Abstract
Background:
Psoriasis is a common inflammatory skin disease characterized by abnormal keratinocyte proliferation and differentiation. Beta-catenin participates in intercellular adhesion. Catenins are proteins found in complexes with cadherin cell adhesion molecules of cells. The role of catenin in regulating keratinocyte stem cell differentiation and hair follicle morphogenesis has been extensively reported.
Aims and Objectives:
is to study β-catenin expression in lesional and non-lesional psoriatic skin to throw light upon its possible role in the pathogenesis of psoriasis.
Materials and Methods:
Biopsies were taken from 20 patients with psoriasis vulgaris and from 10 normal controls. The distribution of Beta catenin was investigated using polycolonal rabbits B-catenin antibody-1 by immunohistochemical method.
Results:
In this study membranous β-catenin expression was significantly demonstrated in the control group then the non-lesional areas in comparison to the lesional areas (P < 0.001). Nuclear β-catenin staining expression was significantly more demonstrated in lesional and non-lesional areas in comparison to the control cases (P < 0.001).
Conclusions:
The down regulation of membranous β-catenin expression in lesional psoriatic skin might reflect a useful phenotypic marker of hyperprolifration of keratinocytes in psoriasis. Moreover, the mild down regulation of membranous β-catenin expression in non lesional psoriatic skin may provide clues about incipient structural abnormalities in the pathogenesis of psoriasis, providing an early diagnostic indicator for evolution to a generalized form of the disease. Nuclear β-catenin expression was not found in the control group but was demonstrated in lesional and moderately in non-lesional reflecting its role in kerationcyte proliferation.
Keywords: Beta-catenin, psoriasis, pathogenesis-immunohistochemical
INTRODUCTION
Psoriasis is a common inflammatory skin disease characterized by abnormal keratinocyte proliferation and differentiation, angiogenesis, immune activation, and inflammation.[1,2] Although there is evidence for immune activation with lymphocyte infiltration in the psoriatic epidermis, there also exists evidence of primary keratinocyte defects.[3] In the psoriatic epidermis, proteins that are expressed in the early stage of keratinocyte differentiation such as keratinocyte transglutaminase are expressed earlier and in a greater amount.[4] In contrast, the expression of proteins that are usually expressed in the later stages of kertinocyte differentiation such as filaggrin are downregulated in the epidermis of psoriatic plaques.[5] There is accumulating evidence that proteins that mediate intracellular adhesions are involved in keratinocyte differentiation.[6] One candidate for this is beta-catenin (β-catenin) a 94-kDa protein that participates in intercellular adhesion, β-catenin links E-cadherin to the actin cytoskeleton through alpha-catenin.[7] Catenins are proteins found in complexes with cadherin cell adhesion molecules of cells. The first two catenins that were identified became known as α-catenin and β-catenin. Alpha-catenin can bind to β-catenin and can also bind actin. β-catenin binds the cytoplasmic domain of some cadherins.[8] Overexpression of the basal layer cadherins in suprabasal epidermis leads to increased cytoplasmic activity of β-catenin and increased β-catenin transcriptional activation. This overexpression of the basal layer cadherins results in abnormal keratinocyte differentiation. The role of β-catenin in regulating keratinocyte stem cell differentiation and hair follicle morphogenesis has been extensively reported. Doglioni et al.[9] studied β-catenin in human skin tumors and noted that β-catenin was occasionally present in both nuclear and cytoplasmic locations in the basal cells, scattered differentiated keratinocytes and luminal cells of the sebaceous glands. To gain an insight into the role of β-catenin on the regulation of keratinocytes and further investigate the pathogenesis of psoriasis, we compared the expression of β-catenin in normal human and psoriatic skin.
MATERIALS AND METHODS
This study was carried out in 20 psoriasis vulgaris patients with 10 non-psoriatic subjects as controls. These patients were selected randomly from the dermatology outpatient clinic, Faculty of Medicine, Menoufia University and Sheibin El-Kom Educational Hospital during the period from April 2009 to June 2010. All patients instructed to stop all forms of medication one month prior to optaining a skin biopsy. Patients were subjected to the following: History taking and clinical examination. Two skin biopsies were taken - one from the representative psoriatic lesion and the other from nonlesional skin under local xylocaine anesthesia. The diagnosis of psoriasis was based on both clinical and histopathological examination by hematoxylin and eosin stain. Informed consent was obtained from all patients. This study was approved by the Ethics Committee of Menoufia University.
Immunohistopathological
The improved biotin-streptavidin amplified detection system was used in the work. The primary antibody was rabbit polyclonal antibody (0.1 ml) (Lab-vision, Westinghouse, USA).
Interpretation of beta-catenin expression
Beta-catenin expression was assessed according to:
Intensity: Weak (+) moderate (++) or strong expression (+++)
Cell counting: The number of β-catenin positive nuclei and total nuclei in the upper half of the suprabasal region were counted in four individual fields from each biopsy specimen. Then the percent positivity was calculated. For further statistical analysis, cases were divided into two groups: Suprabasal nuclear β-catenin staining <50%, and >50%
Location: Membranous or nuclear location of expression.
Statistical analysis
Data was collected and statistically analyzed using Statistical Package (IBM Corporation) for Social Sciences version 9 program and StatView software (IBM Corporation). The following statistical measures were employed:
Arithmetic mean (X) for central tendency
-
Percentage (%). B-Analytic statistics:
- Student's t-test to compare two quantitative variables
- Chi-square test (χ2) to compare between qualitative variables P < 0.05 was considered as statistically significant (S).
RESULTS
The age of the patients ranged from 12 to 75 years with a mean age 49.7 ± 15.2 years. Among 20 patients suffering from psoriasis, 8 were males and 12 were females. The duration of the disease varied from 6 months to 19 years with a mean of 5.67 ± 0.5 years. In all clinically diagnosed patients, histopathology revealed parakeratosis, absent granular cell layer, Munro microabscesses, acanthosis, spongiosis, and papillomatosis in the epidermis. Dermal capillary dilatation and inflammatory cells were present in all cases and edema in some cases [Figure 1a and b].
Figure 1.
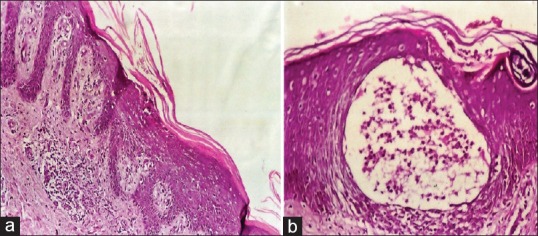
(a) Psoriasis lesion with Munro microabscess in psoriasis (H and E, ×200). (b) Psoriasis lesion with epidermal, parakeratosis, acanthosis, spongiosis, absent granular cell layer and dermal capillary dilatation and inflammatory cells (H and E, ×400)
Immunohistochemical results of beta-catenin staining expression
In the present study, we made a comparative study of the structural organization of the epidermis and in particular the intercellular junctions between lesional and nonlesional skin of psoriasis patients. The presence and distribution of β-catenin was analyzed by immunohistochemistry of normal skin, and lesional and nonlesional psoriatic skin. In the normal epidermis β-catenin was detected solely on the cell membrane of the basal cells and lower spinous cells [Table 1, Figure 2a]. This protein showed a continuous well defined distribution at the borders of these cells. In nonlesional psoriatic skin, there were a few suprabasal cells showing strong nuclear β-catenin expression [Table 2, Figure 2b]. In lesional psoriatic skin, there was a positive nuclear suprabasal β-catenin expression and negative β-catenin expression in parakeratotic cells [Table 2, Figure 3a]. There was a strong nuclear with membranous β-catenin expression in psoriatic skin [Figure 3b]. At the junction between nonlesional and lesional skin there was membranous β-catenin expression in nonlesional skin and both membranous and nuclear β-catenin expression in lesional psoriatic skin [Figure 4a]. In nonlesional psoriatic skin, there was a membranous β-catenin expression along with a positive dermal inflammatory component [Figure 4b]. There was positive nuclear and cytoplasmic β-catenin expression in normal hair follicles [Figure 4c].
Table 1.
Comparison between membranous beta-catenin staining expression in control, and lesional and nonlesional skin of psoriasis
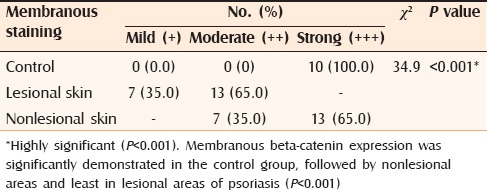
Figure 2.
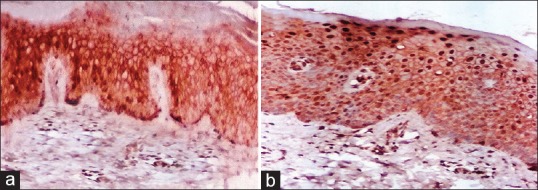
(a) Skin of a normal control showing membranous beta-catenin (β-catenin) staining expression and few suprabasal nuclear stains (IPS, ×200). (b) Few strong nuclear suprabasal β-catenin staining expression in nonlesional skin of psoriasis (Immunoperioxedase stain, ×200)
Table 2.
Comparison between suprabasal nuclear beta-catenin staining expression in controls, and lesional and nonlesional skin of psoriasis
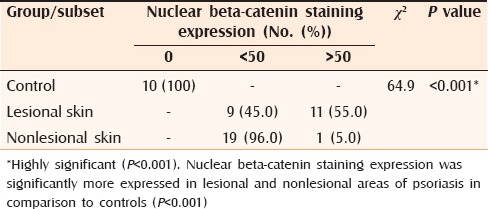
Figure 3.
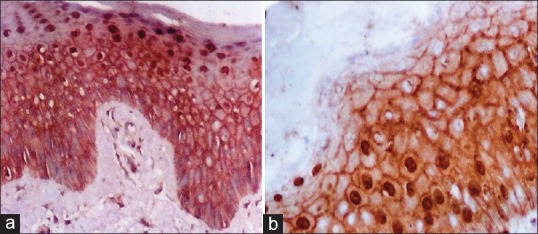
(a) Nuclear suprabasal beta-catenin (β-catenin) staining expression in all layers of epidermis except the parakeratotic and cornified layers of a psoriasis lesion (Immunoperioxedase stain, ×400). (b) Strong nuclear with membranous β-catenin expression and negative expression in the parakeratotic layer of a psoriasis lesion (Immunoperioxedase stain, ×400)
Figure 4.
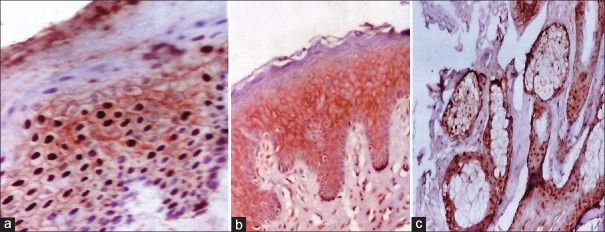
(a) Junction between nonlesional (right) and lesional (left) skin of psoriasis showing only membranous staining (nonlesional) while both membranous and nuclear beta-catenin (β-catenin) expression is seen in lesional skin (IPS, ×400). (b) Membranous β-catenin expression together with positive dermal inflammatory components in nonlesional skin of psoriasis (Immunoperioxedase stain, ×200). (c) Nuclear and cytoplasmic β-catenin expression in normal hair follicle matrix cell (Immunoperioxedase stain, ×400)
DISCUSSION
Catenins are cytoplasmic proteins alpha, beta, gamma, and p120 proteins. They play an important role in the maintenance of normal tissue architecture.[10] Catenins are cell-cell adhesion molecules that also maintain intracellular adhesiveness through cytoplasmic catenin proteins that bind the microfilaments to the cytoskeleton.[11] β-catenin is not required for keratincoyte proliferation, but has been shown to regulate keratinocyte stem cells and hair follicle morophogenesis.[12] With regard to membranous β-catenin staining expression, it was significantly demonstrated in the control group and nonlesional areas in psoriatics as compared to lesional areas in psoriatics. This reflects its role of β-catenin as an adhesion molecule in uninvolved and normal skin. Nuclear β-catenin staining expression was significantly demonstrated in lesional and nonlesional skin of psoriatics as compared to controls. This finding agreed with previous work done by Stojadinovic et al.,[13] who studied the role of β-catenin in the inhibition of epithelization and wound healing. They did not find any nuclear β-catenin in normal epidermis. Furthermore in the study done by Butz and Larue,[14] it was found that β-catenin was detected only on the cell membrane of the normal epidermis and normal hair follicles with no nuclear β-catenin staining detected. Our findings are in agreement with those of Hampton et al.,[15] who found increased nuclear β-catenin in suprabasal lesional psoriatic epidermis. Some nuclear β-catenin was also detected in nonlesional psoriatic skin, but much less than in lesional psoriatic skin. The distribution of β-catenin in the membrane of normal cells more than in the nuclear location may be due to its role in the establishment and regulation of adherens junctions by interaction with E-cadherin through alpha catenin with the actin cytoskeleton.[15] Furthermore, some studies suggest that β-catenin may be translocated from the nucleus back to the cell membrane, presumably through a soluble, cytosolic intermediate state.[16] Watt and Collins,[17] found the same result in their study of the role of nuclear β-catenin in colorectal tumors. Watabe and Miyazono,[17] studied nuclear localization of β-catenin in the hair matrix cells and differentiated keratinocytes. They found less nuclear β-catenin than membranous β-catenin in normal skin cells. Zhou et al.,[18] showed a significant increase in nuclear β-catenin within the suprabasal layers in the involved psoriatic epidermis. Also, Zhou et al.,[18] observed a marked decrease in membranous β-catenin at the same level as increased in the nuclear β-catenin. They suggested that in involved suprabasal psoriatic epidermis, an excess amount of free unphosphrylated β-catenin enters the cytoplasm and subsequently translocates to the nucleus. Zhou et al.,[18] showed in their study that there was no significant increase in the expression of β-catenin in both membranous and nuclear locations in nonlesional suprabasal cells of psoriatics. Normal skin and the uninvolved psoriatic epidermis might transcriptionally active β-catenin released into the cytoplasm that would likely be rapidly targeted for ubiquitination to prevent abnormal signaling events. This leads to little nuclear β-catenin in uninvolved or normal suprabasal epidermis.[18] In a study done by Li et al.,[19] who studied the expression of E-cadherin and β-catenin in the lesional skin of patients with active psoriasis, there was downregulation of β-catenin expression, which was found in the granular layer and basal layer of the psoriatic lesions only.
CONCLUSION
In normal control group β-catenin was mainly demonstrated as membranous pattern of expression; this reflects its role as an adhesion molecule. The downregulation of membranous β-catenin expression in lesional psoriatic skin suggests that it could be a useful phenotypic marker for the hyperprolifration of keratinocytes in psoriasis. Moreover, the mild downregulation of membranous β-catenin expression in nonlesional psoriatic skin may provide clues about impending structural abnormalities in the pathogenesis of psoriasis, providing an early diagnostic indication for evolution to a generalized form of the disease. Nuclear β-catenin expression was not found in the control group but demonstrated in lesional and moderately in nonlesional skin of psoriatics reflecting its role in kerationcyte proliferation. The nuclear and cytoplasmic expression of β-catenin in normal hair follicle provides evidence suggesting that it plays an important role in keratinocyte differentiation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Griffiths CE, Voorhees JJ. Psoriasis, T cells and autoimmunity. J R Soc Med. 1996;89:315–9. doi: 10.1177/014107689608900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louden BA, Pearce DJ, Lang W, Feldman SR. A Simplified Psoriasis Area Severity Index (SPASI) for rating psoriasis severity in clinic patients. Dermatol Online J. 2004;10:7. [PubMed] [Google Scholar]
- 3.Ghorpade A. Linear naevoid psoriasis along lines of Blaschko. J Eur Acad Dermatol Venereol. 2004;18:726–7. doi: 10.1111/j.1468-3083.2004.01066.x. [DOI] [PubMed] [Google Scholar]
- 4.Eckert RL, Sturniolo MT, Broome AM, Ruse M, Rorke EA. Transglutaminase function in epidermis. J Invest Dermatol. 2005;124:481–92. doi: 10.1111/j.0022-202X.2005.23627.x. [DOI] [PubMed] [Google Scholar]
- 5.Iizuka H, Takahashi H, Honma M, Ishida-Yamamoto A. Unique keratinization process in psoriasis: Late differentiation markers are abolished because of the premature cell death. J Dermatol. 2004;31:271–6. doi: 10.1111/j.1346-8138.2004.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 6.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watt FM, Collins CA. Role of beta-catenin in epidermal stem cell expansion, lineage selection, and cancer. Cold Spring Harb Symp Quant Biol. 2008;73:503–12. doi: 10.1101/sqb.2008.73.011. [DOI] [PubMed] [Google Scholar]
- 8.Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–7. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doglioni C, Piccinin S, Demontis S, Cangi MG, Pecciarini L, Chiarelli C, et al. Alterations of beta-catenin pathway in non-melanoma skin tumors: Loss of alpha-ABC nuclear reactivity correlates with the presence of beta-catenin gene mutation. Am J Pathol. 2003;163:2277–87. doi: 10.1016/s0002-9440(10)63585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardman MJ, Liu K, Avilion AA, Merritt A, Brennan K, Garrod DR, et al. Desmosomal cadherin misexpression alters beta-catenin stability and epidermal differentiation. Mol Cell Biol. 2005;25:969–78. doi: 10.1128/MCB.25.3.969-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci. 2010;59:73–80. doi: 10.1016/j.jdermsci.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Korswagen HC, Herman MA, Clevers HC. Distinct β-catenins mediate adhesion and signaling. Journal of Cell Biology. 2006;122:456–89. [Google Scholar]
- 13.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, et al. Molecular pathogenesis of chronic wounds: The role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butz S, Larue L. Expression of catenins during mouse embryonic development and in adult tissues. Cell Adhes Commun. 1995;3:337–52. doi: 10.3109/15419069509081018. [DOI] [PubMed] [Google Scholar]
- 15.Hampton PJ, Ross OK, Reynolds NJ. Increased nuclear beta-catenin in suprabasal involved psoriatic epidermis. Br J Dermatol. 2007;157:1168–77. doi: 10.1111/j.1365-2133.2007.08195.x. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong DD, Wong VL, Esser KA. Expression of beta-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol Cell Physiol. 2006;291:C185–8. doi: 10.1152/ajpcell.00644.2005. [DOI] [PubMed] [Google Scholar]
- 17.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–15. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S, Matsuyoshi N, Liang SB, Takeuchi T, Ohtsuki Y, Miyachi Y. Expression of T-cadherin in basal keratinocytes of skin. J Invest Dermatol. 2002;118:1080–4. doi: 10.1046/j.1523-1747.2002.01795.x. [DOI] [PubMed] [Google Scholar]
- 19.Li ZX, Ji FP, Peng ZH, Wang K. Expression of E-cadherin, beta-catenin and cyclin D1 in epidermal skin lesions of patients with active psoriasis vulgaris. Journal of Cell Biology. 2008;28:545–7. [PubMed] [Google Scholar]


