Abstract
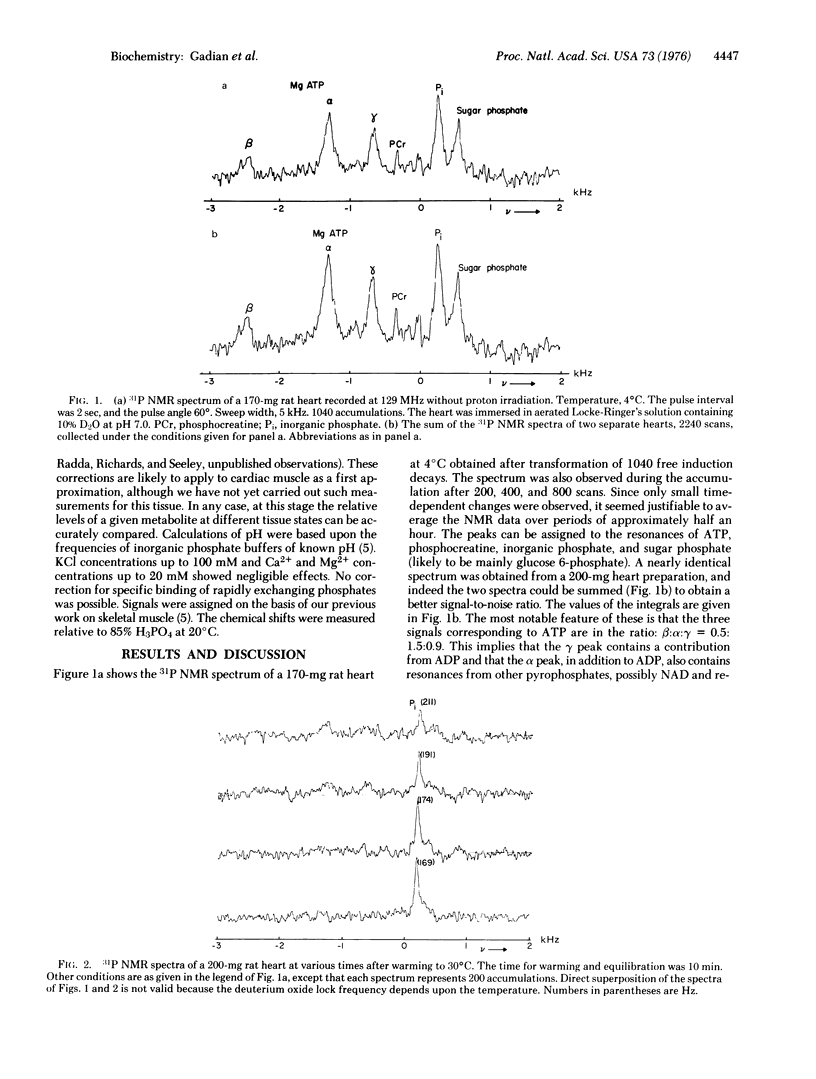
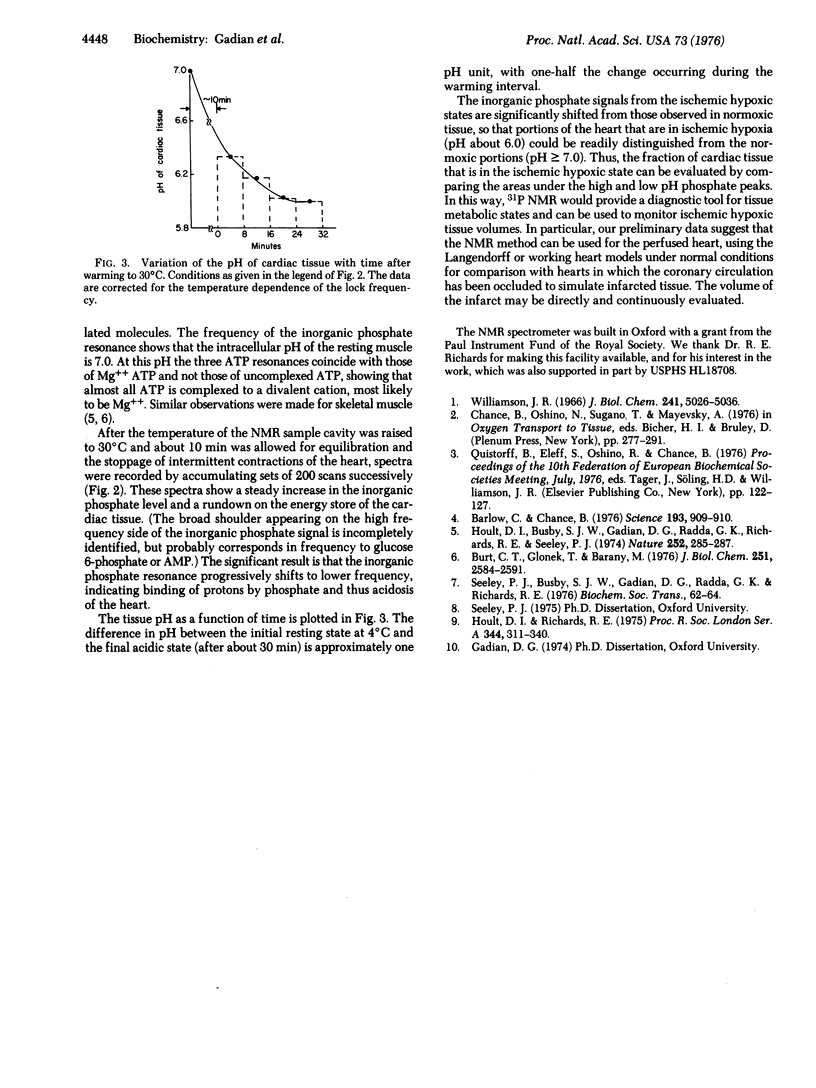
The intact heart of a young rat was excised rapidly and cooled to 0 degree C; its energy-rich compounds were examined by 31P Fourier Transform nuclear magnetic resonance. The heart showed the characteristic spectrum of sugar phosphates, inorganic phosphate, phosphocreatine, and magniesium phates, inorganic phosphate, phosphocreatine, and magnesium ATP, characteristics of the energizing state of the nonbeating tissue. Warming to 30 degrees C imposes an energy load upon the heart consistent with short-term resumption of beating, concomitant intracellular acidosis, and decomposition of all detectable energy-rich compounds. The intracellular acidity causes a shift from pH 7.0 to 6.0. The effects of possible interferences with this pH measurement are considered. The method appears to have wide usefulness in cardiac infarct models for detecting the fraction of the total volume occupied by the infarct and for studying the effect of various proposed therapies upon this infarcted volume.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow C. H., Chance B. Ischemic areas in perfused rat hearts: measurement by NADH fluorescence photography. Science. 1976 Sep 3;193(4256):909–910. doi: 10.1126/science.181843. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Seeley P. J., Busby S. J., Gadian D. G., Radda G. K., Richards R. E. A new approach to metabolite compartmentation in muscle. Biochem Soc Trans. 1976;4(1):62–64. doi: 10.1042/bst0040062. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]


