Abstract
Antidepressants are widely used for the treatment of various neuropathic pain conditions in humans. Recent studies have demonstrated that bupropion is effective for the treatment of neuropathic pain. Also antidepressants like bupropion showed anti-inflammatory properties. So in the present study, the analgesic and anti-inflammatory effects of bupropion in mice and rat were investigated. The acetic acid, formalin and hot plate tests were used in male mice to assess analgesic activity. For evaluation of anti-inflammatory effect, carrageenan-induced rat paw edema and croton oil-induced ear edema were used. Bupropion was administered at the doses of 10, 20 and 40 mg/kg (i.p.). Bupropion at a dose of 40 mg/kg significantly reduced acetic acid-induced abdominal writhes and also was effective in suppression of formalin-induced behavior and showed significant analgesia in hot plate test. While 40 mg/kg bupropion showed considerable anti-inflammatory response in carrageenan test, but no effect was observed in croton oil-induced ear edema. The results showed that bupropion has analgesic and anti-inflammatory effects in animal models and further studies are needed to find out its mechanism of action.
Keywords: Bupropion, Antidepressants, Analgesic, Anti-inflammatory, Animal models
INTRODUCTION
Antidepressant drugs, especially tricyclic drugs, have shown analgesic and anti-inflammatory effects in animal models as well as in clinical settings (1,2,3,4); however, their exact pain killing mechanism has not yet been completely known. It appears that their analgesic effect is independent of the effect on mood and typically for the pain relief they are administered at lower doses than for depression (2,5,6). For the central and peripheral analgesia mechanisms of anti-depressants, a number of pharmacological actions are considered: They block reuptake of noradrenaline and serotonin, have direct and indirect actions on opioid receptors (7), inhibit histamine, cholinergic, serotonin and N-methyl-D-aspartate (NMDA) receptors, inhibit ion channel activity, and block adenosine uptake (8). All these actions modulate neuro-transmitters in the spinal cord that reduce transmission or perception of pain signals (9).
Bupropion is an atypical antidepressant and smoking cessation aid (10). Its pharmacological action is thought to be norepinephrine-dopamine reuptake inhibition. It inhibits the reuptake of dopamine twice as potent as that of norepinephrine reuptake. Bupropion does not inhibit monoamine oxidase or serotonin reuptake. However, it has been shown that it indirectly enhances the firing of serotonergic neurons, via activation of downstream norepinephrine flow (11). It also acts as a noncompetitive nicotinic acetylcholine receptor antagonist (12). Bupropion is metabolized to hydroxy-bupropion (active metabolite) by CYP2B6 that is excreted by the kidneys (13).
Studies observed that bupropion lowers the level of an inflammatory mediator TNF-alpha (by increasing the intracellular cAMP that inhibits TNF-alfa synthesis) and interferon-gamma so bupropion may be useful in inflammatory conditions such as Crohn's disease and psoriasis (14,15,16).
Uncontrolled pilot and placebo-controlled cross over trial showed that bupropion SR (150-300 mg daily) was effective and well tolerated for the treatment of neuropathic pain. Blockade of norepinephrine reuptake may mediate this effect and the role of dopamine reuptake blockade is uncertain (17,18,19). In contrary, one study showed that bupropion SR is not significantly better than placebo in the control of patients with non-neuropathic chronic low back pain (20).
Based on the above controversy regarding the analgesic effect of bupropion, this study was aimed to find more pharmacological evidence for analgesic and anti-inflammatory effects of bupropion in different animal models.
MATERIALS AND METHODS
Experimental animals
Male Swiss mice (weighing 25-35 g) and male Wistar rats (200-250 g) were obtained from the animal house of the Department of Pharmacology, Isfahan University of Medical Sciences, Iran.
Animals were maintained in standard laboratory conditions throughout the study. Animals were housed in polypropylene cages with free access to food and water. All experiments were performed according to the guidelines for the care of laboratory animals of Ethics Committee of Isfahan University of Medical Sciences.
Drugs
All of the chemicals and reagents used were of analytical grade. Acetic acid and formalin were purchased from Merck chemical company (Germany). Morphine (Darou Pakhsh, Iran), bupropion (Raha, Iran), carrageenan (Fluka, Switzerland), croton oil and indomethacin (Sigma, USA) were also used.
Acetic acid-induced writhing test
Mice were divided into five groups of 6 animals each. The control group received normal saline (10 ml/kg, intraperitoneally (i.p.)). The test groups were treated with 10, 20, 40 mg/ kg, i.p. of bupropion while the fifth group received indomethacin as the reference drug at the dose of 10 mg/kg, i.p. After 30 min of drug administration, the mice were treated with 1% acetic acid (10 ml/kg, i.p.). Ten min after acetic acid injection, mice were placed in individual cages and the number of abdominal contractions was counted for each mouse during a period of 10 min. The inhibition percentage of abdominal contractions was calculated and compared (21,22).
Formalin test
The mice were divided into five groups of 7 animals each and were administered with either normal saline (10 ml/kg, i.p.), bupropion (10, 20 and 40 mg/kg, i.p.) or morphine (10 mg/kg, i.p.). Thirty min after this treatment, 20 μl of a freshly prepared 2.5% solution of formalin was injected subcutaneously under the plantar surface of the right hind paw of each mouse. The mice were placed individually in an observation chamber and licking or biting of the injected paw monitored. Analgesic effect was determined in two phases. The early phase (phase 1) was recorded during the first 5 min, while the late phase (phase 2) was recorded during the 10 min with a 15 min interval between both phases (22,23).
Hot plate test
Mice were divided into 4 groups of 6 animals each. Control animals received normal saline, while the standard (reference) group received morphine (10 mg/kg, i.p.) The test groups received bupropion (20, 40 mg/kg, i.p.). Thirty min later, the animals were placed in the hot plate apparatus that maintained at 58 °C and reaction time was recorded every 30 min during 2 h. Reaction time was taken as the period between placing the mice on hot plate and time when they jumped. A cut-off time of 15 sec was used to prevent any thermal injury to mice. Percent of maximal possible antinociceptive effect (MPE%) was calculated using the following formula (24).
MPE%= [test latency (sec)-control latency (sec)] / [cut-off time (sec)- control latency (sec)]×100
Carrageenan-induced paw edema
The rats were divided into five groups of 7 animals each. Acute inflammation was induced by injecting 0.1 ml of (1% w/v) carrageenan into plantar surface of rat hind paw. Bupropion (10, 20 and 40 mg/kg), normal saline (1 ml/kg) and indomethacin (10 mg/kg) as reference agent were administered i.p. 30 min before subplantar injection of carrageenan (100 μl of 1% w/v solution in saline). The paw volume was measured 4 h after carrageenan injection using a mercury plethysmograph (Ugo Basil, Italy) to determine the volume of paw. The difference between the volume of carrageenan-injected paw and control paw was calculated (25).
Croton oil-induced ear edema
The mouse ear edema test was carried out by topical application of croton oil on mouse ear. Mice were divided into 5 groups (6 animals in each group). The control group received normal saline (10 ml/kg, i.p.). The test groups were treated with 10, 20, 40 mg/kg, i.p. of bupropion and the reference group received indomethacin (10 mg/kg, i.p.). Croton oil dissolved in acetone to obtain a concentration of 100 μg/15 μl. Thirty min after drug administration, 15 μL of this solution applied with a sampler on the inner surface of the right ear. Inflammation was allowed to develop for 6 h after which the animals were killed by extra ether inhalation, and a section (6 mm diameter) of the central portion of both ears was obtained and weighed. The swelling induced by croton oil was assessed in terms of the increase in the weight of the right ear punch biopsy over that of the left ear (26).
Statistical analysis
The data are expressed as the mean ± SEM. The statistical significance between control and treated groups were analyzed using one way analysis of variance (ANOVA) using SPSS 13.0 software, where p<0.05 was taken to be significant threshold for all comparisons.
RESULTS
Effect of bupropion on acetic acid-induced writhing test
In acetic acid-induced writhing test, bupropion only at the dose of 40 mg/kg significantly (p<0.05) inhibited abdominal twitches. In this test indomethacin, as the reference drug at a dose of 10 mg/kg produced about 84.2% reduction of writhes (Fig. 1).
Fig. 1.
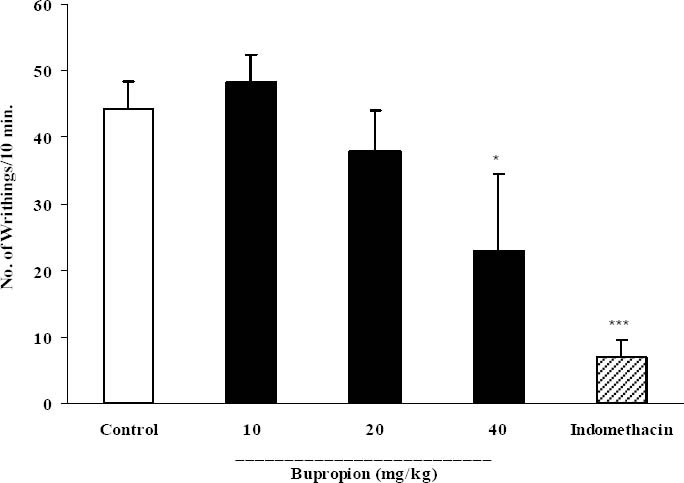
Effect of intrperitoneal injection of bupropion on acetic acid-induced writhing test in mice. Bup (bupropion, 10, 20 and 40 mg/kg), indomethacin (10 mg/kg) and the vehicle were administered 30 min prior to acetic acid (1%) injection, the number of abdominal contractions was counted for each mouse for a period of 10 min starting 10 min after acetic acid injection. The values represent the mean of abdominal twitches ± SEM. *P<0.05; ***p<0.001 compared with control group.
Effect of bupropion on formalin test
In acute phase of formalin test, bupropion just at a dose of 40 mg/kg significantly (p<0.05) inhibited formalin-induced pain and morphine (10 mg/kg) as a standard analgesic drug significantly (p<0.001) reduced pain behavior (Fig. 2).
Fig. 2.
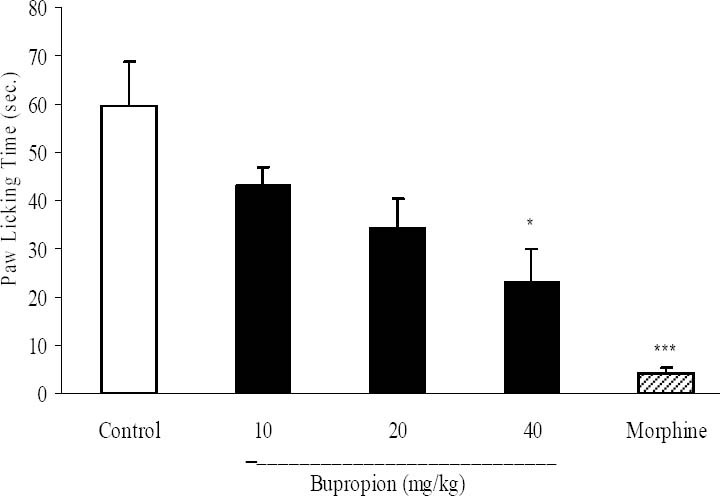
The antinociceptive activity of bupropion on paw licking during acute phase of formalin test. Different doses of buoropion (10, 20, 40 mg/kg) and vehicle were intraperitoneally administered 30 min prior to subplantar injection of formalin and time spent (seconds) for licking was measured during a 0-5 min immediately after formalin injection. Morphine (10 mg/kg, i.p.) was used as reference drug. Data are mean ± SEM. *P<0.05; ***P<0.001 compared with control group.
In chronic phase, bupropion at doses of 20 and 40 mg/kg and morphine caused significant inhibition of formalin-induced licking behavior. In this phase bupropion at doses of 20 and 40 mg/kg produced 74.3% and 83.8% inhibition of paw licking (Fig. 3).
Fig. 3.
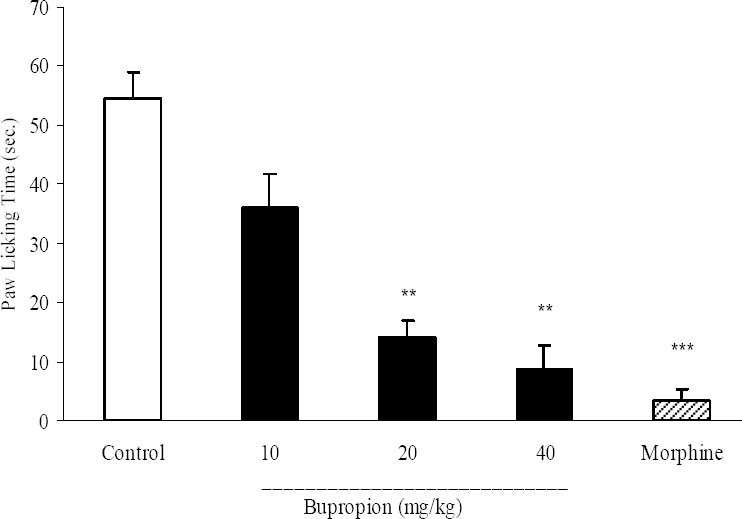
The antinociceptive activity of bupropionin chronic phase of formalin test. Different doses of buoropion (10, 20, 40 mg/kg) and vehicle were intraperitoneally administered 30 min prior to subplantar injection of formalin and time spent for licking was measured during a 20-30 min period after formalin injection. Morphine (10 mg/kg, i.p.) was used as reference drug. Data are mean ± SEM. **P<0.01; ***P<0.001 compared with control group.
Effect of bupropion in hot plate test
In hot plate test, morphine as the standard drug produced significant analgesia 30 min after injection. This analgesic effect remained until 90 min. Bupropion at a dose of 40 mg/kg showed significant (p<0.01) analgesia 30 to 90 min after injection and then the analgesic effect reached to a non significant level (Fig. 4).
Fig. 4.
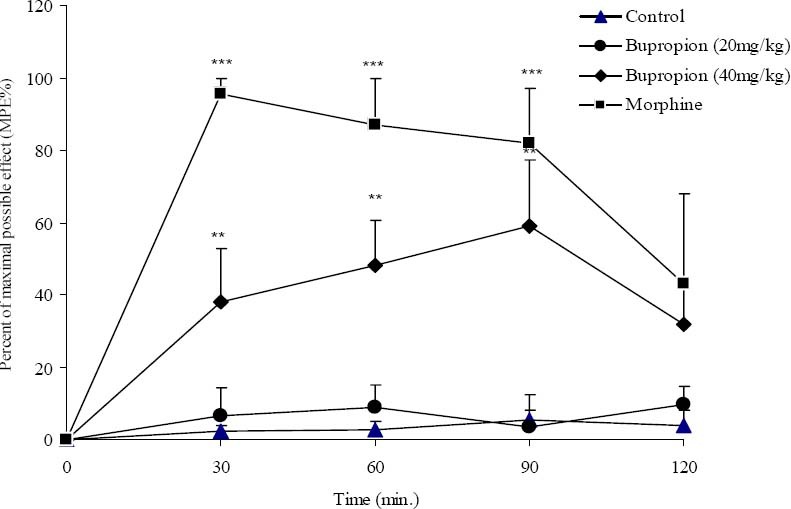
The antinociceptive activity of bupropion in hot plate test. Vehicle and bupropion (20, 40 mg/kg, i.p.) were administered 30 min prior to placement of the animal in hot plate and reaction time of mice was measured at 30 min intervals until 2 h and percent of maximal possible antinociceptive effect (MPE%) was calculated for each time and compared. Morphine (10 mg/kg, i.p.) was used as reference drug. Data are mean ± SEM. **P<0.01; ***P<0.001 compared with control group.
Effect of bupropion on carrageenan-induced paw edema
As illustrated in Fig. 5, i.p. injection of bupropion at a dose of 40 mg/kg significantly inhibited the development of paw edema 4 h after carrageenan injection (P<0.05) as compared to the control group. As expected, the reference drug, indomethacin (10 mg/kg), caused a significant inhibition of edema 4 h post-carrageenan so that an 84.2% inhibition of paw edema was achieved (P<0.001).
Fig. 5.
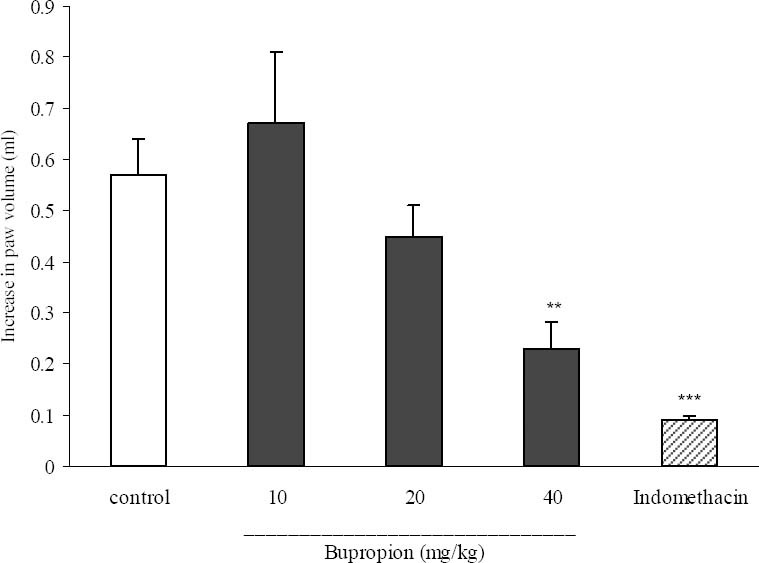
Effect of bupropion on carrageenan induced paw edema in rats. Bupropion (10, 20 and 40 mg/kg), indomethacin (10 mg/kg) and the vehicle were administrated 30 min prior to carrageenan (1%) injection, and the rats were evaluated for paw edema 4 h post-carrageenan injection. The values represent the mean variation in the paw volume ± SEM. **P<0.01; ***P<0.001 compared with control group.
Effect of bupropion on croton oil-induced ear edema
As it is seen in Fig. 6, only indomethacin (10 mg/kg), showed anti-inflammatory activity and bupropion failed to produce any anti-inflammatory effect.
Fig. 6.
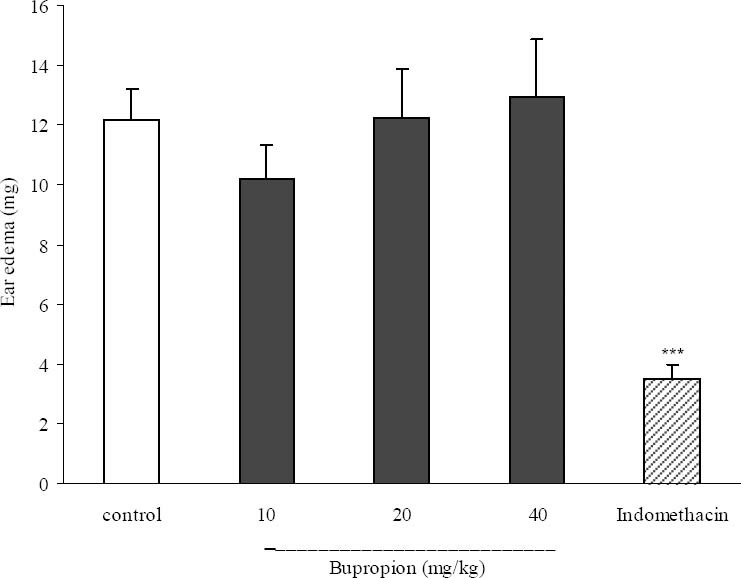
Effect of bupropion on croton oil induced ear edema in mice. Ear edema was measured at 6 h after croton oil treatment. Bup (bupropion, 10, 20 and 40 mg/kg) and indomethacin (10 mg/kg) and the vehicle were administrated 30 min prior to croton oil application. Each bar represents the mean ± SEM. ***P<0.001 compared with control group.
DISCUSSION
The results of the present study indicate that i.p. administration of bupropion at the dose of 40 mg/kg, possess a significant effect against pain in three antinociceptive models of the mice. The antinociceptive evaluation methods used in this study were hot plate (cutaneous thermic stimuli), writhing (chemical visceral stimuli) and formalin (cutaneous chemical stimuli) test. In acetic acid test, that is a non specific test for the assessment of analgesic activity, abdominal constriction is related to the sensitization of nociceptive receptors by prostaglandins particularly PGE2 and PGF2a as well as lipoxygenase products (leucotrienes) (27,28). In this test, bupropion at a 40 mg/kg dose showed significant effect by 43.8% inhibition. Therefore, the analgesic effect of bupropion may be due to either its action on visceral receptors sensitive to acetic acid or the inhibition at the central level of the transmission of painful messages.
The formalin pain model is a valid and reliable model of nociception and is sensitive for various classes of analgesic drugs (23). Two phases in the formalin test have different nociceptive mechanisms. It is suggested that the early phase is due to a direct effect on nociceptors and activation of c-fibers and prostaglandins do not play an important role during this phase. The late phase seems to be dependent on the combination of an inflammatory response with the release of serotonin, histamine, bradykinin and prostaglandins in peripheral tissue and functional changes in the dorsal horn of spinal cord (29). Bupropion, at the highest test dose, significantly suppressed pain response of both phases of formalin test. Drugs which act mainly centrally, such as narcotics, inhibit both phases of formalin-induced pain while peripherally acting drugs, such as aspirin, only inhibit the late phase.
In the hot plate assay, only the higher doses of bupropion produced significant increase in the latency of the animals. The effect of the bupropion on hot plate test provides a confirmation of its central effect since the assay is specific for opioid-induced analgesic effect (24). Bupropion also inhibited acetic acid-induced writhing in mice, hence it can be suggested that the analgesic effect of bupropion is also peripherally mediated.
Inflammation is a complex process and various mediators e.g. prostaglandins, leukotrienes, platelet activating factor and a number of cytokines including TNF-alfa have been reported to be involved in the development of inflammatory process (30).
Carrageenan-induced paw edema in rats and croton oil-induced ear swelling in mice as valid and routine models were selected to assay anti-inflammatory activity of bupropion in this study.
The probable mechanism of action of carrageenan-induced inflammation is three-phasic, the first phase is attributed to the release of histamine and serotonin, the second phase mediated by kinins while the third phase is attributed to the release of prostaglandins and lysosome enzymes in 5 hours of edema (31). The effect of bupropion was investigated at the later phases of the inflammatory response, which indicated that this stage of the hind paw edema is correspond to arachidonic acid metabolites and can be inhibited by aspirin and other arachidonate cyclooxygenase inhibitors; bupropion at dose of 40 mg/kg was capable to inhibit significantly the paw edema in rats. But bupropion at all test doses failed to show any effect in croton oil test. Studies reported croton oil-induced ear swelling is a method to detect the anti-inflammatory potential effects of histamine ligands (26), so this test confirmed that anti-inflammatory effect of bupropion is not mediated by histamine.
CONCLUSION
It is concluded that bupropion possesses analgesic and anti-inflammatory properties, which are probably mediated via inhibition of prostaglandin synthesis as well as central inhibitory mechanisms.
ACKNOWLEDGMENTS
This research was supported by the research council of the Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Sadeghi H, Hajhashemi V, Minaiyan M, Movahedian A, Talebi A. Further studies on anti-inflammatory activity of maprotiline in carrageenan-induced paw edema. Int Immunopharmacol. 2013;15:505–510. doi: 10.1016/j.intimp.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Knotkova H, Pappagallo M. Adjuvant analgesics. Anesthesiol Clin. 2007;25:775–786. doi: 10.1016/j.anclin.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Salam OM, Nofal SM, El-Shenawy SM. Evaluation of the anti-inflammatory and anti-nociceptive effects of different antidepressants in the rat. Pharmacological Res. 2003;48:157–165. doi: 10.1016/s1043-6618(03)00106-3. [DOI] [PubMed] [Google Scholar]
- 4.Korzeniewska-Rybicka I, Płaźnik A. Analgesic effect of antidepressant drugs. Pharmacol Biochem Behav. 1998;59:331–338. doi: 10.1016/s0091-3057(97)00336-5. [DOI] [PubMed] [Google Scholar]
- 5.McQuay HJ, Carroll D, Glynn CJ. Dose-response for analgesic effect of amitriptyline in chronic pain. Anaesthesia. 1993;48:281–285. doi: 10.1111/j.1365-2044.1993.tb06943.x. [DOI] [PubMed] [Google Scholar]
- 6.Sansone RA, Sansone LA. Pain, go away: antidepressants and pain management. Psychiatry (Edgmont) 2008;5:16–19. [PMC free article] [PubMed] [Google Scholar]
- 7.Wattiez AS, Libert F, Privat AM, Loiodice S, Fialip J, Echalier A, et al. Evidence for a differential opioidergic involvement in the analgesic effect of antidepressants: prediction for efficacy in animal models of neuropathic pain. Br J Pharmacol. 2011;163:792–803. doi: 10.1111/j.1476-5381.2011.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96:399–409. doi: 10.1111/j.1742-7843.2005.pto_96696601.x. [DOI] [PubMed] [Google Scholar]
- 9.Sawynok J, Esser MJ, Reid AR. Antidepressants as analgesics: an overview of central and peripheral mechanisms of action. J Psychiatry Neurosci. 2001;26:21–29. [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg MJ, Grandi SM, Gervais A, O’Loughlin J, Paradis G, Rinfret S, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol. 2013;61:524–532. doi: 10.1016/j.jacc.2012.08.1030. [DOI] [PubMed] [Google Scholar]
- 11.Saiz Ruiz J, Gibert J, Gutiérrez Fraile M, Bobes J, Vallejo J, Iglesias C, et al. Bupropion: efficacy and safety in the treatment of depression. Actas Esp Psiquiatr. 2011;39:1–25. [PubMed] [Google Scholar]
- 12.Arias HR. Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? Int J Biochem Cell Biol. 2009;41:2098–2108. doi: 10.1016/j.biocel.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother. 2006;6:1249–1265. doi: 10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- 14.Kast RE, Altschuler EL. Remission of Crohn's disease on bupropion. Gastroenterology. 2001;121:1260–1261. doi: 10.1053/gast.2001.29467. [DOI] [PubMed] [Google Scholar]
- 15.Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol. 2006;6:903–907. doi: 10.1016/j.intimp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Kast RE. Anti- and pro-inflammatory considerations in antidepressant use during medical illness: bupropion lowers and mirtazapine increases circulating tumor necrosis factor-alpha levels. Gen Hosp Psychiatry. 2003;25:495–496. doi: 10.1016/s0163-8343(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 17.Semenchuk MR, Davis B. Efficacy of sustainedrelease bupropion in neuropathic pain: an open-lable study. Clin J pain. 2000;16:6–11. doi: 10.1097/00002508-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 2001;57:1583–1588. doi: 10.1212/wnl.57.9.1583. [DOI] [PubMed] [Google Scholar]
- 19.Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333–336. doi: 10.1177/1049909110361229. [DOI] [PubMed] [Google Scholar]
- 20.Davidson JR, France RD. Bupropion in chronic low back pain. J Clin Psychiatry. 1994;55:362. [PubMed] [Google Scholar]
- 21.Koster R, Anderson M, DeBeer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–417. [Google Scholar]
- 22.Hajhashemi V, Saghaei L, Fassihi A, Mojiri-Froshani H. A study on the analgesic effects of four new derivatives of 3-hydroxy pyridine-4-one. Res Pharmac Sci. 2012;7:37–42. [PMC free article] [PubMed] [Google Scholar]
- 23.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 24.Vogel HG, Vogel WH. Berlin: Springer; 1997. Drug discovery and evaluation; pp. 368–370. [Google Scholar]
- 25.Winter CA, Riselay EA, Nuss GW. Carrageenan-induced oedema in the hind paw of the rats as an assay for anti – inflammatory drugs. Proc soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 26.Coruzzi G, Pozzoli C, Adami M, Grandi D, Guido N, Smits R, et al. Strain-dependent effects of the histamine H4 receptor antagonist JNJ7777120 in a murine model of acute skin inflammation. Exp Dermatol. 2012;21:32–37. doi: 10.1111/j.1600-0625.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 27.Deraedt R, Jonquey S, Delvallee F, Falhout M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 28.Levini JD, Lau W, Kwait G, Goetzl EJ. Leukotriene B4 produces hyperalgesia that is dependent on the polymorphonuclear leucocytes. Science. 1984;225:743–745. doi: 10.1126/science.6087456. [DOI] [PubMed] [Google Scholar]
- 29.Tjolsen A, Berge OG, Hunskaar S, Rosaland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 30.Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35–45. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Rosa M, Giroud JP, Willoughby DA. Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]


