Abstract
The aim of the current study was to design a porous osmotic pump–based drug delivery system for controlling the release of buspirone from the delivery system. The osmotic pump was successfully developed using symmetric membrane coating. The core of the tablets was prepared by direct compression technique and coated using dip-coating technique. Drug release from the osmotic system was studied using USP paddle type apparatus. The effect of various processing variables such as the amount of osmotic agent, the amount of swellable polymer, concentration of the core former, concentration of the plasticizer, membrane thickness, quantum of orifice on drug release from osmotic pump were evaluated. Different kinetic models (zero order, first order and Higuchi model) were applied to drug release data in order to establish the kinetics of drug release. It was found that the drug release was mostly affected by the amount of NaCl as osmotic agent, the swellable polymer; hydroxy propyl methyl cellulose (HPMC), the amount of PEG-400 and cellulose acetate in the coating solution and thickness of the semipermeable membrane. The optimized formulation released buspirone independent of pH and orifice quantum at the osmogen amount of 42%, hydrophilic polymer of 13% and pore size of 0.8 mm on the tablet surface. The drug release of osmotic formulation during 24 h showed zero order kinetics and could be suggested that this formulation as a once-daily regimen improves pharmacokinetic parameters of the drug and enhances patient compliance.
Keywords: Osmotic pump, Buspirone, Controlled release, Release kinetic
INTRODUCTION
The stability and efficacy of drug in oral controlled release dosage forms may be affected by pH, gastric motility, and presence of food (1,2). One practical approach with a potential to overcome these disadvantages, is to prepare the osmotic drug delivery system where osmotic materials have been used extensively in the fabrication of drug delivery systems (3,4). The historical developments of osmotic systems include seminal contributions such as the Rose-Nelson pump, the Higuchi-Leeper pumps, the Alzet osmotic pump, the elementary osmotic pump (EOP) and the push-pull osmotic pump (5,6,7,8,9). Osmotic pumps offer many advantages like they (i) are easy to formulate and simple in operation, (ii) improve patient compliance by reducing dosing frequency and (iii) provide good in-vitro and in-vivo correlation (10). Although different types of oral osmotic systems have been reported in literature, but most important osmotic delivery system is ‘Theeuwes elementary osmotic pump’(EOP) (12,13). In this system, the osmotic core is surrounded by a semipermeable membrane drilled with a drug delivery orifice. Once this system comes in contact with the gastrointestinal fluids, the osmotically driven water enters the system through the semipermeable membrane, dissolves the soluble agents, and exits through the delivery orifice. Because these systems use osmotic pressure for the controlled delivery of the active compound(s), delivery rates are expected to be independent of gastrointestinal conditions (14). The rate at which the core absorbs water depends on the osmotic pressure generated by the core components and the permeability of the membrane coating. As the core absorbs water, it expands in volume, which pushes the drug solution or suspension out of the tablet through one or more delivery ports (15). Unlike the EOP which consists of an osmotic core with the drug surrounded by a semipermeable membrane drilled with a delivery orifice, controlled porosity of the membrane is accomplished by the use of different channeling agents in the coating (16).
Buspirone is a slow-onset anxiolytic agent whose actions are quite different from those of conventional sedative-hypnotics and has selective anxiolytic effects. Buspirone relieves anxiety without causing marked sedative, hypnotic, or euphoric effects. The drug is not effective in blocking the acute withdrawal syndrome resulting from the abrupt cessation of use of benzodiazepines or other sedative-hypnotics. Buspirone has minimal abuse liability.
Buspirone is rapidly absorbed orally but undergoes first-pass metabolism via hydroxylation and dealkylation reactions to form several active metabolites. (17). A multiple-dose study conducted that buspirone has nonlinear pharmacokinetics. Thus, dose increments and repeated dosing may lead to somewhat higher blood levels of unchanged buspirone than would be predicted from results of single-dose studies.
Buspirone is an ideal candidate for a zero-order drug delivery system because it is soluble in water. The average elimination half-life of unchanged buspirone after single doses of 10 mg to 40 mg is about 2 to 4 h (17).
In previous studies, it was indicated that extended release tablet of buspirone has a significant effect on pharmacokinetic modification of this drug such as increasing in bioavailability, half life, and Cmax.
The present work was aimed to design, develop and evaluate an oral osmotic delivery system of buspirone. We prepared buspirone EOP, which can maintain a constant therapeutic concentration and reduce the number of times which the drug must be taken each day. Another improvement is to increase the safety of the drug, which may result in fewer side effects. Therefore, it is essential to prepare buspirone as osmotic pump tablets, providing a safe, effective, and stable controlled-release preparation with strong applicability.
MATERIALS AND METHODS
Buspirone powder was purchased from Tehran Darou Pharmaceuticals (Tehran, Iran). Cellulose acetate with 40% acetyl groups (Fluka, Swizerland) was used as a semipermeable membrane. Hydroxylpropyl methylcellulose (HPMC) (K4M) (Celeron, England), and Polyvinylpirrolidine (PVP K30) (Mowiol, Germany) were used as water swellable and gelling agents. NaCl (Merck Co., Dermastat, Germany) was applied as osmotic active agent. Other chemicals such as Avicel, sodium lauryl sulfate (SLS), magnesium stearate (MgS), PEG-400, potassium dihydrogen phosphate (KH2PO4), sodium hydroxide (NaOH), acetone and ethanol were obtained from Merck Co., Dermastat, Germany.
Preparation of osmotic pump tablets of buspirone
Preparation of core tablets
Core tablets were prepared by direct compression. Formula of different core formulations of buspirone is listed in Table 1. Buspirone was mixed with HPMC for 10 min. After passing this mixture through #30 mesh sieve, osmotic agent (sodium chloride), Avicel and PVP were added in geometric dilution and mixing continued for additional 10 min. To this mix, MgS which passed through #60 mesh sieve, were added and mixing continued for additional 5 min. The blend was then compressed into tablets using a single station tablet punching machine (Kavosh, Iran) fitted with 8.5 mm round standard concave punches. The punched tablets were of 147 ± 2 N hardness on the Monsanto hardness tester. The drug content of tablets was found to be within the limit of 97.98-102.36%.
Table 1.
Composition of osmotic tablet's core.

Coating and drilling
The tablet was coated with cellulose acetate. The composition of coating solutions used for coating of core tablets is given in Table 2.
Table 2.
Composition of coating formulation.

Various components of coating solution were added to solvent mixture in a sequential manner and allowed to dissolve before next one. The cores were coated by dip-coating technique. The cores were immersed in the coating solution about 10 s. To eliminate variation in final coating thickness, the rate of withdrawal of the tablets from the solution was adjusted. Immediately after coating, tablets were rotated for even distribution of the viscous membrane solution, under constant air flow and were kept for 30 s to allow for evaporation of both the solvent (acetone) and non-solvent (ethanol).
This process was repeated until desired thickness achieved. The temperature of air in the environment of the oven was adjusted at 25 °C. Tablets were allowed to dry further for a minimum of 24 h in oven temperature prior to dissolution experiment. Coating process was carried out in the same condition for all tablets and thickness of the coat was periodically controlled using a digital micrometer (Mitotoyo, Japan). The memberane thickness of the basic formulation was regulated in the range of 500 ± 10 μm. For coated tablets, a small orifice was drilled through the one side of each coated tablet by standard mechanical micro-drills (600 μm diameter).
In vitro drug release studies
All drug release experiments were carried out using a dissolution apparatus (Erweka DT 700, Germany), paddle method, rotating at 50 rpm at 37 °C in 500 ml phosphate buffer solution (pH 7.4). The sample of 5 ml which were replaced with fresh medium was taken at distinct time intervals (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 h).
In-vitro analysis of drug concentration in the samples was done by a UV spectrophotometer (Shimadzu-Mini 1240, Japan) at a wavelength of 235 nm. The release tests were performed at least for three separate experiments, the mean values of obtaining data were calculated and the cumulative percentage of drug release was plotted against time. Furthermore, in order to better characterize the drug release process, the mean dissolution time (MDT) was calculated according to the following equation:
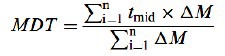
Where i is the dissolution sample number, n is the number of dissolution sample time, t is the time at the midpoint between i and i-1, and M is the additional amount of drug dissolved between i and i-1. To assess the statistical significance between the MDTs, ANOVA with Tukey post test was carried out (17).
RESULTS
Influences of core formulation variables on drug release
To investigate the influences of tablet core formulation variables on drug release, tablets with different formulations were prepared, coated with the same coating solution, and an orifice with a diameter of 600 μm was drilled into the center of the surface.
Influence of amount of NaCl
Osmotic pumps with different amounts of NaCl as an osmotic pressure accelerant in the core were prepared and coated. With an increasing amount of NaCl, the release rate was accelerated, because the increasing osmotic pressure increased drug release rate from the core (Fig. 1). When the coated tablet is exposed to an aqueous environment, water diffuses through the film coating (due to the active gradient of water), hydrating the core. The solvation of the osmotic agents creates the osmotic pressure difference between the core contents and external environment, resulting in the greater buspirone release. The value of MDT was decreased from 7.38 ± 0.09 to 7.11 ± 0.08 h for lower and upper amounts of osmogen (p<0.05).
Fig. 1.

Influence of different contents of NaCl on the drug release profile (n=3, mean ± SD).
Influence of amount of HPMC
Different amounts of HPMC were added to the core for tableting. Fig. 2 indicates that the amount of HPMC had a significant effect on the release rate. The drug release rate was slowed down with decreasing in polymer amount. This can be accounted for by the fact that after extra membranous water was imbibed into the intra membrane, the swelling of HPMC would lead to increasing static pressure inside the membrane, which would accelerate the drug release from the core. The value of MDT was decreased from 7.28 ± 0.09 to 7.11 ± 0.08 h for lower and upper amounts of osmogen (p<0.05). The effect of different grades of HPMC and ethyl cellulose (EC) on the buoyancy and release behavior of buspirone in floating tablets shows that a high viscosity grade of HPMC in combination with EC that is a hydrophobic polymer is necessary to reduce the rate of this freely soluble drug release (19).
Fig. 2.
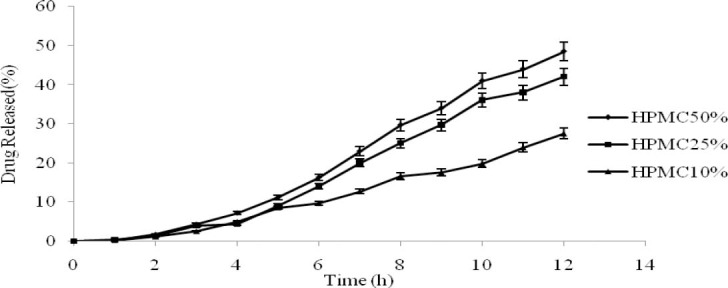
Influence of different content of hydroxylpropyl methylcellulose on the drug release profile (n=3, mean ± SD).
Influence of level of plasticizer on drug release
The effect of plasticizer amount in the coating solution on drug release was evaluated at 0 to 20% w/w of PEG-400 and thickness of 0.8 mm in coded formulations of batch-OP3A and batch OP-3C, respectively. Release profiles of these batches in comparison with batch-OP3B (containing 10% w/w PEG-400) are shown in Fig. 3. It is clearly evident that the level of plasticizer (PEG- 400) has a direct effect on the drug release. As the level of PEG-400 increases, the membrane becomes more porous due to dissolving of water soluble plasticizer in dissolution media resulting in higher drug release (MDT of 7.88 decreased to 6.44 h) (19). This may be explained by considering hydrophilicity improving of the membrane. PEG-400 is a hydrophilic plasticizer, which increases membrane elasticity, fluidity of polymer chains (20,21). Therefore by increasing of PEG-400 concentration as channeling agent, water could be imbibed into the membrane very quickly and accelerating the release rate of the drug (22).
Fig. 3.

Influence of the amount of PEG-400 on the drug release profile (n=3, mean ± SD).
Influence of coating polymer concentration
To study the effect of coating polymer concentrations, core formulation of batch-OP3 were coated with coating formulation D, B and E containing 1.5, 4 and 8% w/w of cellulose acetate respectively assigned as batch -OP3D, OP3B and OP3E. The release profiles of these batches are shown in Fig.4. When the concentration of the coating solution was lower than 1.5%, it was difficult to perform the coating process and the membrane layer was not appropriately formed. By increasing polymer concentration, release rate decreased and MDT was not changed significantly. This behavior was reported by the other researchers (23,24). An increase in the viscosity of the coating solution was reported as a responsible reason for the reduction in the release rates.
Fig. 4.
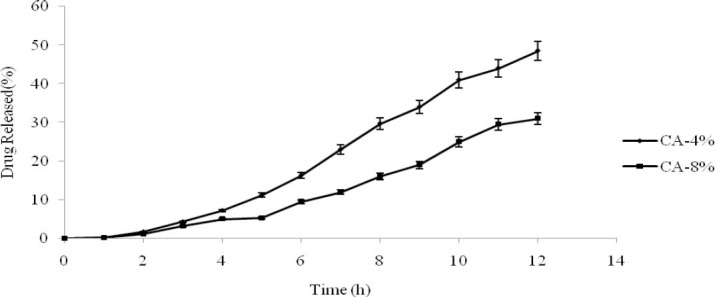
Influence of coating polymer concentrations on the drug release profile (n=3, mean ± SD).
The viscosity increasing of the coating solution in a concentration above 8% w/w resulted in the dense coating membrane layer and lower drug release rate. Hence, based on the results, coating solution with cellulose acetate concentration of 4% in acetone/ethanol solvents had better performance in coating.
Effect of coating thickness on drug release
To study the effect of the coating film thickness on drug release, core formulation of batch-OP3 were coated with coating composition B in different thicknesses (0.5, 0.8 and 1 mm). The release profiles of buspirone from these formulations are shown in Fig. 5.
Fig. 5.

Influence of membrane thickness on the drug release profile (n=3, mean ± SD).
The increase of thickness resulted in an increased resistance to water imbition and consequently causing a decrease in the rate of drug dissolution in the core, and ultimately resulted in a decline in buspirone release (MDT of 6.75, 7.07, 7.11 h, respectively).
It was observed that the coated tablet with 0.3 mm thickness was cracked and fragmented after the exposure to the dissolution medium. This was probably due to the lack of resistance of the prepared thin layer around the tablet, which was unable to endure the internal pressure (1).
Influence of the dissolution medium on drug release
To study the influence of the medium on drug release, core formulation of batch- OP3 were coated with coating composition B. The phosphate buffer solution, simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were chosen to carry out the release test.
The results are shown in Fig. 6 and the values of MDT were 7.11, 6.77, 7.67 h, respectively. It is clear that the rate was unaffected by pH of the medium (P>0.05) and provided further evidence that the osmotic pump tablets aren’t affected by the medium's pH. The pKa value of buspirone HCl is 1.22, 7.32 and this weakly basic compound has a lower solubility in pH 7.2 phosphate buffer solution than in 0.1 M HCl, but statistically, there isn’t any difference between the release profiles in these meda.
Fig. 6.
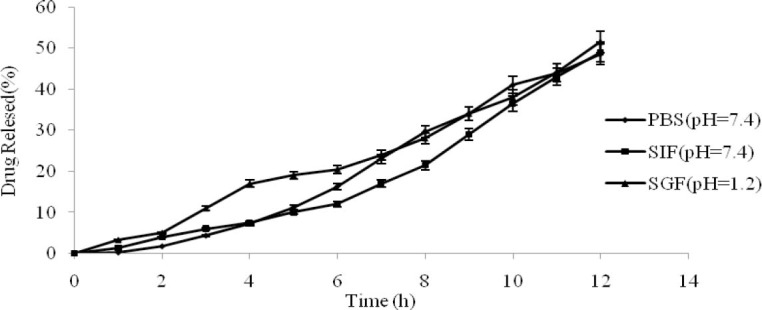
Influence of the dissolution medium on the drug release profile (n=3, mean ± SD).
Influence of the number of release orifices
The effect of the number of release orifices in batch-OP3 with one and two release orifices are presented in Fig. 7. The results showed that the MDT value and release rate didn’t increase significantly with an increasing number of release orifices (P>0.05). It is concluded that the main force for drug release in osmotic pump is the osmotic pressure across the coating membrane.
Fig. 7.
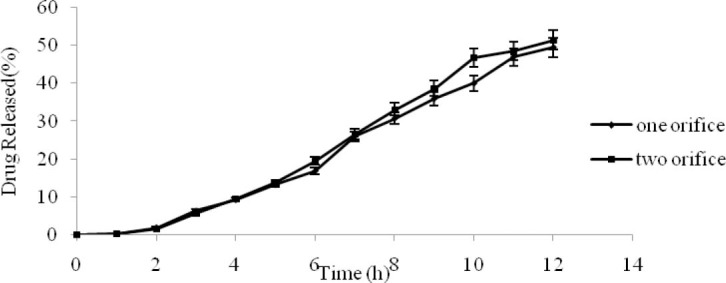
Influence of the number of orifices on the drug release profile (n=3, mean ± SD).
Selecting the optimum formulation
In order to select the optimum formulation, various mathematical models (zero-order, first-order, and Higuchi) were used to describe the kinetics of drug release.
Best goodness-of-fit test (R2) and correlation error percent was taken as criteria for selecting the most appropriate model. Formulation with the highest R2 and lower error for zero-order kinetics was chosen as the optimum formulation.
Correlation error% = (observed data- calculated data)× 100 / observed data
Based on the obtained results, batch-OP3B was the formulation with the highest R2 for zero order kinetics which was close to the desired release (Table 3).
Table 3.
Kinetics of in vitro buspirone release from different batches of osmotic pump tablets.

The optimum formulation (Table 4) released 99% of the drug after 24 h with zero order kinetics and has a minimum lag time of release which is a drawback for osmotic drug delivery system (Fig. 8.).
Table 4.
Optimal tablet core and coat formulation.

Fig. 8.

Release profile of the drug from optimized formulation for 24 h (n=3, mean ± SD).
DISCUSSION
In the present study, EOPand controlled porosity osmotic pump (CPOP) with ethyl cellulose as a semipermeable membrane containing different levels of channeling agent has been developed and evaluated for water soluble drug buspirone. The target release profile (zero order kinetics) was selected and different variables were optimized to achieve the same. The usual dose of buspirone is 5 mg taken three or four times daily. It has a short plasma half life of 2–4 h. Hence, this drug was chosen as a good candidate with the aim of developing a controlled release system for a period of 12 h.
Sakr and coworkers have previously shown that the extended release formulation of buspirone from the two products, had a 70 to 90% greater in vivo bioavailability, 46% higher in the mean steady-state Cmax and the longer mean apparent half-life of buspirone (9.04 and 3.06 h for extended and immediate release tablets, respectively.) There were no significant differences in average pharma-cokinetic metrics observed in men and women. Based on these observations, the potential benefits of once-daily dosing with the ER product in terms of prolonged buspirone plasma concentrations with a lower inter-subject variation with an improvement in the desired therapeutic effects of buspirone could be achieved (25). So the preparation of the novel formulation such as osmotic pump could be supported by these findings.
The drug release from the optimized osmotic pump was significantly affected by the preparation parameters such as the amount of NaCl, the amount of HPMC in the core and concentration of the polymer, plasticizer and the thickness of the membrane. Also, the release from the developed formulation was independent of pH and orifice number. The optimum formulation showed a zero order release during 24 h. There is a relationship between these data and previous results. Kumar and coworkers have previously shown that tramadol hydrochloride release was directly proportional to the level of plasticizer and osmotic pressure generated by an osmotic agent but inversely proportional to the level of swellable polymer within the core and coat thickness of the membrane. Drug release from the developed formulation was independent of pH and agitation intensities of the release media (26).
Kanagale designed a porous osmotic pump-based drug delivery system for controlling release of oxybutinin. It was found that the drug release rate increased with the amount of osmogen because of the increased water uptake, and hence increased driving force for drug release. Oxybutinin release was inversely proportional to the membrane weight gain; however, directly related to the level of pore former, sorbitol, in the membrane. This system was found to deliver drugs at a zero-order rate for 20 h (27).
CONCLUSION
The present work aimed towards the design and development of the extended release formulation of water-soluble drug, buspirone based on osmotic technology. This drug delivery system was successfully developed using symmetric membrane coating and the optimum formulation showed a zero order release during 24 h. The prepared osmotic delivery system could be used as once-daily tablet for efficient treatment of anxiety and improving patient compliance.
ACKNOWLEDGMENTS
This work was performed in partial fulfillment of the requirements for a Pharm. D thesis of Morteza Ghasemnejad Berenji at Faculty of Pharmacy, Kermanshah University of Medical Sciences.
REFERENCES
- 1.Shokri J, Ahmadi P, Rashidi P, Shahsavari M, Rajabi-Siahboomi A, Nokhodchi A. Swellable elementary osmotic pump (SEOP): An effective device for delivery of poorly water soluble drugs. Eur J Pharm Biopharm. 2008;68:289–297. doi: 10.1016/j.ejpb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Che B. Preparation of monolithic osmotic pump system by coating the indented core tablet. Eur J Pharm Biopharm. 2006;64:180–184. doi: 10.1016/j.ejpb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Khan MA, Sastry SV, Vaithiyalingam SR, Agarwal V, Nazzal S, Reddy IK. Captopril gastrointestinal therapeutic system coated with cellulose acetate pseudo latex: evaluation of the main effects of several formulation variables. Int J Pharm. 2000;193:147–156. doi: 10.1016/s0378-5173(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 4.Verma RK, Mishra B, Garg S. Osmotically controlled oral drug delivery. Drug Dev Ind Pharm. 2000;26:695–708. doi: 10.1081/ddc-100101287. [DOI] [PubMed] [Google Scholar]
- 5.Rose S, Nelson JF. A continuous long-term injector. Aust J Exp Biol Med Sci. 1955;33:415–420. doi: 10.1038/icb.1955.44. [DOI] [PubMed] [Google Scholar]
- 6.Theeuwes F, Swanson D, Wong P, Bonson P, Place V, Heimlich K, et al. Elementary osmotic pump for indomethacin. J Pharm Sci. 1982;72:253–258. doi: 10.1002/jps.2600720313. [DOI] [PubMed] [Google Scholar]
- 7.Bittner B, Thelly Th, Isel H, Mountfield RJ. The impact of co-solvents and the composition of experimental formulations on the pump rate of the ALZET osmotic pump. Int J Pharm. 2000;205:195–198. doi: 10.1016/s0378-5173(00)00481-6. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi T, Leeper HM. US Patent; 1976. Osmotic dispenser with means for dispensing active agent responsive to osmotic gradient. No.3, 995, 631. [Google Scholar]
- 9.Prabakaran D, Singh P, Kanaujia P, Jaganathan KS, Rawat A, Vyas SP. Modified push-pull osmotic system for simultaneous delivery of theophylline and salbutamol: development and in vitro characterization. Int J Pharm. 2004;284:95–108. doi: 10.1016/j.ijpharm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Verma RK, Mishra B, Garg S. Osmotically controlled oral drug delivery. Drug Dev Ind Pharm. 2000;26:695–708. doi: 10.1081/ddc-100101287. [DOI] [PubMed] [Google Scholar]
- 11.Makhija S, Vavia P. Controlled porosity osmotic pump-based controlled release systems of pseudoephedrine. I. Cellulose acetate as a semipermeable membrane. J Control Release. 2003;89:5–18. doi: 10.1016/s0168-3659(02)00482-0. [DOI] [PubMed] [Google Scholar]
- 12.Santus G, Baker RW. Osmotic drug delivery: a review of the patent literature. J Control Release. 1995;35:1–21. [Google Scholar]
- 13.Verma RK, Garg S. Current status of drug delivery technologies and future direction. Pharm Tech. 2001;25:1–14. [Google Scholar]
- 14.Theeuwes F. Elementary osmotic pump. J Pharm Sci. 1975;64:1987–1991. doi: 10.1002/jps.2600641218. [DOI] [PubMed] [Google Scholar]
- 15.Thombre AG, Appel LE, Chidlaw MB, Daugherity PD, Dumont F, Evans LAF, et al. Osmotic drug delivery using swellable-core technology. J Control Release. 2004;94:75–89. doi: 10.1016/j.jconrel.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Sapna N Makhija, Pradeep R Vavial. Controlled porosity osmotic pump-based controlled release systems of pseudoephedrine: I. Cellulose acetate as a semipermeable membrane. J Control Release. 2003;89:5–18. doi: 10.1016/s0168-3659(02)00482-0. [DOI] [PubMed] [Google Scholar]
- 17.Kutzung BG. 10th Ed. USA: McGraw-Hill; 2007. Basic & clinical pharmacology; p. 375. [Google Scholar]
- 18.Gohel MC, Panchal MK. Novel use of similarity factors f2 and Sd for the development of diltiazem HCl modified-release tablets using a 32 factorial design. Drug Dev Ind Pharm. 2002;28:77–87. doi: 10.1081/ddc-120001488. [DOI] [PubMed] [Google Scholar]
- 19.Ghaznavi M, Mortazavi S. The effect of different grades of HPMC and ethyl cellulose on the buoyancy and release behavior of buspirone floating table. Research in Pharmaceutical Sciences, 2012;7:S391. [Google Scholar]
- 20.Kumar P, Singh S, Rajinikanth PS, Mishra B. An overview of osmotic pressure controlled release formulation. J Pharm Res. 2006;5:34–45. [Google Scholar]
- 21.Liu l, Khang G, Rhee J, Lee H. Monolithic osmotic tablet system for nifedipine delivery. J Control Release. 2000;67:309–315. doi: 10.1016/s0168-3659(00)00222-4. [DOI] [PubMed] [Google Scholar]
- 22.Sanjeri Dasankoppa F, Ningangowdar M, Sholapur H. Formulation and evaluation of controlled porosity osmotic pump for oral delivery of ketorolac. J Basic Clin Pharma. 2013;4:2–9. doi: 10.4103/0976-0105.109398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altinkaya S, Yenal H. In vitro drug release rates from asymmetric membrane tablet coating: prediction of phase- inversion dynamic. J Biochem Eng. 2006;28:131–139. [Google Scholar]
- 24.Garg A, Gupta M, Bhargava H. Effect of formulation parameters on the release characteristics of propranolol from asymmetric membrane coated tablets. Eur J Pharm Biopharm. 2007;67:725–731. doi: 10.1016/j.ejpb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Sakr A, Andheria M. Pharmacokinetics of buspirone extended-release tablets: a single-dose study. J Clin Pharmacol. 2001;41:783–789. doi: 10.1177/00912700122010582. [DOI] [PubMed] [Google Scholar]
- 26.Kumar P, Singh S, Mishra B. Development and evaluation of elementary osmotic pump of highly water soluble drug: tramadol hydrochloride. Current Drug Delivery. 2009;6:130–139. doi: 10.2174/156720109787048249. [DOI] [PubMed] [Google Scholar]
- 27.Kanagale P, Lohray BB, Misra A, Davadra P, Kini R. Formulation and optimization of porous osmotic pump-based controlled release system of oxybutinin. AAPS Pharm Sci Tech. 2007;8:E53. doi: 10.1208/pt0803053. [DOI] [PMC free article] [PubMed] [Google Scholar]


