Abstract
Background:
Finasteride acts by reducing dihydrotestosterone levels, thereby inhibiting miniaturization of hair follicles in patients with androgenetic alopecia (AGA). Oral finasteride is associated with side effects such as decreased libido, sexual dysfunction, and gynecomastia.
Aim:
The aim of the following study is to assess the efficacy of maintaining hair growth with 5% topical minoxidil fortified with 0.1% finasteride in patients with AGA after initial treatment with 5% topical minoxidil and oral finasteride for two years.
Materials and Methods:
A retrospective assessment was done in 50 male patients aged 20-40 years with AGA. All the patients had been initially treated with topical minoxidil and oral finasteride for a period of two years, after which the oral finasteride was replaced with topical minoxidil fortified with finasteride. Five of 50 patients had discontinued the treatment for a period of 8-12 months and were then resumed with only topical minoxidil fortified with finasteride. The patients’ case sheets and photographs were reviewed by independent observers and the efficacy of minoxidil-finasteride combination was assessed.
Results:
Of the 45 patients who underwent a continuous treatment for AGA, 84.44% maintained a good hair density with topical minoxidil-finasteride combinatio. Of the five patients who discontinued oral finasteride for 8-12 months, four demonstrated good improvement in hair density when treatment was resumed with topical minoxidil-finasteride combination.
Conclusion:
Topical finasteride can be considered for hair density maintenance after initial improvement with oral finasteride, thereby obviating the indefinite use of oral finasteride.
Keywords: Androgenetic alopecia, maintenance of hair density, oral finasteride, topical finasteride, topical minoxidil
INTRODUCTION
Androgenetic alopecia (AGA) is the most common form of hair loss experienced in genetically predisposed individuals. It occurs due to the stimulation of genetically susceptible hair follicles by dihydrotestosterone (DHT). DHT causes follicular miniaturization, resulting in decreased hair density.
Finasteride reduces DHT level by inhibiting type II 5α reductase,[1] the enzyme responsible for conversion of testosterone to DHT, thereby inhibiting miniaturization of hair follicles. It also promotes the anagen phase of hair growth. Oral intake of finasteride reduces DHT levels in the blood and causes side effects such as decreased libido, erectile dysfunction, etc., These side effects are temporary and normalize with continued use.[1,2,3] Once oral finasteride is stopped, the DHT level rises and reverses its effects, thereby resulting in less dense hair. This accounts for an indefinite period of oral finasteride treatment.[4]
Studies show that topical finasteride appears to have results equivalent to oral finasteride in AGA,[5,6] and has better tolerance. Therefore, the we conducted a study to assess the efficacy of topical minoxidil fortified with finasteride in the maintenance of hair density post- treatment with oral finasteride.
MATERIALS AND METHODS
A retrospective assessment was done in 50 male patients with AGA. The photographs and case sheets of AGA patients with Hamilton grading 5a and 6; and Ludwigs pattern II and III falling in the age group of 20-40 years were selected for this assessment.
The patients had undergone treatment with oral finasteride and twice daily application of topical minoxidil 5% for a period of two years. After a considerable improvement in hair density was noticed, the patients were advised to discontinue oral finasteride and were shifted to topical minoxidil 5% fortified with 0.1% finasteride. Forty-five of 50 patients had resumed the treatment with topical finasteride and minoxidil without interruption. Five of 50 patients had discontinued the treatment for a period of 8-12 months resulting in a decrease in hair density to the initial level. These patients were then started only with topical minoxidil fortified with finasteride; oral finasteride was not represcribed. The patients were followed up once every four months for a period of 12 months. At each follow up, the patients’ photographs were taken and side effects if any noted.
Photographs taken at baseline, i.e. when oral treatment was stopped, and the 4th month, 8th month, and 12th month of topical medication were assessed [Table 1]. The assessment was done by the investigator and an independent observer to avoid bias.
Table 1.
The assessment of hair density maintenance after replacing oral finasteride with topical minoxidil and finasteride combination

RESULTS
Of the 45 patients (on continuous treatment), 25 patients’ hair density was moderately maintained, as given in Table 1. These patients when shifted from oral to topical finasteride initially experienced some hair loss and then reached a plateau phase. They had no further hair loss and the hair density was well maintained [Figure 1a-d]. Seven patients had a slight decline in hair density when shifted from oral finasteride to topical treatment alone as seen in [Figure 2a-c] and 13 patients did not experience a decline and even showed an improvement in the hair density.
Figure 1.
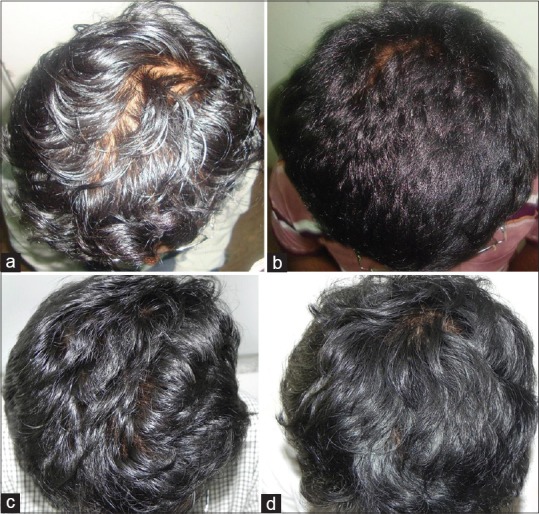
(a) Initial stage before starting treatment, (b) Improvement after oral finasteride treatment at eight months, (c) Initial decline in hair density when oral finasteride was stopped, (d) Plateauing of hair loss with well maintained hair density
Figure 2.
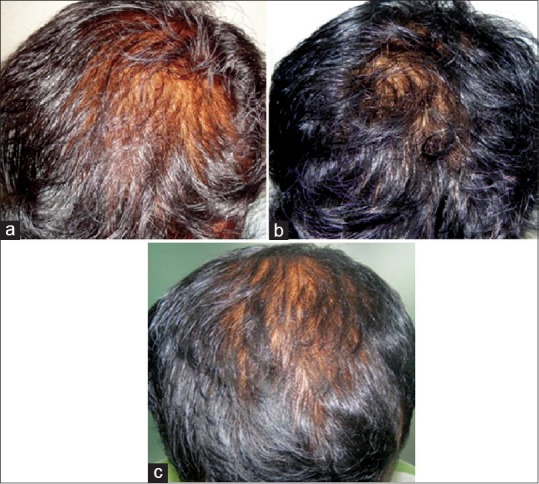
(a) Initial phase before starting hair fall treatment, (b) Improvement noticed after oral finasteride treatment, (c) Decline in hair density after switching from oral to topical finasteride
Five of 50 patients had stopped all treatment for hair loss and showed a decrease in hair density over a period of 8-12 months [Figure 3a-c] after stopping treatment. All these patients improved when started with topical minoxidil fortified with finasteride as seen in Figure 3d. Four of these patients had a good improvement in hair density after one year of follow up [Table 2].
Figure 3.
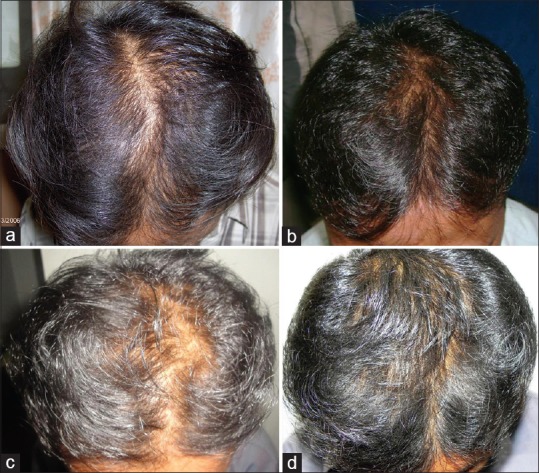
(a) Initial phase before starting treatment, (b) Improvement noticed after oral finasteride treatment, (c) Decline in hair density after stopping the treatment for a period of eight months, (d) Good improvement in hair density after restarting topical medication
Table 2.
The improvement of hair density in patients who had discontinued the treatment for a year and started with topical minoxidil and finasteride combination

DISCUSSION
AGA is an androgen mediated event and is the most common type of alopecia in men. It is genetically inherited and keeps progressing. The main cause is the androgenic hormone DHT. The scalp DHT level is higher in a bald scalp when compared to a hairy scalp.[7] The Food and Drug Administration approved treatment options for male AGA are topical minoxidil and oral finasteride.
5α reductase causes conversion of testosterone to DHT and finasteride is an inhibitor of type II 5α reductase, thereby reducing the formation of DHT molecules.[1] Hence, patients on oral finasteride have lower concentrations of DHT in blood, thereby a reduced DHT level is noticed in the scalp. This in turn reduces the follicular miniaturization and increases the anagen: telogen ratio.[3]
A study conducted by Kaufman et al., determined the effect of oral finasteride treatment for two years and the effect of withdrawal of treatment after one year. The results showed that the effect of oral finasteride was much better than the placebo group and there was reversal in the hair density after withdrawing the oral finasteride. This was because of the increased levels of DHT once finasteride had been stopped.[4]
A study to compare the efficacy of oral vs topical finasteride conducted by Hajheydari et al. says that the efficacy of topical finasteride was at par with that of oral finasteride.[5]
However, the penetration of topical finasteride is a matter of concern. Therefore, minoxidil, a vasodilator and a potassium channel opener was used in combination with finasteride to aid in better absorption. A study conducted by Tanglertsampan comparing the efficacy of 3% minoxidil alone and a combination of 3% minoxidil and 0.1% finasteride topically in the treatment of AGA revealed minoxidil and finasteride combination to be better than minoxidil alone.[8]
In our study, 84.44% patients maintained the density well, showing the effectiveness of the combination in maintaining hair growth. In five patients who had discontinued the treatment, it was noted that 80% of patients had good improvement in a year upon restarting the treatment with topical finasteride in combination with minoxidil. This shows that the topical medication alone is beneficial in maintaining hair density as well as improving hair growth.
Oral finasteride is known to cause side effects such as erectile dysfunction and decreased libido due to decreased in blood DHT.[4,9] Though this occurs in a minority of patients, we noticed that most of our patients were apprehensive about using oral finasteride on a long term basis. The case sheets of the five patients who had discontinued treatment, when reviewed, revealed that they were averse to taking oral finasteride because of its side effects, and hence had discontinued treatment. The patient compliance was good with topical finasteride. However, the limitation of this study is that the serum and scalp DHT levels could not be obtained.
CONCLUSION
A majority of patients in this study who discontinued oral finasteride and adopted topical minoxidil fortified with finasteride showed a minimal decline in hair density reaching a plateau phase, after which there was no further decline in the hair density. Furthermore, it was well tolerated with no psychological fear of oral medication and good compliance. The five patients who had discontinued the treatment for a period of 8-12 months when resumed on topical minoxidil and finasteride combination showed good improvement in hair density. The combination of topical minoxidil and finasteride can thus be considered as a beneficial treatment strategy to maintain hair density after achieving initial improvement with oral finasteride, thereby obviating the use of oral finasteride indefinitely.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. An overview on 5alpha-reductase inhibitors. Steroids. 2010;75:109–53. doi: 10.1016/j.steroids.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Leyden J, Dunlap F, Miller B, Winters P, Lebwohl M, Hecker D, et al. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999;40:930–7. doi: 10.1016/s0190-9622(99)70081-2. [DOI] [PubMed] [Google Scholar]
- 3.Van Neste D, Fuh V, Sanchez-Pedreno P, Lopez-Bran E, Wolff H, Whiting D, et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol. 2000;143:804–10. doi: 10.1046/j.1365-2133.2000.03780.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, Bergfeld W, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39:578–89. doi: 10.1016/s0190-9622(98)70007-6. [DOI] [PubMed] [Google Scholar]
- 5.Hajheydari Z, Akbari J, Saeedi M, Shokoohi L. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2009;75:47–51. doi: 10.4103/0378-6323.45220. [DOI] [PubMed] [Google Scholar]
- 6.Mazzarella F, Loconsole F, Cammisa A, Mastrolonardo M, Vena GA. Topical finasteride in the treatment of androgenetic alopecia: Preliminary evaluation after a 16-month therapy course. J Dermatol Treat. 1997;8:189–92. [Google Scholar]
- 7.Dallob AL, Sadick NS, Unger W, Lipert S, Geissler LA, Gregoire SL, et al. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J Clin Endocrinol Metab. 1994;79:703–6. doi: 10.1210/jcem.79.3.8077349. [DOI] [PubMed] [Google Scholar]
- 8.Tanglertsampan C. Efficacy and safety of 3% minoxidil versus combined 3% minoxidil/0.1% finasteride in male pattern hair loss: A randomized, double-blind, comparative study. J Med Assoc Thai. 2012;95:1312–6. [PubMed] [Google Scholar]
- 9.Arca E, Açikgöz G, Taştan HB, Köse O, Kurumlu Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology. 2004;209:117–25. doi: 10.1159/000079595. [DOI] [PubMed] [Google Scholar]


