Abstract
Pharmacological activities of 6-(4-hydroxy-3-methoxyphenyl)-hexanonic acid (HMPHA), a phenolic compound, isolated from the extract of Pycnocycla spinosa was investigated on ileum motility in vivo and in vitro. Ileum motility was examined by measuring charcoal movement through the gut in mice. In addition, antidiarrhoeal activity of HMPHA was assessed and compared with standard drug; loperamide (2 mg/kg) and the hydroalcoholic extract of P. spinosa (2 mg/kg). Furthermore, concentration response curve to contraction induced by acetylcholine (ACh), 5-hydroxy triptamine (5-HT) and electrical field stimulation (EFS) were obtained after incubation of ileum segment with various concentrations of HMPHA or hydroalcoholic extract of P. spinosa. HMPHA (2 mg/kg and 5 mg/kg, orally) significantly inhibited gut movements in vivo and reduced diarrhoea induced by castor oil or sulphate magnesium. In addition, HMPHA reduced ileum contraction induced by ACh (IC50=33 ± 6 μg/ml), 5-HT (IC50=87 ± 12 μg/ml) and EFS (IC50=36 ± 3 μg/ml) in vitro in a concentration-dependent manner. The inhibitory effect was reversible following washing off the drug. These studies indicate that HMPHA as an active component of P. spinosa extract has significant antispasmodic and antidiarrhoeal activities and therefore, has the potential as a lead compound for further development of a new spasmolytic remedy.
Keywords: Pycnocycla spinosa extract, 6-(4-hydroxy-3-methoxyphenyl)-hexanonic acid, Antispasmodic, Antidiarrhoeal
INTRODUCTION
Irritable bowel syndrome (IBS) is a digestive tract diseases associated with intestinal movement problems. IBS can present with pain, constipation, or diarrhoea (1,2). A laxative may be needed to relieve constipation (3,4). Anti-motility drugs such loperamide may relieve diarrhoea and antispasmodic drugs reduce intestinal spasm and relieve the pain (4,5,6). Antimuscarinic agents also are used for gastrointestinal smooth muscle spasm (4,5,6). Dicyclomine has less antimuscarinic adverse effects than atropine and may also have some direct action on smooth muscle. The smooth muscle relaxant properties of antimuscarinic and other antispasmodic drugs make them useful remedy in IBS. Plant derivatives extract has also been used as a remedy and many clinically useful drugs had been derived from plant materials (6,7). Alverine, mebeverine and peppermint oil are other antispasmodic agents believed to be direct relaxant of intestinal smooth muscle and may relieve the pain in IBS and diverticular disease (8,9).
Pycnocycla spinosa Decne. exBoiss. (P. spinosa) (10,11) extract is a smooth muscle relaxant of intestinal smooth muscles (12,13,14) and it could be an alternative remedy for the treatment of IBS, since its extract has shown to posses both antispasmodic and antidiarrhoeal activities (12,13,14,15). The hydroalcoholic extract of P. spinosa are enriched in a number of substances including isovanilline and isoacetovanilone with antispasmodic and anti-diarrhoeal activities (16,17,18). 6-(4-hydroxy-3-methoxyphenyl)-hexanonic acid (HMPHA) is another active compound which has been identified in P. spinosa extract (19,20). However, its effect on ileum motility has not yet been investigated. Therefore, the objective of the present study was to examine the relaxant activity of HMPHA on ileum contractions induced by acetylcholine (ACh), 5-hydroxy triptamine (5-HT) and electrical field stimulation (EFS). In addition to antispasmodic activity, anti-diarrhoeal action and anti-motility of this compound was also investigated in vivo.
MATERIALS AND METHODS
Plant materials
The aerial parts of the plant in flowering season (Jun 2012) were collected from Isfahan University campus located in the base of the Sofah mountain in south of Isfahan-Iran. The plant was identified as Pycnocycla spinosa Decne. exBoiss. (Fam. Umbelliferae) in Biology Department of Isfahan University. A voucher specimen of the plant (A24) was deposited in the herbarium of the Faculty of Pharmacy and Pharmaceutical Sciences at the Isfahan University of Medical Sciences (Iran).
Drugs and solutions
The following drugs were used in antidiarrhoeal studies: loperamide (Sigma), castor oil (Dine Company, Iran), alcoholic extract of P. spinosa obtained by maceration (21). HMPHA (Fig. 1) was isolated form P. spinosa extract as described before (19,20). Loperamide and extract of P. spinosa were initially prepared as 10 mg/ml stock solution in 70% ethanol. HMPHA was made up as 1 mg/ml suspension solution in 70% ethanol. MgSO4 (Merck) was used as 10% solution in distilled water.
Fig. 1.
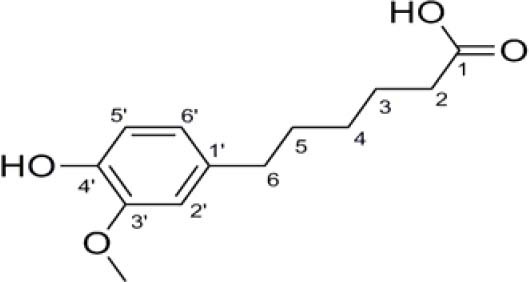
Chemical structure of 6-(4-hydroxy-3-methoxyphenyl)- hexanonic acid.
Charcoal (3%) and tragacanth powder (5%) suspension were prepared in distilled water. The stock solutions were prepared in such a way that about 0.5 ml of the solution contained the appropriate dose of each drug.
The following drugs or chemicals were used for antispasmodic studies: ACh and 5-HT(Sigma) were dissolved in distilled water. Tyrode's solution with the following composition (mM), NaCl; 136.9, KCl; 2.68, CaCl2; 1.8, MgCl2; 1.05, NaHCO3; 11.9, NaH2PO4; 0.42 and glucose; 5.55 were made up in distilled water. Unless stated, all other chemicals were from Merck.
Antidiarrhoeal studies
Male albino mice (25-30g) bred in School of Pharmacy and Pharmaceutical Sciences animal house were kept at room temperature. The animals were fasted overnight prior to the experiments with free access to water. All animals were handled in accordance with the internationally accepted principles for laboratory animal use and care, as recommended by university authorities (22).
In this study antidiarrhoeal effect of HMPHA (isolated from P. spinosa extract) were assessed on castor oil and MgSO4 induced diarrhea and compared with hydroalcoholic extract. In addition, effect of this substance on ileum movement was assessed by charcoal meal transit test. The following doses of drugs were examined: HMPHA (2 mg/kg and 5 mg/kg), loperamide (2 mg/kg), and hydroalcoholic extract of P. spinosa (2 mg/kg). In addition, equivalent volume of vehicle was used as control group. All the drugs were given orally to each mice using special feeding needle.
Castor oil and magnesium sulphate induced diarrhoea
The testing drugs or vehicle were given orally to groups of 10 mice. Thirty min after drug administration, diarrhoea was induced by oral administration of 0.5 ml castor oil. Then each mouse was placed under a large glass funnel and number of wet defecation on tissue paper was recorded over 3.5 h. In separate groups of animals diarrhoea was induced by oral administration of MgSO4 solution (2 g/kg) and half an hour later drugs or vehicle were given by gavage to the animals and incidence of diarrhoea was assessed as above.
Charcoal meal transit test
In this test movement of charcoal meal in the intestine was assessed. For this purpose each test drug was given orally to mice and 30 min later, 0.5 ml of charcoal meal containing 3% charcoal and 5% tragacanth suspension was administered orally. An hour after charcoal meal administration, the animal was killed and distance of charcoal movement was measured for each mouse.
Anti spasmodic studies on isolated ileum
Male Wistar rats (200-220 g) were killed and longitudinal strips of ileum were taken and placed in oxygenated Tyrode's solution. Each strip was suspended between parallel platinum electrodes in 50 ml organ bath under 1 g weight tension. The bathing fluid was Tyrode's solution at 37 °C and gassed with oxygen. The ileum tension was monitored with an isotonic transducer and displayed on a pen recorder device (Harvard). Electrical field stimulation was performed using rectangular pulses produced by a stimulator (made in the university workshop). Tissues were stimulated with maximum of 6 volts using 1 s trains of stimuli at 50 Hz. ACh (0.5 μM) and 5-HT (2 μM) were added into the bath with contact time of 20 s. Stimulation was performed once every 10 or 15 min as appropriate.
Measurement and statistical analysis
Number of wet defecation over the course of study was used for the assessment of diarrhoea index. Ileum transit was expressed as percentage of charcoal moved from pylorus to caecum relative to whole length of ileum.
Contraction to EFS or to added spasmogens were measured relative to the tissue baseline and expressed as percentage of initial response prior to addition of the testing agent.
The IC50 value (drug concentration causing 50% of maximum response) was determined by plotting a full concentration response curve for each tissue. Values are presented as mean ± standard error of mean (SEM). Statistical analysis was performed using Student's t-test and/or one way analysis of variance (ANOVA) as appropriate.
RESULTS
Plant materials
The hydroalcoholic extract had green-brownish color. The yield of extract was 10% (w/w). In this separation process 260 mg pure HMPHA (0.002% w/w of dried plant materials) was obtained.
Castor oil and magnesium sulphate-induced diarrhoea
In the vehicle treated control groups within 1.5 h after administration of castor oil all the animals had shown sign of diarrhoea. The incidence of diarrhoea peaked 2 h after the treatment and then gradually subsided down. Loperamide (2 mg/kg) substantially suppressed castor oil induced diarrhoea (Fig. 2), in fact only one mouse showed sign of diarrhoea.
Fig. 2.
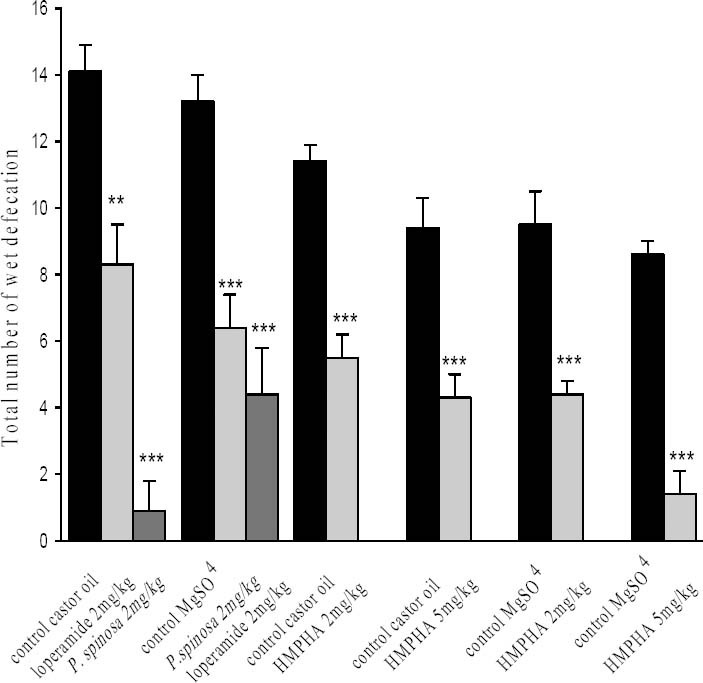
Antidiarrhoeal activity of P. spinosa hydroalcoholic extract, loperamide and 6-(4-hydroxy-3- methoxy-phenyl)-hexanonic acid on mice with castor oil (0.5 ml, orally) and MgSO4 (0.5 ml, 10% solution) induced diarrhoea. Incident of diarrhoea were assessed as number of wet defecation following castor oil administration. The control groups were treated with the corresponding vehicle (0.7%, 7% & 17.5% ethanol) respectively. Data are mean ± SEM (n=10) for each group. **P<0.01, ***P<0.001 in comparison with corresponding vehicle treated control group (Student's t-test).
Hydroalcoholic extract of P. spinosa (2 mg/kg) in comparison with the control group reduced the incidence of diarrhoea by 41% (Fig. 2). HMPHA (2 mg/kg & 5 mg/kg) significantly reduced castor oil induced diarrhoea in mice by 52% and 54% respectively in comparison with corresponding control groups (Fig. 2). An hour after oral administration of MgSO4, first sign of diarrhoea was observed in the animals and peak of diarrhoea was achieved within 1.5 h before it gradually subsided down. Loperamide (2 mg/kg) both delayed the induction of diarrhoea (Table 1) and attenuated diarrhoea by 67% (Fig. 2). In this group 4 mice had no incidence of diarrhoea at all. P. spinosa extract (2 mg/kg) reduced the incidence of diarrhoea by 52% (Fig. 2). HMPHA (2 mg/kg and 5 mg/kg) dose dependently inhibited MgSO4 induced diarrhoea in mice by 54% and 84% respectively in comparison with corresponding control groups (Fig. 2). In addition it caused a significant delay in induction of diarrhoea in mice (Table 1).
Table 1.
Induction time of diarrhoea following oral administration of laxatives in mice.
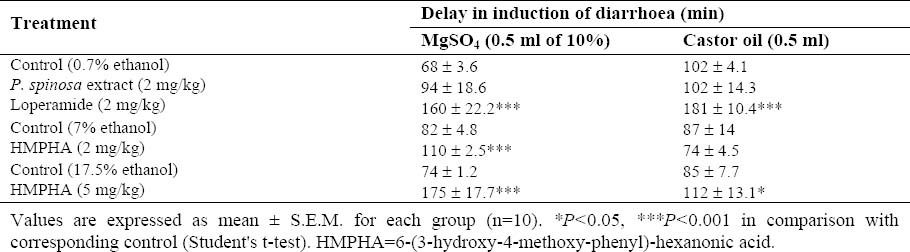
In the group treated with 5 mg/kg HMPHA, half of the animals had no sign of diarrhoea during the course of the study.
Charcoal meal transit test
In the control group, 1 h after charcoal feeding, the black meal moved up to 90% of the ileum length.
All the tested compounds significantly reduced the distance moved by the charcoal meal while loperamide (2 mg/kg) was the most effective antispasmodic drug (Fig. 3). HMPHA (2 mg/kg and 5 mg/kg) also reduced the movement of charcoal meal in the ileum for about 33% and 35% respectively in comparison with the corresponding control groups (Fig. 3).
Fig. 3.
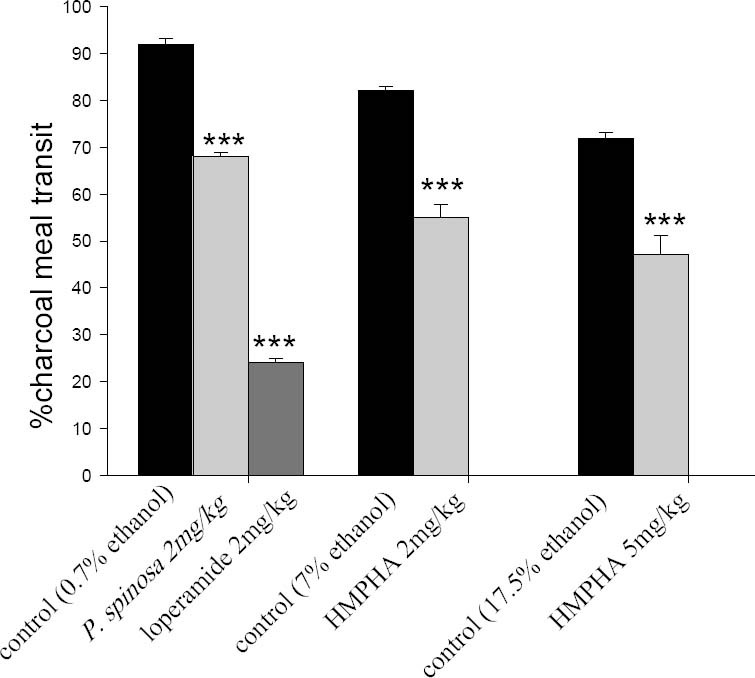
Effect of P. spinosa hydroalcoholic extract, loperamide and 6-(4-hydroxy-3-methoxy-phenyl)- hexanonic acid on small intestinal transit (0.5 ml of charcoal meal). Gastrointestinal transit was expressed as the percentage of distance that charcoal moved relative to whole length of small intestine over an hour. Data are mean ± SEM (n=10) for each group. ***P<0.001 in comparison with corresponding vehicle treated control group (Student's t-test).
P. spinosa extract (2 mg/kg) reduced distance of charcoal meal moved down the ileum by only 24% (Fig. 3).
Anti spasmodic studies on isolated ileum
Electrical field stimulation produced a biphasic contractile responses (EFS-1 and EFS-2) as described before (23,24,25) while ACh and 5-HT caused a rapid contraction. Loperamide inhibited the contractile response to ACh, 5-HT and EFS in concentration dependent manner (Figs 4–6), abolishing the response at its highest used concentration. 6-(4-hydroxy-3-methoxyphenyl)-hexanonic acid (5-160 μg/ml) concentration dependently inhibited the contractile responses to 5-HT and ACh with IC50 values of 87 ± 12 μg/ml (336 ± 50 μM, n=6) and 33 ± 6 μg/ml (140 ± 20 μM, n=6) respectively (see Figs 4 and 5). HMPHA reduced both responses to EFS with IC50 values 36 ± 3 μg/ml (152 ± 10 μM, n=6) and 41 ± 5 μg/ml (170 ± 20 μM, n=6) respectively (Fig. 6). After washing, contractile responses were gradually restored.
Fig. 4.
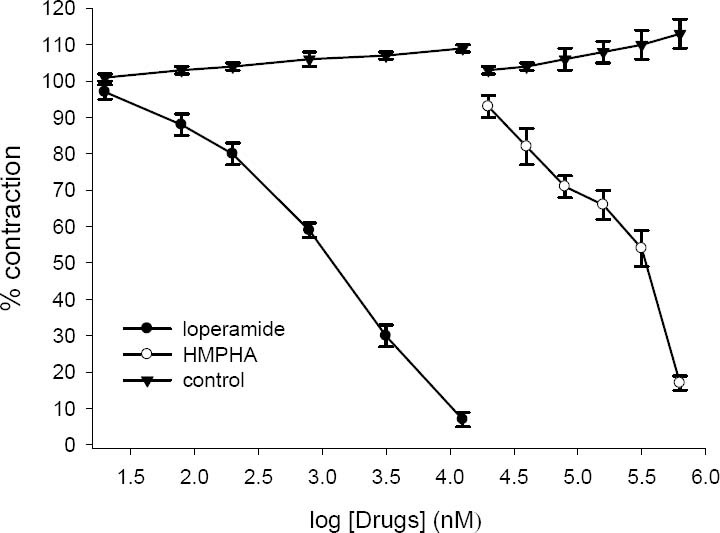
Effect of loperamide (20 nM-12.8 μM) and 6-(4- hydroxy-3-methoxyphenyl)-hexanonic acid (20-630 μM) on tension development to 5-HT (2 μM) in isolated ileum of rat. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of drugs. The points are mean and the vertical bars show the SEM (n=6). There is no statistical significant differences in vehicle treated (DMSO) time-matched control over the course of study (ANOVA).
Fig. 6.
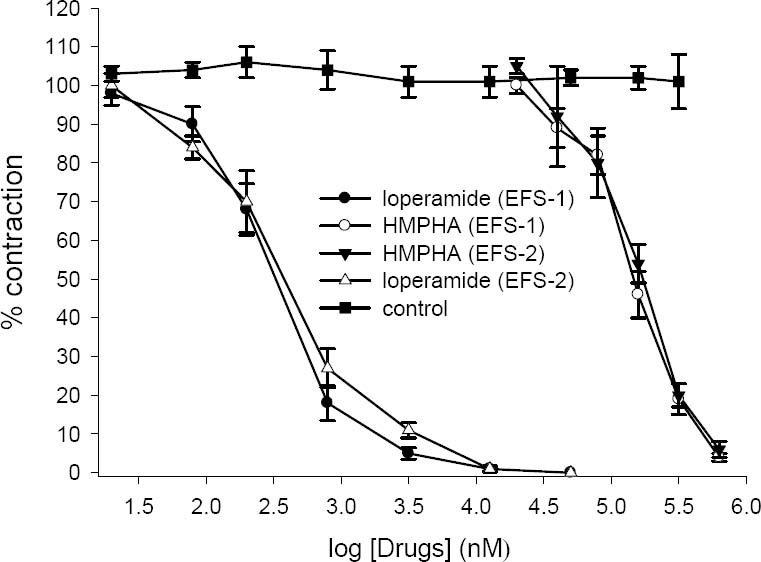
Effect of loperamide (20 nM-50 μM) and 6-(4-hydroxy-3-methoxyphenyl)-hexanonic acid (20-630 μM) on tension development to first (EFS-1) and second (EFS-2) contractile responses to electrical field stimulation (6 V, 50 Hz, 1 s duration) in isolated ileum of rat. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of drugs. The points are mean and the vertical bars show the SEM (n=6). There is no statistical significant differences in vehicle treated (DMSO) time-matched control over the course of study (ANOVA).
Fig. 5.
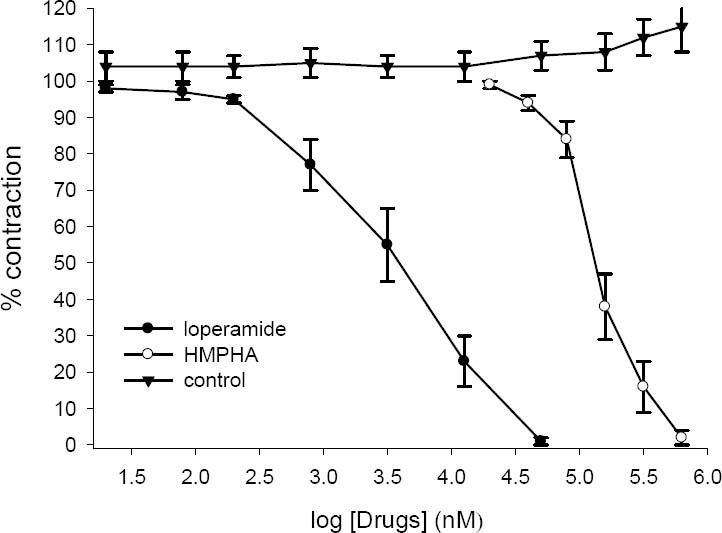
Effect of loperamide (20 nM-50 μM) and 6-(4- hydroxy-3-methoxyphenyl)-hexanonic acid (20-630 μM) on tension development to acetylcholine (0.5 μM) in isolated ileum of rat. Ordinate scale: ileum contractile response expressed as percent of initial control response. Abscissa scale: log10 concentration of drugs. The points are mean and the vertical bars show the SEM (n=6). There is no statistical significant differences in vehicle treated (DMSO) time-matched control over the course of study (ANOVA).
Loperamide in concentration dependent manner inhibited the response to 5-HT (IC50=1.2 ± 0.1 μM, n=6), ACh (IC50=4.3 ± 0.9 μM, n=6) and EFS (IC50=335 ± 42 nM, n=6). In the vehicle treated time matched control tissues, DMSO (0.05-1.6%) caused a slight increase in contractile responses to ACh, 5-HT and EFS but these changes was not statistically significant (ANOVA).
DISCUSSION
Irritable bowel syndrome is a disorder that leads to abdominal pain, cramping, and changes in bowel movements. People with IBS may switch between constipation and diarrhoea, or mostly have one or the other. People with diarrhoea will have frequent, loose, watery stools (1,2,3). Patients often have an urgent need to have a bowel movement, which may be hard to control.
The use of antispasmodic drugs may help patients especially those with cramps or diarrhea (4,5,6). Antimuscarinic such as propanthline or dicyclomine, only reduce the responses to parasympathic nerve fibers but also have other side effects (4,5,6). Antimuscarinic drugs reduce intestinal motility and they are used for the management of IBS (4,5,6). However, their value has not been established and responses are variable. Drugs which act directly at the smooth muscle of the gastrointestinal tract could relieve spasm without affecting normal gut function. Since this action is not mediated by the autonomic nervous system, the usual anticholinergic side effects are absent.
Antidiarrheal agents such as loperamide are used in diarrhoea-predominant IBS (4,5,6). Opioids act by several mechanisms, mediated principally through either μ- or δ-opioid receptors on enteric nerves, epithelial cells and smooth muscles (26,27,28). We have shown that loperamide not only inhibits diarrhoea but also reduce the intestinal movement due to its anti-spasmodic activity on intestinal smooth muscle. HMPHA also had anti-diarrhoeal activity comparable to that of loperamide, reduced diarrhoea induced by both castor oil and MgSO4 solution.
Furthermore, it reduced movement of charcoal meal in the ileum which also reflects its antispasmodic activity. In addition, HMPHA removed the contractile response to EFS, ACh and 5-HT. Drugs affecting 5-HT in the intestines can help reduce symptoms of IBS (29). 5-HT antagonists are also effective in post-infectious IBS and diarrhoea dominant IBS. The reason for their benefit might be because of excessive serotonin in the gut that is believed to play a role in the pathogenesis of some subtypes of IBS (29). 5-HT is spasmogenic agent on intestinal smooth muscle and can stimulate the gut motility and therefore drugs like HMPHA can help diarrhea-predominant IBS.
Benefits may include reduced diarrhoea and abdominal cramps. It could reduce the frequency of bowel movements and relieve rectal urgency in patient with mild disease. As with other antispasmodic agents care should be taken if obstruction is suspected.
Hydroalcoholic extract of P. spinosa also slowed down gastrointestinal motility by affecting smooth muscles in the intestine. Some part of its antidiarrhoeal effect may be due to a reduction in gastrointestinal secretion, because it reduced the diarrhoea induced by MgSO4. MgSO4 is osmotic laxative and by creating osmotic pressure prevent water absorption form gut and in such way it cause diarrhoea (28).
Castor oil contains two known ingredient ricin and a triglyceride of ricinoleic acid. The triglyceride hydrolysed in the small intestine by the action of lipases into glycerol and the active agent, ricinoleic acid, which acts primarily in the small intestine to stimulate secretion of fluid and electrolytes and speed intestinal transit (8) In this research we have demonstrated that P. spinosa extract and its active component HMPHA, reduced diarrhoea induced by castor oil or MgSO4. Reduction in diarrhoea most likely is due to anti spasmodic activity of the agent since gut motility was reduced both in vivo and in vitro. This new compound may have advantage over antimuscarinic agent to the treatment of IBS, since this agent in addition to ACh, inhibited contraction due to 5-HT as well as neural stimulation of the intestine.
Primary studies on P. spinosa extract indicate that this extract possessed selective action on ileum in comparison with other smooth muscle including uterus and bladder (30,31). At anti-diarrheal dose, P. spinosa extract had no significant effect on rat blood pressure (32), central nervous system (33) renal or hepatic functions (34). As the dose of HMPHA and P. spinosa extract are relatively similar, other active materials also must be present in the P. spinosa extract. Therefore, P. spinosa extract and its component are recommended for clinical trial for IBS treatment.
CONCLUSION
6-(4-hydroxy-3-methoxyphenyl)-hexanonic acid is an active agent from P. spinosa. Pharmacological properties of HMPHA are similar to those of loperamide. Therefore, it could be a suitable lead compound for further drug development. However, toxicological tests are required before it could be used for clinical studies in the man.
ACKNOWLEDGMENTS
We are grateful to the Isfahan Pharmaceutical Sciences Research Center, Isfahan University of Medical Sciences, Isfahan, I.R. for financial support.
REFERENCES
- 1.Malagelada JR. A symptom-based approach to making a positive diagnosis of irritable bowel syndrome with constipation. Intern J Clin Pract. 2006;60:57–63. doi: 10.1111/j.1368-5031.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- 2.Cash BD, Schoenfeld P, Chey WD. The utility of diagnostic tests in irritable bowel syndrome patients: A systematic review. Am J Gastroentrol. 2002;97:2812–2819. doi: 10.1111/j.1572-0241.2002.07027.x. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med. 2008;358:1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesbros-Pantoflickova D, Michetti P, Fried M, Beglinger C, Blum A. Meta-analysis: The treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:1253–1269. doi: 10.1111/j.1365-2036.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 5.Jailwala J, Imperiale T, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–147. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 6.Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;8:CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasricha PJ. Treatment of disorders of bowel motility and water flux; antiemetics; agents used in biliary and pancreatic disease. In: Hardman J.G, Limbird L.E, editors. Goodman & Gilmans The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. pp. 983–1008. [Google Scholar]
- 9.Rang HP, Dale MM, Ritter JM, Flower RJ. 6th ed. London: Churchill Livingstone Elseveire; 2007. The gastrointestinal tract (chapter 25). In Rang & Dale's Pharmacology; pp. 385–396. [Google Scholar]
- 10.Mozaffarian V. Tehran: Farhng Moaser; 1996. A dictionary of Iranian plant names; pp. 443–444. [Google Scholar]
- 11.Jalili A, Jamzad Z. Tehran: Research Institute of Forests and Rangelands; 1999. Red data book of Iran, A preliminary survey of endemic, rare and endangered plant species in Iran; pp. 689–690. [Google Scholar]
- 12.Sadraei H, Asghari G, Naddafi A. Relaxant effect of essential oil and hydro-alcoholic extract of Pycnocycla spinosa Decne. exBoiss. on ileum contractions. Phytother Res. 2003;17:645–649. doi: 10.1002/ptr.1217. [DOI] [PubMed] [Google Scholar]
- 13.Sadraei H, Asghari G, Hekmatti AA. Antispasmodic effect of three fraction of hydroalcoholic extract of Pycnocycla spinosa. J Ethnopharmacol. 2003;86:187–190. doi: 10.1016/s0378-8741(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 14.Sadraei H, Asghari G, Khazael M. Relaxant effect of four fractions separated from alkaloid extract of Pycnocycla spinosa on rat isolated ileum. Res Pharm Sci. 2008;3:79–86. [Google Scholar]
- 15.Sadraei H, Asghari G, Shams M. Antidiarrhoeal action of hydroalcoholic extract of Pycnocycla spinosa in comparison with loperamide and dicyclomine. Iranian J Pharm Res. 2011;10:835–841. [PMC free article] [PubMed] [Google Scholar]
- 16.Sadraei H, Asghari G, Behzad S. Bioactivity-guided isolation of spasmolytic components of Pycnocycla spinosa Decne ex Boiss. Res Pharm Sci. 2011;6:81–86. [PMC free article] [PubMed] [Google Scholar]
- 17.Sadraei H, Ghanadian M, Asghari G, Madadi E. Antispasmodic activity of isovanillin and isoacetovanillon in comparison with Pycnocycla spinosa extract on rat ileum. Res Pharm Sci. 2014;9(3):178–192. [PMC free article] [PubMed] [Google Scholar]
- 18.Sadraei H, Ghanadian M, Asghari G, Azali N. Antidiarrhoeal activity of isovanillin and iso-acetovanillon, two bioactive compounds from Pycnocycla spinosa extract. Res Pharm Sci. 2014;9:83–89. [PMC free article] [PubMed] [Google Scholar]
- 19.Azali N. Pharm. D thesis, Isfahan University of Medical Sciences; 2013. Study of anti diarrheal action of 6-(4-hydroxy-3-methoxyphenyl)-hexanoic acid and, 3-hydroxy-4-methoxybenzaldehyde, 1-(3-hydroxy-4- methoxyphenyl)-ethanone as bioactive compounds from Pycnocycla spinosa extract. [Google Scholar]
- 20.Ghanadian M, Sadraei H, Yousuf S, Asghari G, Jahed M. New diterpene polyester and phenolic compounds from Pycnocycla spinosa Decne. Ex Boiss with relaxant effects on KCl-induced contraction in rat ileum. Phytochem Lett. 2014;7:57–61. [Google Scholar]
- 21.Samuelsson G. 4th ed. Stockholm: Swedish Pharmaceutical Press; 1999. Drug of natural origin; pp. 48–49. [Google Scholar]
- 22.Washington DC: The National Academies Press; 2010. Committee for the update of the guide for the care and use of laboratory animals, National Research Council. Guide for the Care and use of Laboratory animals; pp. 11–37. [Google Scholar]
- 23.Sadraei H, Asghari G, Emami S. Inhibitory and excitatory action of Rosa damascena Mill flower extract and essential oil on rat ileum contraction. Res Pharm Sci. 2013;8:17–23. [PMC free article] [PubMed] [Google Scholar]
- 24.Sadraei H, Shokoohinia Y, Sajjadi SE, Mozafari M. Antispasmodic effect of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Res Pharm Sci. 2010;5:29–39. [PMC free article] [PubMed] [Google Scholar]
- 25.Ekblad E, Sundler F. Motor responses in rat ileum evoked by nitric oxide donors vs. field stimulation: Modulation by pituitary adenylate cyclase-activating peptide forskolin and guanylate cyclase inhibitors. J Pharmacol Exp Ther. 1997;283:23–28. [PubMed] [Google Scholar]
- 26.Holzer P. Opioids and opioids receptors in the enteric nervous system. From a problem in opioid analgesia to a possible new prokinetic therapy in the human. Neurosci lett. 2004;361:192–195. doi: 10.1016/j.neulet.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Daugherty LM. Loperamide hydrochloride. Am Pharm. 1990;NS30(12):45–48. doi: 10.1016/s0160-3450(15)31396-9. [DOI] [PubMed] [Google Scholar]
- 28.Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988;40:121–162. [PubMed] [Google Scholar]
- 29.Talley N. Serotoninergic neuroenteric modulators. Lancet. 2001;358:2061–2068. doi: 10.1016/S0140-6736(01)07103-3. [DOI] [PubMed] [Google Scholar]
- 30.Sadraei H, Asghari G, Andisha M. Antispasmodic effect of Pycnocycla spinosa seed and aerial part extract on rat ileum and uterus smooth muscle contractions. Daru. 2008;13:160–163. [Google Scholar]
- 31.Sadraei H, Asghari G, Arabzadah A. Effect of hydroalcoholic extract of Pycnocycla spinosa on rat isolated bladder contraction. Iranian J Pharm Res. 2004;4:237–241. [Google Scholar]
- 32.Sadraei H, Asghari G, Hajhashemi V, Nezami M. Evaluation of cardiovascular effect of Pycnocycla spinosa Decne. exBoiss., var. spinosa extracts in anaesthetized rat. Daru. 2008;14:11–14. [Google Scholar]
- 33.Sadraei H, Asghari G, Zeinodin M. Evaluation of systemic effect of Pycnocycla spinosa extract in mice using Irwin test. Res Pharm Sci. 2006;1:22–29. [Google Scholar]
- 34.Palizban AA, Sadraei H, Asghari G, Zinalzadeh SJ. Assessment of the effect of Pycnocycla spinosa hydroalcoholic extract on rat renal function. J Bio Sci. 2008;8:205–208. [Google Scholar]


