBS-induced colitis in rats. A; Normal colon treated with normal saline (mucus layer and crypts are normal and leukocyte infiltration is absent), B; Normal saline treated (control) colitis (mucosal and sub-mucosal inflammation as well as crypt damage and leukocyte infiltration are evident) C. Prednisolone (4 mg/kg, i.p.) treated colitis, D; Apricot kernel extract treated colitis (400 mg/kg p.o.). Hematoxyllin and eosin staining with low power (× 10) magnification.
DISCUSSION
To investigate the etiology of IBD, animal models of experimental colitis have been developed and are frequently used to evaluate new anti-inflammatory and anticolitis treatments. Several models of experimental colitis that demonstrate various patho- physiological aspects of the human disease have been described. Colitis induction by hapten reagent TNBS was chosen due to the similarities in the pattern of pathogenesis with human IBD (26).
Several traditional medicines have been studied for their effects on TNBS-induced colitis such as Cydonia oblonga (19), Rosmarinus officinalis (21) and Ginkgo biloba (27). In this study apricot kernel was chosen due to its anti-inflammatory and antioxidant effects suggested in previous researches (9,14). Ulcerations of the mucosa, edema, and influx of inflammatory cells are the most important local pathologic changes observed during colitis produced by TNBS (28). In this model, increased Th1 cytokines production including interferon-gamma (IFN-γ), activation of macrophages, production of tumor necrosis factor (TNF-α) and IL-1β occur (28,29). IL-4 also plays an important role in inflammation and regulation of immune response (29). Amygdalin present in the apricot kernel can inhibit TNF-α and IL-1β which can be attributed to the transcriptional suppression of the mRNA expression of pro-inflammatory cytokines (30). TNBS can also increase the formation of free radicals in colon tissue (31). Studies have shown that apricot kernel extract with high propensity for radical scavenging can stabilize the cell membrane and prevent the oxidation of membrane lipids (14,32).
Polyphenolic compounds of apricot kernel extract isolated in this study consist of a large group of active materials like tannins which could repair intestinal mucosal layers by precipitation of the microproteins on them protecting the layers against chemical injuries and proteolytic enzymes (33,34).
Pectin is another ingredient found in significant amount in apricot kernel extract (11,12). It is supposed that pectin has prominent role in protection against the chemical induced colitis as have been reported previously (35). Pectin can be fermented by microorganisms within the gut to form short chain fatty acids and these fatty acids can be consumed by epithelial cells to stimulate cell proliferation on colon mucosal layers (36). The beneficial effect of pectins against peptic ulcers has been previously suggested by Hamauzu and coworkers in the study of Cydonia oblonga anti-ulcerative effect (37).
Control groups demonstrated highest level of inflammation, necrosis and infiltration of the immune cells considering macroscopic and histopathologic evaluations and confirmed the method's efficacy. Prednisolone treated groups showed significant reduction in all macroscopic and histopathologic scores; however, it was not able to normalize the affected areas. This could be attributed to the short period of treatment and/or acute method of chemically induced model of colitis (21,22).
The results of the study, especially those obtained with the dose of 100 mg/kg, showed that intraperitoneal treated groups demonstrated better outcomes than those treated orally which can be attributed to the higher bioavailability of active components after intraperitoneal injection. By comparing the results of groups treated with extract and extract/oil fraction, it is concluded that oil fraction did not exhibit ameliorating effect whilst it was safe and did not interfere with beneficial anti-inflammatory and anti-ulcerative effects of the extract. It should be remembered that apricot kernel oil is an important and inseparable component of apricot kernel and whole apricot kernel extract could be a good alternative for IBD alleviation. More experiments with oil fraction alone are highly recommended to clarify the efficacy of this component on diminishing the bowel inflammation and its probable role in IBD therapy or prevention.
CONCLUSION
Our results suggest that apricot kernel extracts can ameliorate the colon inflammation and ulcers in TNBS experimental model of colitis in rats. Different active components and mechanisms may be involved in these beneficial effects, thus more detailed studies are needed for more accurate clarification. Results of further studies can support the application of apricot kernel fractions as complementary medicines in colitis treatment.
ACKNOWLEDGMENTS
We acknowledge research council of Isfahan University of Medcial Sciences for financial support of this work.
REFERENCES
- 1.Xavier RJ, Podolsky DK. Unraveling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Andreoli TE, Fayette RL. 6th ed. Philadelphia: WB Saunders; 2004. Cecil essentials of medicine. [Google Scholar]
- 4.Kenneth R, McQaid MD. Drugs used in the treatment of gasterointestinal diseases. In: Katzung BG, editor. Basic and clinical pharmacology. 9th ed. New York: McGraw-Hill; 2004. pp. 1029–1033. [Google Scholar]
- 5.D’Inca R, Garribba AT, Vettorato MG, Martin A, Martines D, Di Leo V, et al. Use of alternative and complementary therapies by inflammatory bowel disease patients in an Italian tertiary referral center. Dig Liver Dis. 2007;39:524–529. doi: 10.1016/j.dld.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Thomas KJ, Nicholl JP, Coleman P. Use and expenditure on complementary medicine in England: a population based survey, complementary therapies in medicine. 2001;9:2–11. doi: 10.1054/ctim.2000.0407. [DOI] [PubMed] [Google Scholar]
- 7.Jones SB, Luchsinger AAE. 2nd ed. New York: McGraw-Hill; 1979. Plant systematic. [Google Scholar]
- 8.Geneva: World Health Organization Publications; 2007. WHO Monographs on Selected Medicinal Plants. [Google Scholar]
- 9.Gokhandurmaz MA. Antioxidant properties of roasted apricot (Prunus armeniaca L.) kernel. Food Chem. 2007;100:1177–1181. [Google Scholar]
- 10.EL-Aal MHA, Hamza MA, Rahma EH. Apricot kernel oil: Characterization, chemical composition and utilization in some baked products. Food Chem. 1986;19:287–289. [Google Scholar]
- 11.Sefer F, Misirli A, Gulcan R. A research on phenolic and cyanogenic compounds in sweet and bitter apricot kernels. Proceedings of XII international symposium on apricot culture and decline, ISHS Acta Hort. 2006;701:167–170. [Google Scholar]
- 12.Femenia A, Rosello C, Mulet A, Canellas J. Chemical composition of bitter and sweet apricot kernels. J Agric and Food Chem. 1995;43:356–361. [Google Scholar]
- 13.Tonekaboni SMM. Tehran: Publications of Traditional Medicine Research Center of Shahid Beheshti University of Medical Sciences; 2006. Tohfatalmomenin. [Google Scholar]
- 14.Yiğit DD, Yiğit N, Mavi A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Braz J Med Biol Res. 2009;42:346–352. doi: 10.1590/s0100-879x2009000400006. [DOI] [PubMed] [Google Scholar]
- 15.Miyagoshi M, Amagaya S, Ogihara Y. Antitussive effects of L-ephedrine, amygdalin, and makyokansekito (Chinese traditional medicine) using a cough model induced by sulfur dioxide gas in mice. Planta Medica. 1986;52:275–278. doi: 10.1055/s-2007-969151. [DOI] [PubMed] [Google Scholar]
- 16.Waterhouse AL. Determination of total phenolics. In: Wrolstad RE, editor. Current protocols in food analytical chemistry. 3rd ed. New York: John Wiley; 2001. [Google Scholar]
- 17.1st ed. Tehran: Iranian Ministry of Health Publications; 2002. Iranian Herbal Pharmacopoeia Committee, Iranian Herbal Pharmacopoeia. [Google Scholar]
- 18.Stallmach A, Wittig B, Giese T, Pfister K, Hoffmann JC, Bulfone-Paus S, et al. Protection of trinitrobenzene sulfonic acid–induced colitis by an Interleukin 2–IgG2b fusion protein in mice. Gastroenterology. 1999;117:866–876. doi: 10.1016/s0016-5085(99)70345-8. [DOI] [PubMed] [Google Scholar]
- 19.Minaiyan M, Ghannadi AR, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res Pharma Sci. 2012;7:103–110. [PMC free article] [PubMed] [Google Scholar]
- 20.Minaiyan M, Ghannadi AR, Afsharipour M, Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- 21.Morris GP, Beck PL, Herridge M, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 22.Minaiyan M, Ghannadi A, Mahzouni P, Nabi-Meibodi M. Anti-ulcerogenic effect of ginger (rhizome of Zingiber officinale Roscoe) hydroalcoholic extract on acetic acid-induced acute colitis in rats. Res Pharm Sci. 2008;3:15–22. [Google Scholar]
- 23.Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, et al. On the benefits of silymarin in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Cent Eur J Biol. 2009;4:204–213. [Google Scholar]
- 24.Williams KL, Fuller CR, Dieleman LA, DaCosta CM, Haldeman KM, Sartor RB, et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that over-express growth hormone. Gastroenterology. 2001;120:925–937. doi: 10.1053/gast.2001.22470. [DOI] [PubMed] [Google Scholar]
- 25.Yousefi I, Adibmoradi M. 2nd ed. Tehran: Tehran University Publishings; 2002. Comparative histology and histotechniques. [Google Scholar]
- 26.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong Zhou Y, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, et al. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-α, NF-κBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. 2006;5:1–9. doi: 10.1155/MI/2006/92642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, et al. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-α production in mice. Gastroenterology. 2001;121:1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- 29.Guang-Bijin H, Yeshino GV, Miuram M, Meada T, Shigefumi CH, Yong J. Protective effects of polygalae root in experimental TNBS–induced colitis in mice. J Ethnopharmacol. 2002;79:341–346. doi: 10.1016/s0378-8741(01)00399-3. [DOI] [PubMed] [Google Scholar]
- 30.Hwang HJ, Kim P, Kim CJ, Lee HJ, Shim I, Yin CS, et al. Antinociceptive effect of amygdalin isolated from Prunus armeniaca on formalin-induced pain in rats. Biol Pharm Bull. 2008;31:1559–1564. doi: 10.1248/bpb.31.1559. [DOI] [PubMed] [Google Scholar]
- 31.Ademuglu E, Erbil Y, Barbaros TBV, Ilhan E, Olga V, Torkoglu MV. Do vitamin E and selenium have beneficial effects on trinitrobenzene sulfonic acid-induced experimental colitis? Dig Dis Sci. 2004;49:102–108. doi: 10.1023/b:ddas.0000011610.47179.0b. [DOI] [PubMed] [Google Scholar]
- 32.Jahanban Sfahlan A, Mahmoodzadeh A, Hasanzadeh A, Heidari R, Jamei R. Antioxidant and antiradicals in almond hull and shell (Amygdalus communis L.)as a function of genotype. Food Chem. 2009;115:529–533. [Google Scholar]
- 33.Al-Rehailya AJ, Al-Howirinya TS, Al-Sohaibanib MO, Rafatullaha S. Gastroprotective effects of ‘Amla’ Emblica officinalis on in vivo test models in rats. Phytomedicine. 2002;9:515–522. doi: 10.1078/09447110260573146. [DOI] [PubMed] [Google Scholar]
- 34.Cobzac S, Moldovan M, Olah NK, Bobos L, Surducan E. Tannin extraction efficiency, from Rubus idaeus, Cydonia oblonga and Rumex acetosa, using different extraction techniques and spectrophotometric quantification. Seria F Chemia. 2005;8:55–59. [Google Scholar]
- 35.Rolandelli RH, Saul SH, Settle RG, Jacobs DO, Trerotola SO, Rombeau JL. Comparison of parenteral nutrition and enteral feeding with pectin in experimental colitis in the rat. Am J Clin Nutr. 1988;47:715–721. doi: 10.1093/ajcn/47.4.715. [DOI] [PubMed] [Google Scholar]
- 36.Roediger WE. The starved colon diminished mucosal nutrition, diminished absorption, and colitis. Dis Colon Rectum. 2010;33:858–862. doi: 10.1007/BF02051922. [DOI] [PubMed] [Google Scholar]
- 37.Hamauzu Y, Irie M, Kondo M, Fujita T. Antiulcerative properties of crude polyphenols and juice of apple and Chinese quince extracts. Food Chem. 2008;108:488–495. doi: 10.1016/j.foodchem.2007.10.084. [DOI] [PubMed] [Google Scholar]


