Abstract
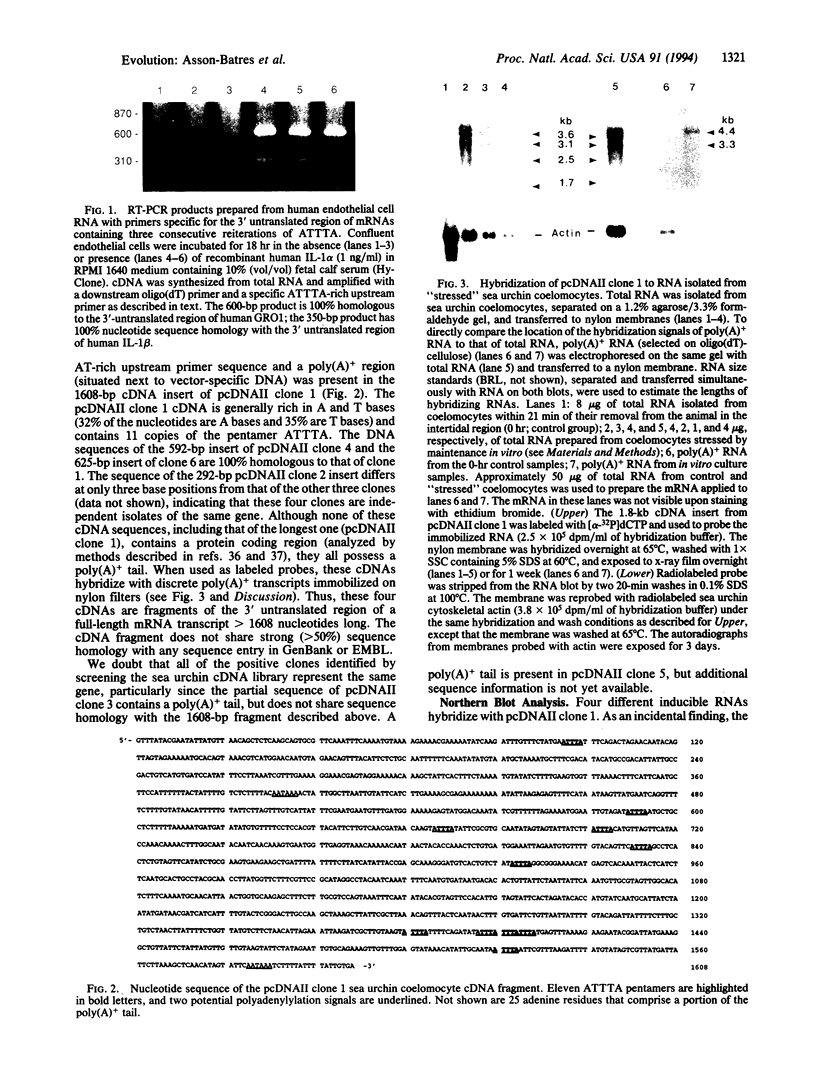
AU-rich sequence motifs (specifically sequences containing reiterations of AUUUA) are found in the 3' untranslated region of mammalian mRNAs encoding cytokines, adhesion molecules, and protooncogenes. Because these AU-rich elements (3'AURE) have been observed to reduce the stability and translational efficiency of transcripts that contain them, and because many of these transcripts accumulate in cells exposed to inflammatory stimuli, we reasoned that mRNAs with 3'AURE may be highly conserved and that the AURE is a marker of mRNAs that are inducible by environmental stressors. To test this hypothesis, we developed a polymerase chain reaction (PCR) strategy to isolate specifically mRNAs with 3'AURE. We first validated the effectiveness of this approach by selectively amplifying two mRNAs containing 3'AURE from interleukin 1 (IL-1)-induced human endothelial cells, then used the same primers in reverse transcriptase-PCR of sea urchin RNA, and used the radiolabeled reaction products to screen a cDNA library prepared from endotoxin-exposed sea urchin coelomocytes. We identified 124 positive clones and isolated a 1608-base-pair fragment that contains an AU-rich consensus sequence upstream from a poly(A) tail. This sea urchin transcript hybridizes with immobilized poly(A)(+)-selected RNA prepared from living coelomocytes maintained in vitro for 8.5-13 h but not with RNA prepared from freshly harvested coelomocytes. Our results provide support for the growing body of evidence that 3' AURE are both conserved and functional and indicate further that isolation and short-term in vitro culture of sea urchin coelomocytes is sufficient to induce the expression of transcripts containing 3'AURE.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K., Natori S. Molecular cloning, sequencing, and characterization of cDNA for sarcotoxin IIA, an inducible antibacterial protein of Sarcophaga peregrina (flesh fly). Biochemistry. 1988 Mar 8;27(5):1715–1721. doi: 10.1021/bi00405a050. [DOI] [PubMed] [Google Scholar]
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Shaw G., Heinrich M. C., Hefeneider S., Brown M. A., DeLoughery T. G., Segal G. M., Band L. Interleukin-1 stimulation stabilizes GM-CSF mRNA in human vascular endothelial cells: preliminary studies on the role of the 3' AU rich motif. Prog Clin Biol Res. 1990;352:233–239. [PubMed] [Google Scholar]
- Beck G., Habicht G. S. Isolation and characterization of a primitive interleukin-1-like protein from an invertebrate, Asterias forbesi. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7429–7433. doi: 10.1073/pnas.83.19.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G., O'Brien R. F., Habicht G. S. Invertebrate cytokines: the phylogenetic emergence of interleukin-1. Bioessays. 1989 Aug-Sep;11(2-3):62–67. doi: 10.1002/bies.950110206. [DOI] [PubMed] [Google Scholar]
- Beutler B., Thompson P., Keyes J., Hagerty K., Crawford D. Assay of a ribonuclease that preferentially hydrolyses mRNAs containing cytokine-derived UA-rich instability sequences. Biochem Biophys Res Commun. 1988 May 16;152(3):973–980. doi: 10.1016/s0006-291x(88)80379-6. [DOI] [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorssers L., Burger H., Bot F., Delwel R., Geurts van Kessel A. H., Löwenberg B., Wagemaker G. Characterization of a human multilineage-colony-stimulating factor cDNA clone identified by a conserved noncoding sequence in mouse interleukin-3. Gene. 1987;55(1):115–124. doi: 10.1016/0378-1119(87)90254-x. [DOI] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugitsch H. W., Prieschl E. E., Kalthoff F., Huber N. E., Baumruker T. A novel transiently expressed, integral membrane protein linked to cell activation. Molecular cloning via the rapid degradation signal AUUUA. J Biol Chem. 1992 Jun 5;267(16):11267–11273. [PubMed] [Google Scholar]
- Gillis P., Malter J. S. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine, and oncogene mRNAs. J Biol Chem. 1991 Feb 15;266(5):3172–3177. [PubMed] [Google Scholar]
- Han J., Brown T., Beutler B. Endotoxin-responsive sequences control cachectin/tumor necrosis factor biosynthesis at the translational level. J Exp Med. 1990 Feb 1;171(2):465–475. doi: 10.1084/jem.171.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Cole M. D. Rapid cytoplasmic turnover of c-myc mRNA: requirement of the 3' untranslated sequences. Mol Cell Biol. 1987 Dec;7(12):4513–4521. doi: 10.1128/mcb.7.12.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabnick K. S., Housman D. E. Determinants that contribute to cytoplasmic stability of human c-fos and beta-globin mRNAs are located at several sites in each mRNA. Mol Cell Biol. 1988 Aug;8(8):3244–3250. doi: 10.1128/mcb.8.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Shott R. J., Rose S. J., 3rd, Thomas T. L., Britten R. J., Davidson E. H. Sea urchin actin gene subtypes. Gene number, linkage and evolution. J Mol Biol. 1984 Jan 15;172(2):149–176. doi: 10.1016/s0022-2836(84)80035-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Paco-Larson M. L., Garen A. DNA homology between the 3'-untranslated regions of a developmentally regulated Drosophila gene and a mouse alpha-interferon gene. Gene. 1986;46(1):79–88. doi: 10.1016/0378-1119(86)90169-1. [DOI] [PubMed] [Google Scholar]
- Nishida T., Nishino N., Takano M., Kawai K., Bando K., Masui Y., Nakai S., Hirai Y. cDNA cloning of IL-1 alpha and IL-1 beta from mRNA of U937 cell line. Biochem Biophys Res Commun. 1987 Feb 27;143(1):345–352. doi: 10.1016/0006-291x(87)90671-1. [DOI] [PubMed] [Google Scholar]
- Rosen K. M., Lamperti E. D., Villa-Komaroff L. Optimizing the northern blot procedure. Biotechniques. 1990 Apr;8(4):398–403. [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Segal G. M., McCall E., Bagby G. C., Jr Erythroid burst-promoting activity produced by interleukin-1-stimulated endothelial cells is granulocyte-macrophage colony-stimulating factor. Blood. 1988 Oct;72(4):1364–1367. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stoeckle M. Y., Hanafusa H. Processing of 9E3 mRNA and regulation of its stability in normal and Rous sarcoma virus-transformed cells. Mol Cell Biol. 1989 Nov;9(11):4738–4745. doi: 10.1128/mcb.9.11.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonouchi N., Miwa K., Karasuyama H., Matsui H. Deletion of 3' untranslated region of human BSF-2 mRNA causes stabilization of the mRNA and high-level expression in mouse NIH3T3 cells. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1056–1062. doi: 10.1016/0006-291x(89)92328-0. [DOI] [PubMed] [Google Scholar]
- Vakalopoulou E., Schaack J., Shenk T. A 32-kilodalton protein binds to AU-rich domains in the 3' untranslated regions of rapidly degraded mRNAs. Mol Cell Biol. 1991 Jun;11(6):3355–3364. doi: 10.1128/mcb.11.6.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virca G. D., Northemann W., Shiels B. R., Widera G., Broome S. Simplified northern blot hybridization using 5% sodium dodecyl sulfate. Biotechniques. 1990 Apr;8(4):370–371. [PubMed] [Google Scholar]
- Vogeli G., Horn E., Laurent M., Nath P. Recombinant DNA techniques: storage and screening of cDNA libraries with large numbers of individual colonies from initial transformations. Anal Biochem. 1985 Dec;151(2):442–444. doi: 10.1016/0003-2697(85)90202-7. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., Rechavi G. Differential mRNA stability to reticulocyte ribonucleases correlates with 3' non-coding (U)nA sequences. Eur J Biochem. 1988 Mar 1;172(2):333–340. doi: 10.1111/j.1432-1033.1988.tb13891.x. [DOI] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]





