Abstract
Bark extract of Pinus pinaster contains numerous phenolic compounds such as catechins, taxifolin, and phenolic acids. These compounds have received considerable attentions because of their anti-inflammatory, antimutagenic, anticarcinogenic, antimetastatic and high antioxidant activities. Although P. pinaster bark has been intensely investigated in the past; there is comparably less information available in the literature in regard to P. eldarica bark. Therefore, the aim of this study was to determine the chemical composition of P. eldarica commonly found in Iran. A reversed-phase high pressure liquid chromatography (RP-HPLC) method for the determination of catechin, caffeic acid, ferulic acid, and taxifolin in P. pinaster and P. eldarica was developed. A mixture of 0.1% formic acid in deionized water and 0.1% formic acid in acetonitrile was used as the mobile phase, and chromatographic separation was achieved on a Nova pack C18 at 280 nm. The two studied Pinus species contained high amounts of polyphenolic compounds. Among four marker compounds, the main substances identified in P. pinaster and P. eldarica were taxifolin and catechin, respectively. Furthermore, the composition of the bark oil of P. eldarica obtained by hydrodistillation was analyzed by gas chromatography/mass spectroscopy (GC/MS). Thirty-three compounds accounting for 95.1 % of the oil were identified. The oils consisted mainly of mono- and sesquiterpenoid fractions, especially α-pinene (24.6%), caryophyllene oxide (14.0%), δ-3-carene (10.7%), (E)-β-caryophyllene (7.9%), and myrtenal (3.1%).
Keywords: P. pinaster, P. eldarica, HPLC, GC/MS, Pine bark extract
INTRODUCTION
Nowadays, there has been an intense scientific interest in discovering new natural antioxidant agents (1,2). Pinus pinaster bark extract has been used worldwide as herbal remedy and nutrition supplemental food in many kinds of chronic and degenerative diseases (3,4). It contains numerous phenolic compounds such as catechins, taxifolin, and phenolic acids. The structures of catechin, caffeic acid, ferulic acid, and taxifolin are shown in Fig. 1. These compounds have received considerable attentions because of their anti-inflammatory, antimutagenic, anticarcino-genic, antimetastatic and high antioxidant activities (3,4,5). Several researches indicated the pharmaceutical and nutriceutical effects of polyphenolic components investigated in this study (3,4,5). For instance, catechins might be useful in body fat and malondialdehyde- modified low density lipoprotein (LDL) reduction, and in the prevention and improvement of other lifestyle-related diseases (6). Green tea catechins might have potential to inhibit the endonuclease activity of influenza A virus RNA polymerase (7).
Fig. 1.
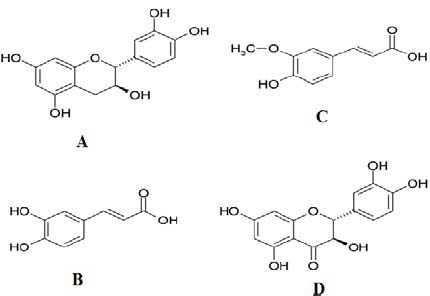
Structures of phenolic compounds detected in pine bark extracts. A; catechin, B; caffeic acid, C; ferulic acid, D; taxifolin.
It was also reported that polyphenolic compounds including catechins, ((-)-epigallocatechin gallate, (-)-epicatechin gallate and (-)-epigallocatechin from green tea had antiviral effect against influenza virus. This effect is mediated not only by specific interaction with HA protein, but altering the physical properties of viral membrane (8). Taxifolin was found to inhibit the synthesis and secretion of triacylglycerol and phospholipids, and inhibit cholesterol synthesis in a dose- and time-dependent manner. It also suppressed HMG-CoA reductase activity and cholesteryl ester formation, down-regulated the expression of intercellular adhesion molecule-1 (ICAM-1), and decreased the secretion of apoB into LDL-like particles (9,10,11). Furthermore, the identified phenolic acids (ferulic and caffeic acids) have antioxidants activity against reactive oxygen radicals which cause damage to cell membranes and DNA. These components might be useful in prevention of cancers, cardiovascular disorders, and neurodegenerative diseases (1,3).
Pinus pinaster (Pinaceae) is a tree of 20-35 m tall (medium-sized tree); the bark is orange-red, thick and deeply fissured at the base of the trunk, somewhat thinner in the upper crown. The needles are in pairs, 12-22 cm long and bluish-green to distinctly yellowish-green. The cones, 10-20 cm long and 4-6 cm broad at the base when closed, are green at first, ripening to glossy red-brown when 24 months old.
There are more than 4 million hectares of P. pinaster forest all around the occidental Mediterranean basin (Portugal, Spain, France, Italy, Tunisia, Algeria and Morocco). P. pinaster also grows in the north of Iran (especially Nowshahr, Chaloos, and Kiashahr). This pine is especially exploited for its wood and resin products. The use of pine needles to produce the essential oil is another possibility for integral profit of pine forests (12,13). P. eldarica is one of the most common pines in Iran. It is a medium-sized tree, reaching 12-15 m high. The bark is brownish-gray or light gray, not flaking; head broad-topped. The leaves are stiff, 6-9 cm long and green. The cones are pedunculate, solitary or in pairs and light reddish-brown. Scales irregularly rhombic, glossy, smooth, the whitish-gray apophysis concave: seeds blackish, 6-7 mm long, the reddish-brown wing 18-28 mm long (14). Although P. pinaster bark has been intensely investigated in the past, there is comparably less information available in the literature in regard to P. eldarica bark. Therefore, the aim of this study was to determine the chemical composition of P. eldarica commonly found in Iran.
MATERIALS AND METHODS
Oil preparation
Pinus eldarica powders (100 g) were hydro-distilled (with 1.2 liter of water) in a Clevenger-type apparatus for 4 h according to British Pharmacopoeia (15). The volatile oil was collected with pentane and dried over anhydrous sodium sulfate and stored in sealed vial at 4 °C until analysis. The yield of oil was calculated based on dried weight of plant material. Pale yellow oil from the bark was obtained (0.15 % v/w).
Gas chromatography/mass spectroscopy analysis
GC-MSanalysis was performed on a Hewlett Packard 5792A mass selective detector coupled with a Hewlett Packard 6890 gas chromatograph, equipped with a HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm). The GC operating conditions were as follows: carrier gas; helium with a flow rate of 2 mL/min, column temperature; 60-280 °C at 4 °C/min, injector and detector temperatures; 280 °C, volume injected; 0.1 mL of the oil, split ratio; 1:50. The MS operating parameters were as follows: ionization potential; 70 eV, ion source temperature; 250 °C, resolution; 1000, ionization current; 750 μA, mass range; 35-425.
Identification of the components of the essential oils
Identification of the constituents was based on computer matching against the library spectra (Library Database Wiley 275L), their retention indices with reference to an n-alkane series in a temperature programmed run, interpreting their fragmentation pattern and comparison of the mass spectra with those reported in the literatures (16,17).
High pressure liquid chromatography analysis
Reagents
The reagents were as follows: a; H2O-deionized high pressure liquid chromatography (HPLC) water, b; acetonitrile (Merck, for liquid chromatography), c; methanol (Merck, for liquid chromatography), d; formic acid (Merck), e; chemical reference standards containing catechin (≥98% HPLC), caffeic acid (≥99.0% HPLC), ferulic acid (≥99.0% HPLC), and taxifolin (≥98% HPLC) all from Sigma Aldrich, f; HPLC mobile phase which mobile phase A was 0.1 % formic acid in H2O, and mobile phase B was 0.1 % formic acid in acetonitrile.
Plant material
Pine bark specimens were collected from two different locations in Iran: P. pinaster from Nowshahr/Chaloos (36°39′11.47″N 51°29′56.93″E / 36°39′22”N 51°25′40”E) and P. eldarica from Isfahan (32°38′N 51°39′E, altitude, 1590 m). The samples were collected between August and September 2009. The specimens were dried at room temperature, ground by using a conventional grinder and stored at +4 °C.
Preparation of pine bark extracts
Pine bark extract was obtained by the method developed by Masquelier (18). Pine bark (100 g) was ground for 1 min, at a speed setting of 2 using a mixer to obtain coarse powder, extracted with 600 mL of boiling water, and then cooled down to 20 °C.
After filtration, 250 mL of liquid were collected and sodium chloride was added up to saturation, and the precipitate formed was removed by filtration. Subsequently, the filtrate was extracted three times with ethyl acetate (10 mL filtrate per 1 mL ethyl acetate (v/v)).
The ethyl acetate phase was collected and dried using anhydrous sodium sulfate and reduced to 1/5 of its volume in a rotary vacuum evaporator. The extract was then poured into three volumes of chloroform, while stirring mechanically. The proanthocyadins were precipitated and collected by filtration. The light beige color powder obtained was stored at −20 °C. All chemicals were of analytical grade purity.
Standard preparation
Stock solutions of catechin, caffeic acid, ferulic acid and taxifolin were prepared in methanol (1 mg/ml) which were stable for a week in the dark and at 0 °C. The stock solutions were used for working standards (10, 20, 60, 80, and 100 μg/ml) and standard calibration curves. All the standards were injected at different times (n value=9).
HPLC conditions
The HPLC system consisted of a manual injector, a HPLC pump (515 Waters), a C18 Nova pack reversed-phase- column (3.9 × 150 mm, 4 μm), a spectrophotometer UV-Vis detector (Dual absorbance, 2448 Waters) controlled by computer software (Waters Empower).
The HPLC gradient profile comprising A; 0.1% formic acid in H2O and B; 0.1% formic acid in acetonitrile, was as follows: 0-40 min; linear gradient from 92:8 (v/v) to 34:66 (v/v), 40-45 min; linear gradient from 34:66 (v/v) to 98:2 (v/v, column rinsing), 45-50 min; 98:2 isocratic, 50-52 min; 98:2 (v/v) to 92:8 (v/v), 52-57; 92:8 (v/v) isocratic. The flow rate was 1.0 ml/min and the analysis was carried out at room temperature. The wavelength for UV detection was set at 280 nm, and the injection volumes were 20 μL.
Accuracy
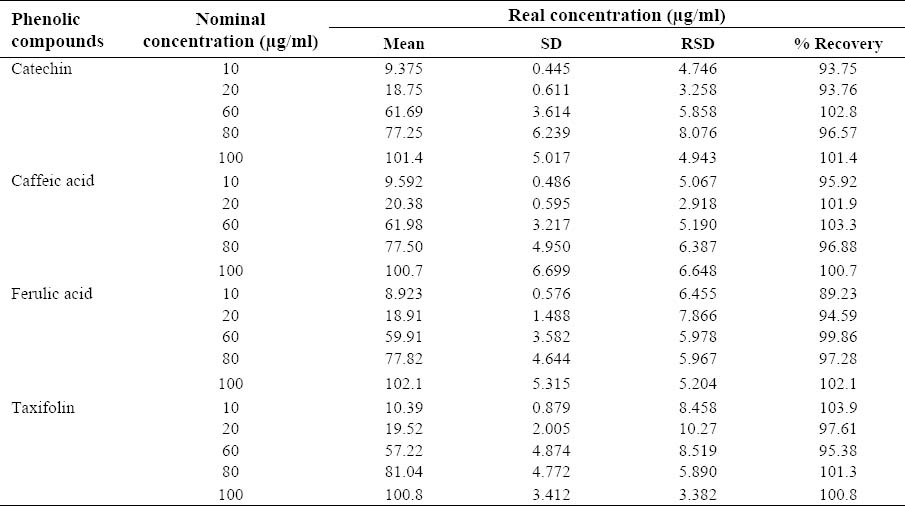
Accuracy was measured as the percent of deviation from the nominal concentration. Accuracy solutions of catechin, caffeic acid, ferulic acid, and taxifolin (10, 20, 60, 80, and 100 μg/ml) were accurately prepared and injected to the HPLC in triplicate. The response was used for back calculation of concentration according to the calibration curve equation. The back calculated concentration was compared to the nominal concentration and the percent deviation was calculated.
Statistical analysis
The data were processed using Microsoft Excel for averages and standard deviation. Data are presented as mean values ± SD.
RESULTS
HPLC analysis
Sample solutions were prepared in methanol, and analyzed by HPLC assay (19). We noticed that determination at 280 nm was much more suitable (19). Four components (catechin, caffeic acid, ferulic acid, and taxifolin) were identified in HPLC chromatograms of P. pinaster and P. eldarica samples based on the retention time by comparison with retention time of reference standard (Figs 2 and 3). The individual signals were assigned by addition of 30 micrograms of each standard (catechin, caffeic acid, ferulic acid, and taxifolin) into P. pinaster sample (Fig. 4). In order to obtain quantitative results, the standard of the four identified compounds were used to establish calibration curves. The correlation between the peak area ratios and the standard concentrations was evaluated over the range 10-100 μg/ml, and was found to be linear (Table 1).
Fig. 2.
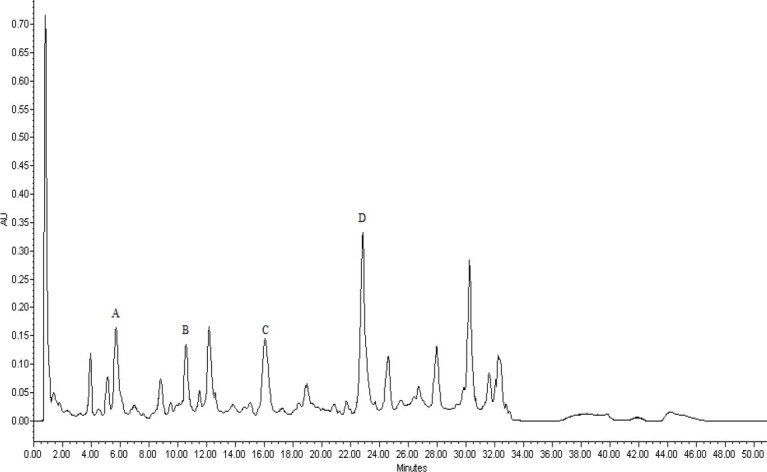
HPLC chromatogram of P. pinaster sample investigated. A; catechin, B; caffeic acid, C; ferulic acid, D; taxifolin.
Fig. 3.
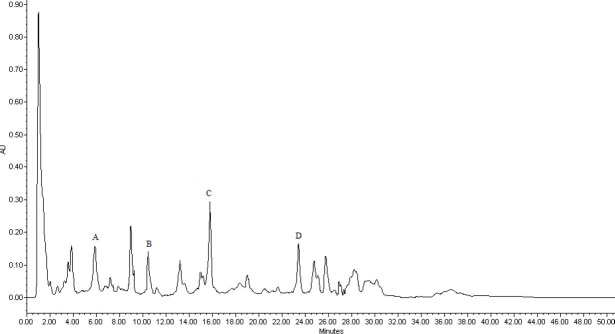
HPLC chromatogram of P. eldarica sample investigated. A; catechin, B; caffeic acid, C; ferulic acid, D; taxifolin.
Fig. 4.
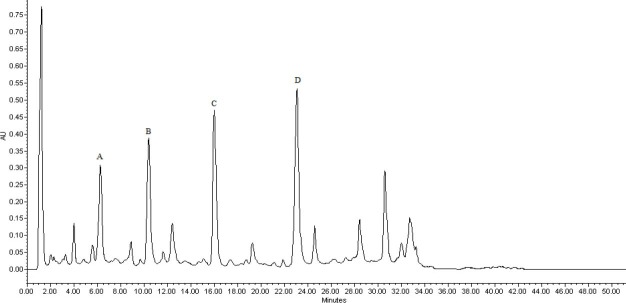
HPLC chromatogram of standards. A; catechin, B; caffeic acid, C; ferulic acid, D; taxifolin.
Table 1.
Linear regression analysis data of catechin, caffeic acid, ferulic acid, and taxifolin (n value = 9).

The four polyphenolic compounds content of P. pinaster and P. eldarica bark extracts are shown in Table 2. The extraction yield for P. pinaster is 20% and for P. eldarica is 22%. The accuracy of the developed method was evaluated by back calculation method. The results are expressed as percent recoveries of catechin, caffeic acid, ferulic acid, and taxifolin in the samples (Table 3). As shown in table 3, the minimum of recovery was 89.23%. In higher concentrations of each compound, the recovery percent is higher than 100% which is due to some errors.
Table 2.
Quantitative results for P. pinaster and P. eldarica (percentage of each compound in the respective pine bark extract; Mean ± SD; n=3).
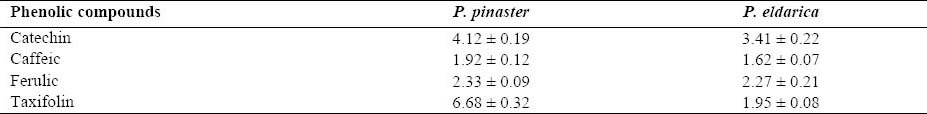
Table 3.
Evaluation of accuracy of the proposed method for determination of catechin, caffeic acid, ferulic acid, and taxifolin (n=3).
Essential oil composition of the bark of Pinus eldarica Medw.
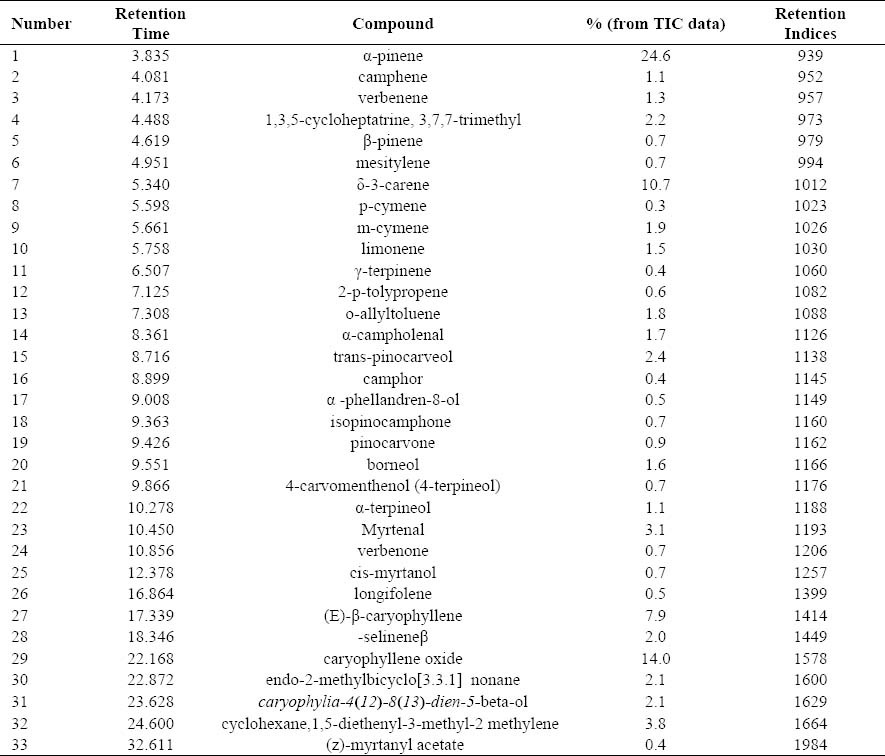
Thirty-three constituents, representing 95.1 % of the bark oil were identified (Table 4). α-pinene (24.6 %), δ-3-carene (10.7 %), and myrtenal (3.1 %) were the main constituents of the monoterpenoid fraction of the bark oil, while (E)-β-caryophyllene (7.9 %) and caryophyllene oxide (14.0 %) were the main components of sesquiterpenoid fraction of the same oil. The composition of P. eldarica bark oil is characterized by high content of monoterpenes (62.2 %).
Table 4.
Percentage composition of the bark oil of the P. eldarica Medw.
DISCUSSION
HPLC analysis
Among the four marker compounds investigated, the major substances identified in P. eldarica and P. pinaster bark extracts were catechin (3.414%) and taxifolin (6.68%), respectively. The total amounts of the four identified compounds in P. pinaster and P. eldarica bark extracts were 15.055% and 9.273%, respectively. Previous studies also reported that the main substances identified in P. pinaster bark extract were taxifolin, catechin, and ferulic acid (20,21). It was demonstrated that the herbal extracts that showed strong antioxidant activity, also possessing high amounts of phenolic compounds, especially catechins and taxifolin (22).
Yesil-Celiktas and co-workers analyzed the polyphenolic composition of four pine bark extracts (P. brutia, P. nigra, P. sylvestris, and P. pinea). They suggested that the other Pinus species like P. brutia which contained extremely high concentrations of taxifolin (approximately 18.5%) had high biological activities, and could be used for commercial applications instead of French P. pinaster (22).
GC/MS analysis
It was reported that the main constituents of the leaf oil of P. eldarica were germacrene D (26.6%), β-caryophyllene (17.1%), α-pinene (11.8%), β-pinene (7.9%), elemicin (4.3%), and α-humulene (4.2%). Main components of the fruit oil were β-caryophyllene (34.0%), α-pinene (16.3%), longifolene (10.5%), α-humulene (6.4%), δ-3-carene (6.3%), and β-pinene (3.8%) (23).
The compounds of turpentine oil from Russian P. eldarica are relatively stable, and the oil content of the resin has been reported to be 15% to 19%. The main components of Monoterpenes were α-pinene (31.5% to 64.2%), δ-3-carene (16.9% to 42.8%), and β-pinene (2.5% to 11.7%) (24).
In another study, the major components of the monoterpene and sesquiterpene fractions of the oil obtained from the terpenoid resin of P. eldarica were α-pinene and β-caryophyllene (25). Furthermore, we analyzed the composition of essential oil obtained from the bark of P. pinaster (26).
As a result, the composition of the oil is characterized by high content of monoterpenes (70.6%). α-pinene (63.9%) was the main constituent of the monoterpenoid fraction while junipene (7.5%) and β-caryophyllene (14.3%) were the main components of sesquiterpenoid fraction of the bark oil (26).
CONCLUSION
This study shows that RP-HPLC method enables the identification and quantification of catechin, caffeic acid, ferulic acid, and taxifolin in P.eldarica and P. pinaster bark extracts. Previous studies introduced P. pinaster as a source of polyphenolic compounds. Our results demonstrated that P. eldarica bark extracts can be used as an effective source of polyphenolic compounds in the food and pharmaceutical industries, as well. Furthermore, because of the high percentage of α-pinene in the volatile oils obtained from the bark of P. eldarica, it can be used in industries and especially in production of plasticizers, camphor, insecticides, perfumes, and solvents.
ACKNOWLEDGMENTS
This study was a part of the Pharm.D thesis of Pharmacognosy department, Faculty of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences (Registration No. 388288).
REFERENCES
- 1.Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from the pine (Pinus maritima) bark, Pycnogenol. Free Rad Biol Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 2.Grimm T, Schafer A, Hogger P. Antioxidant activity and inhibition of matrix metalloproteinases by metabolites of maritime pine bark extract (Pycnogenol) Free Rad Biol Med. 2004;36:811–822. doi: 10.1016/j.freeradbiomed.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158–168. doi: 10.5414/cpp40158. [DOI] [PubMed] [Google Scholar]
- 4.Iravani S, Zolfaghari B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res Pharm Sci. 2011;6:1–11. [PMC free article] [PubMed] [Google Scholar]
- 5.Blazso G, Gabor M, Sibbel R, Rohdewald P. An anti-inflammatory and superoxide radical scavenging activities of a procyanidins containing extract from the bark of Pinus pinaster sol. and its fractions. Pharm Parmacol Lett. 1994;3:217–220. [Google Scholar]
- 6.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005;81:122–129. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- 7.Kuzuhara T, Iwai Y, Takahashi H, Hatakeyama D, Echigo N. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. 2009;1 doi: 10.1371/currents.RRN1052. RRN1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song JM, Lee KH, Seong BL. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005;68:66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Krecman V, Skottova N, Walterova D, Ulrichova J, Simanek V. Silymarin inhibits the development of diet-induced hypercholesterolemia in rats. Planta Med. 1998;64:138–142. doi: 10.1055/s-2006-957391. [DOI] [PubMed] [Google Scholar]
- 10.Casaschi A, Rubio BK, Maiyoh GK, Theriault AG. Inhibitory activity of diacylglycerol acyltransferase (DGAT) and microsomal triglyceride transfer protein (MTP) by the flavonoid, taxifolin, in HepG2 cells: potential role in the regulation of apolipoprotein B secretion. Atherosclerosis. 2004;176:247–253. doi: 10.1016/j.atherosclerosis.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Wang YH, Wang WY, Liao JF, Chen CF, Hou YC, Liou KT, et al. Prevention of macrophage adhesion molecule-1 (Mac-1)-dependent neutrophil firm adhesion by taxifolin through impairment of protein kinase-dependent NADPH oxidase activation and antagonism of G protein-mediated calcium influx. Biochem Pharmacol. 2004;67:2251–2262. doi: 10.1016/j.bcp.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Chagné D, Brown G, Lalanne C, Madur D, Pot D, Neale D, et al. Comparative genome and QTL mapping between maritime and loblolly pines. Mol Breed. 2003;12:185–195. [Google Scholar]
- 13.Ribeiro MM, Plomion C, Petit R, Vendramin GG, Szmidt AE. Variation in chloroplast single-sequence repeats in Portuguese maritime pine (Pinus pinaster Ait. Theor Appl Genet. 2001;102:97–103. [Google Scholar]
- 14.Parsa A. Vol. 1. Tehran: 1978. flora of iran. sponsord by ministry of science & higher. Education of iran; pp. 448–449. [Google Scholar]
- 15.Vol. 2. London: HMSO; 1998. British Pharmacopoeia; pp. A137–A138. [Google Scholar]
- 16.Adams RP. Illinois: Allured Publishing Corporation; 1995. Identification of essential oil components by gas chromatography/mass spectroscopy; pp. 69–351. [Google Scholar]
- 17.Swigar AA, Silverstein RM. Wisconsin: Aldrich Chemical Company; 1981. Monoterpenes. infrared, mass, 1H-NMR, 13C-NMR spectra and kovats indices; pp. 1–121. [Google Scholar]
- 18.Masquelier J. US Patent; 1987. Plant extract with proanthocyanidins content as therapeutic agent having radical scavenger effect and use thereof. 4,698,360. [Google Scholar]
- 19.Chen P, Song F, LZ L. Chromatographic fingerprint analysis of Pycnogenol dietary supplements. J AOAC Int. 2009;92:624–632. [PubMed] [Google Scholar]
- 20.Sarikaki V, Rallis M, Tanojo H, Panten I, Dotsiva Y, Laukas YL. In vitro percutaneous absorption of pine bark extract (PYC) in human skin. J Toxicol Cutaneous Ocular Toxicol. 2004;23:149–158. [Google Scholar]
- 21.Romani A, Ieri F, Turchetti B, Mulinacci N, FF V, Buzzini P. Analysis of condensed and hydrolysable tannins from commercial plant extracts. J Pharm Biomed Anal. 2006;41:415–420. doi: 10.1016/j.jpba.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Yesil-Celiktas O, Ganzera M, Akgun I, Sevimli C, KS K, Erdal B. Determination of polyphenolic constituents and biological activities of bark extracts from different Pinus species. J Sci Food Agric. 2009;89:1339–1345. [Google Scholar]
- 23.Afsharypour S, San’aty F. Essential oil constituents of leaves and fruits of Pinus eldarica Medw. L. J Essent Oil Res. 2005;17:327–328. [Google Scholar]
- 24.Chundnyi AV, Rudenko B-A. Composition of turpentine oil from P. eldarica Medw. Rastit Resur. 1982;18:252–255. [Google Scholar]
- 25.Drebushchack TD, Shmidt EN, Khan VA, Dubovenko ZV, Kemertelidze EP, Pentegova VA. Terpenoids of oleoresins of Pinus pityusa and P. eldarica. Chem Natural Comp. 1982;18:316–320. [Google Scholar]
- 26.Zolfaghari B, Iravani S. Essential oil constituents of the bark of Pinus pinaster from Iran. J essent oil-bearing plants. 2012;15:348–351. [Google Scholar]


