Abstract
In a biomedical research environment, research or management procedures may render continuous full contact pairing of rhesus macaques (Macaca mulatta) unfeasible. This study aimed to determine whether separation on a frequent basis or housing in adjacent cages with tactile contact interferes with the behavioral benefits of continuous full contact. Behavioral data (1260 hours) were collected from 32 adult females and 16 adult males housed at two National Primate Research Centers. Subjects were studied in four housing conditions: single housing, full contact pair housing, intermittent contact pair housing, and protected contact housing. After introduction, each pair was housed in each of the three social housing conditions in varying order. Among females, but not males, introducing animals into full and intermittent contact reduced levels of abnormal behavior. There was a trend toward this reduction in protected contact. In both females and males, full and intermittent contact was associated with lower levels of anxiety-related behavior, but protected contact was not. Females spent more time inactive in protected contact than either full or intermittent contact, and males showed a trend toward less inactivity following introduction into full contact. Both sexes showed less affiliation in protected contact compared to the other forms of social housing. Agonistic behavior among females was not affected by housing condition; among males, levels were equivalent in full and intermittent contact but were higher in intermittent than protected contact. Frequent separation of pairs does not appear to detract from the behavioral benefits of pair housing. Separation by a barrier permitting tactile contact is inferior to other forms of social housing but showed modest improvements over single housing nonetheless. This study can guide the provision of social contact to rhesus macaques under conditions restricting pairs from continuous full contact.
Keywords: primate, behavioral management, social, introduction, cage design
INTRODUCTION
In the United States, approximately three-quarters of laboratory primates are housed socially. Among laboratory rhesus macaques (Macaca mulatta), about half are housed socially, generally in isosexual pairs; this figure that has changed little in the past decades according to available literature [Baker et al., 2007; Reinhardt, 1994]. It is well established that socially housed rhesus macaques demonstrate well-being that is superior to that of their singly housed counterparts; pair-housed rhesus macaques display more affiliative interactions, physical activity, play, and exploration than singly housed individuals [Baker et al., 2012a; Eaton et al., 1994; Schapiro et al., 1996] and less abnormal, stereotyped, and self-injurious behavior [Baker et al., 2012a; Lutz et al., 2003; Novak, 2003; Schapiro et al., 1996]. Improved welfare as a result of social housing has been demonstrated in both female–female and male–male adult pairs [Baker et al., 2012a; Doyle et al., 2008; Eaton et al., 1994]. Pair and group housing may buffer stress for rhesus macaques as they are exposed to research events [Gilbert & Baker, 2011], removed from social groups [Gust et al., 1994], or transported to different facilities [Fernström et al., 2008].
Prior studies have evaluated pairs maintained in continuous contact in a shared space. However, rhesus macaque pairs assigned to research projects are often subject to frequent separation and reunion because one or both members of a pair may be removed from its primary housing for research procedures. Responses to such repeated short-term separation from social partners have been studied only in adolescent macaques. Such separations result in abnormal and depressive behaviors, and with repeated separation these responses intensify, both during the separation period and for several days after reunions [Mineka et al., 1981]. Adult females show stress responses to single separations from large social groups [Gust et al., 2000]. In contrast, adult males appear to tolerate removal from large social groups better, though they engage in agonistic interactions and react with physiological signs of stress when returned to their groups [Gust et al., 1993]. Juveniles show pronounced stress responses to both a single separation event and an eventual reunion, and receive aggression upon reunion [Gordon et al., 1992; Gordon & Gust, 1993]. Taken together, these results suggest that repeated, short-term separations and reunions could detract from the benefits of pair housing by creating distress and increased aggression within pairs, requiring a careful assessment of the effects of moving animals from single housing to intermittent contact, and between intermittent and continuous full contact.
In response to concerns over the potential impact of intermittent separations on distress or aggression, as well as for a variety of research or medically related purposes, laboratory macaques may be housed in a configuration of cages that permits social contact without access to social partners’ cages. Crockett et al. [1997] were the first to publish the idea of providing tactile contact through grooming-contact partitions that prevents entry into neighboring cages. Providing tactile contact through a barrier, hereafter termed “protected contact,” is less common than providing full contact [Baker et al., 2007]. In addition to providing for a broad range of social behavior, protected contact housing has the potential to reduce stress resulting from the inability to control proximity in a restricted space, and may also protect subordinate individuals from possible food monopolization and unwanted social interaction that may occur with full contact. Protected contact can also accommodate research requirements for different diets or feeding and watering schedules [Thom & Crockett, 2004] or increase the ease with which animals can be monitored for food intake or feces output. Because social incompatibility and research protocol constraints are the most common challenges associated with social housing programs [Baker et al., 2007], the assessment of protected contact as a viable alternative form of pair housing compared to repeated intermittent contact is especially important.
In addition, a careful comparison of protected contact and single housing is also imperative to determine whether significant welfare improvements result from the protected contact style of housing. The potential advantages of protected contact housing over single housing may be at odds with the stance of the 2011 edition of the Guide for the Care and Use of Laboratory Animals and merits objective assessment. The language of the new Guide calls into question the categorization of protected contact housing as a form of social housing, as suggested in the following quotation [National Research Council, 2011, p. 64]:
Single housing of social species should be the exception and justified based on experimental requirements or veterinary-related concerns about animal well-being. In these cases, it should be limited to the minimum period necessary, and where possible, visual, auditory, olfactory, and tactile contact with compatible conspecifics should be provided. Characterizing protected contact as a form of single housing logically implies that one would therefore expect that the benefits of full contact pair housing would be absent in protected contact.
When evaluating a behavioral management technique for macaques, it is important to remember that the population housed in laboratories is diverse and that response to changes in management may potentially vary with such characteristics as age, rearing, and prior social experience. With respect to social housing, one source of variation that may be particularly salient to the responses to social housing is the behavioral pathology termed “self-injurious behavior” (SIB), which consists of repetitive, self-directed biting that can lead to tissue damage and mutilation. Among individually housed rhesus macaques, between 5% and 13% of the population spontaneously demonstrate self-biting and/or wounding [Bayne et al., 1992, 1995; Novak, 2003]. Social housing is one of the few potentially effective approaches to treating SIB [Weed et al., 2003]. However, SIB may function to reduce arousal when stressed [Davenport et al., 2008; Tiefenbacher et al., 2000]. Because some social interactions are stressful, it is possible that interaction with another monkey could elicit self-injury or interfere with normal social responses. For this reason, some people managing macaques may be hesitant to introduce individuals with histories of SIB for fear of triggering self-biting or wounding, or might be inclined to use protected contact as an alternative to full contact in hopes of reducing this risk. For macaques that engage in SIB, it is especially critical to determine the social setting that either best controls the expression of this abnormal behavior or avoids triggering it.
The objective of the current study was to measure the behavioral responses of rhesus macaques introduced into isosexual pairs and maintained in three different arrangements intended to provide social contact: continuous full-contact pair housing condition (hereafter termed “full contact”), intermittent full-contact pair housing condition (hereafter termed “intermittent contact”), and contact via a perforated panel preventing entry into partners’ cages (hereafter termed “protected contact”). Based on the literature reviewed above, the following hypotheses were tested.
Introduction into full contact pair housing will diminish the expression of undesirable behaviors (abnormal behavior, anxiety-related behavior, and inactivity) and increase species-typical locomotion.
The frequent separations and reunions characteristic of intermittent contact housing will blunt the benefits of continuous full contact pair housing, and result in increased anxiety-related behavior and aggression in comparison to full contact.
Restricted contact through a barrier will not blunt the benefits of full contact nor result in undesirable behaviors hypothesized to result from intermittent contact.
Because of the stress involved in social housing, rhesus macaque subjects with histories of SIB will show fewer behavioral benefits in full contact (i.e., smaller reductions in abnormal behavior, in anxiety-related behavior, and reduced levels of affiliative behavior) than those without such histories, and will show increases in anxiety-related behavior and self-biting when living in intermittent contact housing.
In summary, we predict that for normal study subjects (i.e., those without a history of self-wounding or -biting), full contact housing will show the most benefits to captive rhesus macaques. We also predict that protected contact will have more benefits than intermittent contact, though both of these housing conditions are expected to be better than single housing. However, the benefits of the various forms of social housing may differ for individuals with this history.
METHODS
This study was designed as a collaborative research project between the Tulane National Primate Research Center (TNPRC) and the Yerkes National Primate Research Center (YNPRC), United States. This study was conducted between September 2001 and May 2008. Methodologies at the two centers were identical except where stated below.
Subjects and Housing
The initial subject pool consisted of 54 female and 18 male rhesus macaques from the TNPRC and the YNPRC. Rearing was unknown for four females previously imported from another facility. Most subjects were mother reared in breeding groups. However, seven females were nursery reared and/or housed with only their mothers in cages for all or part of infancy. Not all of the introductions implemented for this study were successful; 20% of female–female introductions were terminated because of persistent agonism, food monopolization, or wounding. An additional two pairs were later separated due to agonism before the entire phase of data collection in social housing had been completed, and were therefore not used in the subject pool. All but one male–male introductions were successful, and no pairs required separation prior to completion of the study. Four females subjects who failed introductions were introduced to alternative partners and data on these successful pairs were used in the study. Otherwise, no data from individuals that could not be paired due to initial or delayed incompatibility were included in the current study. The remaining subject pool consisted of 32 females and 16 males (Table I).
TABLE I.
Study Subjects
| Rearing | TNPRC
|
YNPRC
|
||||
|---|---|---|---|---|---|---|
| Mother-rearing | Other rearing | Unknown | Mother-rearing | Other rearing | Unknown | |
| Females | 18 | 4 | 2 | 8 | 0 | 0 |
| Males | 10 | 0 | 0 | 6 | 0 | 0 |
Females ranged in age from 4.8 to 14.3 years (mean 8.9 ±0.5 years [standard error]) and males from 3.7 to 7.1 years (mean 5.1 ±0.3 years). While all subjects had been housed socially at some point in their lives, the duration of the most recent period of single housing at the onset of the study ranged from 4 months to 9.7 years among females (mean 3.1 ±0.4 years) and from 1 month to 4.9 years among males (mean 1.1 ±0.4 years). The variability in these durations reflects the different histories of assignment to past research protocols. Among the study animals, seven females (22% of subjects) and four males (25% of subjects) had a history of repetitive self-biting; most had incurred at least one documented incident of self-wounding prior to the onset of the study. While self-biting was recorded during the study period, no self-wounding occurred during that time.
All aspects of management and research use conformed to applicable US federal regulations and the guidelines described in the Guide for the Care and Use of Laboratory Animals [National Research Council, 1996] and the US Department of Agriculture’s Animal Welfare regulations [1991] and adhered to the study’s protocol as approved by the TNPRC and YNPRC Institutional Animal Care and Use Committees. In addition, methods adhered to the guidelines and principles of the American Society of Primatologists for the ethical treatment of non-human primates.
The subjects were housed indoors in rooms maintained on a 12:12-hr light:dark cycle and ambient temperature between 18–22°C with a relative humidity of 30–70%. All subjects weighed less than 10 kg. Stainless steel cages had a height of 0.8–0.9 m and floor space of 0.4–0.8 m2 which met or exceeded federal animal welfare regulations. During the single housing phase of the study, subjects were housed in the same room and next to their future social partner in order to reduce potential confounds relating to variations in the external environment (e.g., number of animals in the room, identity of caregiver or animals in visual contact).
Animal care staff provided nutritionally complete food biscuits twice daily, and fresh water was available ad libitum. Three to five times per week, husbandry staff distributed fruits, vegetables, and other food treats as feeding enrichment. Each cage included a perch and a manipulable object such as a toy, PVC piece or hardwood segment. Some animals also had foraging or grooming devices at the time of enrollment in this study. Devices were neither removed nor added to subjects’ cages once data collection had commenced.
Pairing Process
For over half of the pairs studied, the identity of the pair-mate was entirely dictated by research constraints (i.e., pairs could only be derived from the same research project and treatment group). When there were options for the composition of potential pairs, no more than two possible pair-mates were available. Pairs were chosen so that individuals were not closely matched in body weight, since greater weight disparities have been associated with a higher success rate at the TNPRC [K. Baker, personal observation]. Individuals were also not matched for similarity in temperamental characteristics such as aggressive or fearful responses to humans. All pairs were isosexual.
Social introductions between potential pair mates began with subjects being placed into protected contact by replacing the solid panel separating them with a panel permitting limited contact. This introductory phase lasted for 1 or 2 weeks. At the TNPRC, protected contact involved 7 consecutive days in which individuals could touch through a panel with a 32 cm-deep by 53 cm-high area containing 12 oblong holes, each measuring 5.1 ×8 cm. This rectangular area of holes was at the front of the cage, leaving a 33-cm-deep solid area in the back of the cage to serve as a visual barrier. The rectangle was placed in such a way that animals could look through the holes whether sitting or standing (the lowest point was approximately 27 cm from the cage floor and extended to a height of 80 cm. As part of the YNPRC introduction process, three panel styles were used with increasing sizes of the openings to increase access as the introduction progressed. As with the TNPRC panels, all perforated areas were located toward the front of the cage. Subjects spent one 24-hr period with a panel with a large number of 1-cm holes (permitting visual access and only fingertip contact), 6 days with a panel with two rows of 3 ×15 cm oblong holes, and 7 days with a panel with three 5 ×15 cm oblong holes. This last panel was the type used long term in the protected contact phase of the study. Individuals could touch through the panel, which included a 25-cm-deep by 15-cm high area containing the three oblong holes. This rectangular area of holes was at the front of the cage, leaving a 50-cm-deep solid area in the back. The bottom of the holes was 38 cm from the cage floor and extended to a height of 53 cm. Panels were then removed to provide monkeys with unrestricted full contact. Intermittent contact involved the placement of a solid panel between pair-mates for two 24-hr periods per week; no data were collected on the days in which pairs were reunited. Protected contact at the TNPRC involved the placement of the same panel employed in the introduction process (with the 3 ×15 cm oblong holes). At the YNPRC, the panel style with 5 ×15 cm oblong holes was used.
Pairs were closely monitored in person and via videotape for injurious, persistent, and/or escalating aggression, which were criteria for separation. Introductions proceeded only for pairs that did not display these problems. Alternative social partners were not available for the majority of individuals involved in failed introductions, and these subjects were dropped from the study.
The current study assessed four housing conditions: a baseline condition involving single housing prior to any social introduction, and then the three experimental social housing conditions, the order of which varied between pairs:
full contact (continuous sharing of cages);
intermittent contact (housing in share cages but separated by a solid panel for two separate 24-hr periods per week);
protected contact (continuous access through a panel containing 12 oblong holes measuring 5.1 cm ×8 cm [TNPRC] or six 5 ×15 cm oblong holes [YNPRC]).
While all subjects were first studied in single housing, the order of the three social housing styles was predetermined before any social introductions commenced so that it varied across pairs and all possible sequences were implemented. No phases were repeated. The order of the social housing phases were varied to avoid introducing consistent confounds in duration and type of prior familiarity in each style of social housing. Due to the variation in order of social housing phases, prior familiarity at the onset of each style of social housing varied considerably, although it was predetermined and balanced. For example, when protected contact was the first phase of social housing, subjects had initially been introduced as recently as 6 weeks previously (including the introduction process and the planned 4 weeks delay in the onset of data collection). For other animals, the protected contact phase may have begun over nine months following initial social introduction. The variation in prior familiarity was similar for the other forms of social housing and was necessary in order to use pairs as their own control and avoid order effects.
The present study describes the behavior of the 48 subjects that were maintained in social housing without evidence of incompatibility. After completion of the study, animals were placed in full contact pair housing to support their well-being. Study aims did not include an assessment of separation stress or a reassessment of baseline conditions.
Data Collection
Data were collected and coded by four individuals with interobserver reliability both within facilities and between at least two observers at different facilities, with a minimum of 85% agreement. After the collection of baseline data, the initial social introductions were performed. Following the initial introduction as well as changes in social setting, data collection commenced no sooner than 4 weeks after each change in housing setting in order to avoid the period of short-term adjustment and potentially transient effects. On average, the duration from the change in housing until the onset of data collection was 7 weeks. For most subjects, data were collected over a subsequent 4- to 8-week period, and the transition to the next housing phase was undertaken promptly after this period. In a few cases, this schedule was disrupted for reasons unrelated to the present study, such as availability of appropriate caging, avoidance of potentially confounding conditions such as nearby maintenance noise or a significantly lower level of human activity associated with the suspension of some research activities following Hurricane Katrina. For these reasons, the data collection period was extended due to interruption mid-phase, or subjects remained in their current environment for up to seven months before transitioning to the new style of social housing.
Videotaping was employed to collect 60-min focal observations with start times held steady across conditions for each individual, in recognition of the effect of time of day on behavior. Start times varied between pairs (between 10 a.m. and 3 p.m.) to facilitate data collection on multiple pairs. Data collection was scheduled to avoid daily feedings, routine husbandry, and research procedures. For most subjects, 6–8 hr of data were collected per each of the housing conditions. However, this quantity of data was not achieved for several potential subjects, who were excluded from the study if fewer than 4 hr were collected in any phase. Phases during which as little as 4 hr of data were collected comprised approximately 6% of the total phases. A total of approximately 1260 hours of observational data were collected. Data were coded with an ethogram including 62 behaviors, using instantaneous sampling with a 15-sec inter-sample interval. Predetermined decision rules were applied for priority of data entry for samples in which more than one behavior occurred (i.e., abnormal >social >anxiety-related behavior > other non-social). Point samples for individual subjects in each study phase were pooled across observation periods, and statistical analyses were performed using percentages of samples for each behavior in each study phase. Behaviors of interest in the current study were collapsed into six categories for analysis (see Table II for operational definitions). Not all behaviors included in the ethogram were analyzed in the current study so percentages of time spent performing the behaviors of interest do not sum to 100%.
TABLE II.
Behavioral Categories Analyzed (in Bold)
| Abnormal |
| Appetitive: coprophagy, feces paint, regurgitate, urine drink |
| Locomotor stereotypies: bizarre posture, flip, floating limb, head toss, jump, pace, rock |
| Non-injurious self-directed: eye-poke, self-clasp, self-mouth |
| Overgroom: pluck hair from self with a quick and forceful movement, using hands or teeth; may include ingesting hair |
| Self-injurious: self-bite, self-slap (no self-wounding occurred during the study) |
| Anxiety related |
| Body shake: rapid shaking of head and shoulders |
| Scratch: vigorous strokes of the hair |
| Self-groom: any picking, stroking and/or licking of one’s own body hair without pulling hair out (see overgroom [above], includes biting or chewing on nails |
| Yawn: monkey opens mouth wide, often exposing teeth |
| Affiliative |
| Contact affiliative: groom, social play, cling, mount, genital explore, rest in contact (inactive with body surface [usually trunk] touching another individual) |
| Non-contact affiliative: attempt to touch, lip-smack, present |
| Agonistic |
| Contact aggression: moderate aggressive contact (pushing, pulling, grabbing, minor scratching), severe aggressive contact (biting with injury) |
| Non-contact aggression: bob, cage shake, cringe, crook tail, ear flick, fear grimace, flee, grab, jaw snap, lunge, open-mouth stare, rapid glances, rump present, stare, teeth grinding, attempt to bite |
| Inactive: passive, awake or asleep |
| Locomotion: walk, climb, jump |
During the intermittent contact phase, data were not collected during periods of separation because most animals received ketamine anesthesia and were involved in research procedures during the separation.
Statistical Analysis
A within-subjects design was used to compare the four conditions. All statistics were calculated using Statistica 10.0 for Windows. For all categories of behavioral data analyzed, measures of skewness, kurtosis, and homogeneity of variance failed to meet required assumptions for parametric tests, so data were transformed using an arcsin square root transformation prior to analysis. Separate analyses were conducted on nonsocial and social behavior. Since the expression of social behavior was prevented in single housing (prospective pair mates being housed side by side with no visual contact), it would have skewed results pertaining to the proportion of time spent in nonsocial behavior across all four phases of the study if incorporated into one statistical analysis. Statistical tests were performed on nonsocial behaviors across all four housing conditions (single housing, full contact, intermittent protected contact, and protected contact) and on social behaviors across only the three social housing conditions, excluding the single housing phase.
Nonsocial behavior
For the analysis of the four nonsocial behavioral categories (abnormal, anxiety-related, inactivity, and locomotion), multivariate analyses of variance (MANOVA) for repeated measures with sex as a grouping factor were used across the four housing conditions with alpha set at 0.025 to control for multiple comparisons. Significant sex differences were detected across the nonsocial behaviors (see below) so data were analyzed separately for each sex. Following an overall significant MANOVA result, its univariate results identified which behavioral categories differed significantly, and t-tests (with alpha set at 0.01, with a trend between 0.01 and 0.02) were used to identify significant pair-wise differences. Among female subjects, an additional MANOVA followed by ANOVAs were performed, adding history of SIB as an independent variable. While a similar proportion of male and female subjects displayed SIB, the number of males with a history of SIB (n = 4) was insufficient for performing this analysis.
Social behavior
Sex differences in affiliative and agonistic behavior were assessed separately. Significant sex differences were detected in both social behaviors (see below) so data were analyzed separately for each sex. For each behavior, an ANOVA for repeated measures was employed across the three social housing conditions. Among females, an additional ANOVA using SIB was performed on each behavior. For ANOVA analyses, significance was defined as above, but t-test thresholds for significance and trends (0.02 and 0.03, respectively) were set higher than those set for nonsocial behaviors, since fewer pair-wise comparisons were conducted.
RESULTS
Nonsocial Behaviors
A MANOVA applied to the four categories of nonsocial behavior showed a trend toward an interaction between sex and study phase (F12,35 = 2.24; P = 0.03) so each sex was examined separately for analyses involving these behaviors.
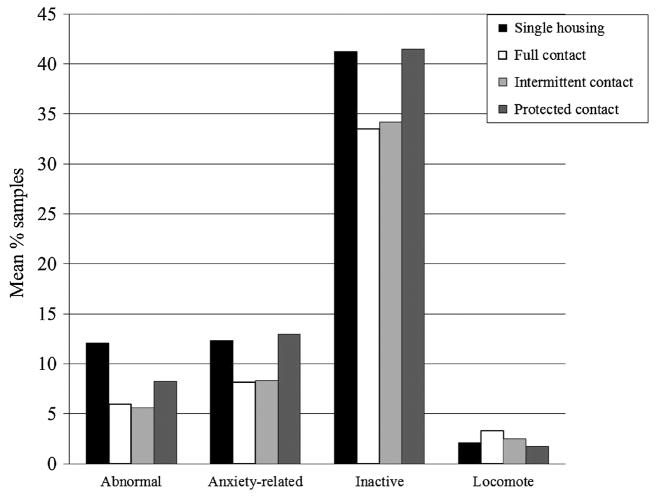
Among females, the overall MANOVA yielded a significant result (F12,20 = 42.98, P = 0.001), due to significant changes in levels of all categories of behavior across the four housing types (Table III and Fig. 1). Subjects in full and intermittent contact pair housing spent significantly less time performing abnormal behavior than they did in single housing. A trend toward this same pattern was found in the comparison between protected contact and single housing, as well as a trend toward a lower level of abnormal behavior in full contact than in protected contact. Compared to single housing, subjects spent less time performing anxiety-related behaviors in full contact and intermittent contact, but levels did not vary between single housing and protected contact. Levels of anxiety-related behaviors in protected contact were significantly higher than in full contact and intermittent contact. Subjects were inactive in protected contact more often than in either of the other forms of pair housing. Subjects in full contact spent more time locomoting than when they were singly housed or maintained in protected contact; levels in protected contact were lower than those in intermittent contact as well.
TABLE III.
Effects of Housing Condition on Nonsocial Behavior Among Female Subjects
| F3,29 | P | t | P | |
|---|---|---|---|---|
| Abnormal | 112.74 | 0.001 | ||
| Single housing >full contact | 3.85 | 0.001 | ||
| Single housing >intermittent contact | 3.95 | 0.001 | ||
| Single housing >protected contact | 2.64 | [0.013] | ||
| Full contact <protected contact | −2.66 | [0.012] | ||
| History of SIB: F3,28 = 5.56, P = 0.004 | ||||
| SIB subjects: no pair-wise contrasts significant | ||||
| Anxiety | 56.65 | 0.001 | ||
| Single housing >full contact | 3.43 | 0.002 | ||
| Single housing >intermittent contact | 3.28 | 0.003 | ||
| Full contact <protected contact | −6.26 | 0.001 | ||
| Intermittent contact <protected contact | −3.60 | 0.001 | ||
| Inactivity | 67.36 | 0.001 | ||
| Full contact <protected contact | −3.50 | 0.001 | ||
| Intermittent contact <protected contact | −3.25 | 0.003 | ||
| Locomotion | 190.23 | 0.001 | ||
| Single housing <full contact | −3.58 | 0.001 | ||
| Full contact >protected contact | 5.72 | 0.001 | ||
| Intermittent contact >protected contact | 3.48 | 0.002 | ||
| History of SIB: F3,28 = 3.99, P = 0.017 | ||||
| SIB subjects | ||||
| Single housing <full contact | −3.90 | 0.008 | ||
| Full contact >intermittent contact | 3.21 | [0.018] | ||
| Full contact >protected contact | 4.58 | 0.004 | ||
| Subjects without SIB | ||||
| Full contact >protected contact | 4.39 | 0.001 | ||
| Intermittent contact >protected contact | 3.03 | 0.006 |
Note: Univariate ANOVAs (α = 0.025) followed by paired t-tests (α = 0.01; trends [α = 0.02] are shown in brackets); results for all female subjects and for the subset of females with a history of self-injurious behavior (SIB).
Fig. 1.
Nonsocial behaviors among female subjects (backtransformed arcsin square root means). The three social housing phases varied in order across subjects but are presented in the same order in this and all subsequent figures.
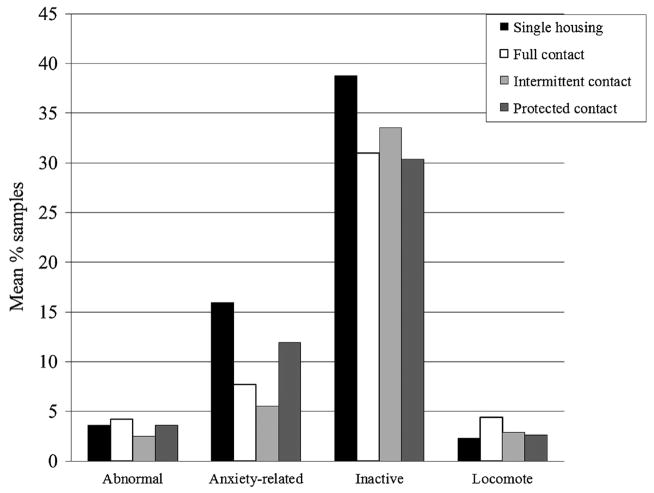
History of SIB among females showed a trend toward an interaction effect with housing condition (F12,19 = 2.34, P = 0.047) (Table III and Fig. 2). The ANOVA involving abnormal behavior showed a significant interaction between study phase and SIB status but among this subset of females, contrasts in levels of abnormal behavior did not reach statistical significance. No interaction effect was detected for anxiety-related behavior or inactivity. Subjects with SIB showed the same increase in locomotion following introduction into full contact as the full set of subjects, as well as the same elevation in full contact in comparison to protected contact (single housing: 1.21% of samples; pair housing: 4.41%; intermittent contact: 2.36%; protected contact: 1.20%). The SIB subset of subjects showed a trend toward more locomotion in full contact in comparison to intermittent contact, but a significant contrast between intermittent and protected contact was not seen.
Fig. 2.
Nonsocial behaviors among male subjects (backtransformed arcsin square root means).
Among males, the overall MANOVA was significant (F12,4 = 13.60, P = 0.02); univariate results showed significant differences in levels of all nonsocial behaviors except abnormal behavior (Table IV and Fig. 2). Compared to single housing, subjects spent less time performing anxiety-related behaviors in both full contact and intermittent contact, but not in protected contact. Levels of anxiety-related behaviors in protected contact were significantly higher than in intermittent contact. Males spent less time inactive in full contact than in single housing. Lastly, in comparison to single housing, subjects spent more time locomoting in full contact but not in intermittent or protected contact. Levels in protected contact were lower than those in full contact, but there was no difference between full contact and intermittent contact. Unlike females, males showed no desirable changes in behavior between single housing and protected contact.
TABLE IV.
Effects of Housing Condition on Nonsocial Behavior Among Male Subjects
| F3,13 | P | t | P | |
|---|---|---|---|---|
| Abnormal | 46.09 | 0.001 | ||
| No pair-wise contrast significant | ||||
| Anxiety | 20.74 | 0.001 | ||
| Single housing >full contact | 3.92 | 0.001 | ||
| Single housing >intermittent contact | 7.03 | 0.001 | ||
| Intermittent contact <protected contact | −3.11 | 0.007 | ||
| Inactivity | 46.09 | 0.001 | ||
| Single housing >full contact | 2.61 | [0.02] | ||
| Locomotion | 36.29 | 0.001 | ||
| Single housing <full contact | −3.38 | 0.004 | ||
| Full contact >protected contact | 3.78 | 0.002 |
Note: Univariate ANOVAs (α = 0.025) followed by paired t-tests (α = 0.01; trends [α = 0.02] are shown in brackets).
Social Behaviors
During pair housing, sex differences were detected for both affiliative behavior (F2,92 = 51.09, P = 0.001) and agonistic behavior (F2,92 = 7.27, P = 0.002) so the sexes were analyzed separately.
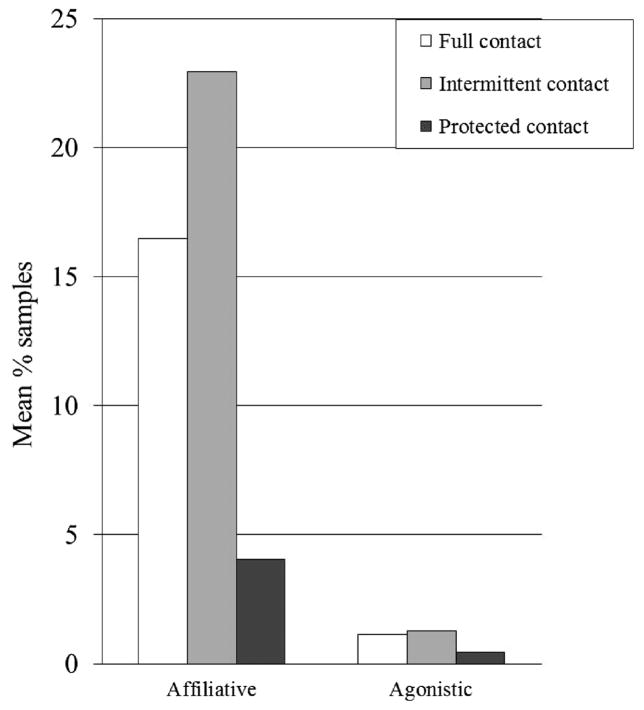
Among females, levels of affiliative behavior were lower in protected contact than either of the other forms of pair housing. Levels of affiliation trended toward being higher in intermittent than full contact. Among females, there was no effect of housing on agonistic behavior (Table V and Fig. 3).
TABLE V.
Effects of Housing Condition on the Social Behavior
| F | P | t | P | ||
|---|---|---|---|---|---|
| Affiliative | |||||
| Females | 60.02 (df = 2,92) | 0.001 | |||
| Full contact >protected contact | 9.20 | 0.001 | |||
| Full contact <intermittent contact | −3.04 | [0.029] | |||
| Intermittent contact >protected contact | 8.29 | 0.001 | |||
| History of SIB: | 0.16 (df = 2.60) | 0.85 | |||
| Males | 10.72 (df = 2,30) | 0.001 | |||
| Full contact >protected contact | 3.72 | 0.002 | |||
| Intermittent contact >protected contact | 4.27 | 0.001 | |||
| Agonistic | |||||
| Females | 1.50 (df = 2,62) | 0.23 | |||
| History of SIB: | 0.27, (df = 2,60) | 0.77 | |||
| Males | 5.01 (df = 2,30) | 0.01 | |||
| Intermittent contact >protected contact | 2.84 | 0.012 | |||
Note: Univariate ANOVAs (α = 0.025) followed by paired t-tests (α = 0.02; trends [α = 0.03] are shown in brackets).
Fig. 3.
Social behaviors among female subjects (backtransformed arcsin square root means).
History of SIB among females did not show an interaction effect with housing condition for either affiliative behavior (F2,60 = 0.16, P = 0.85) or agonistic behavior (F2,60 = 0.27, P = 0.77).
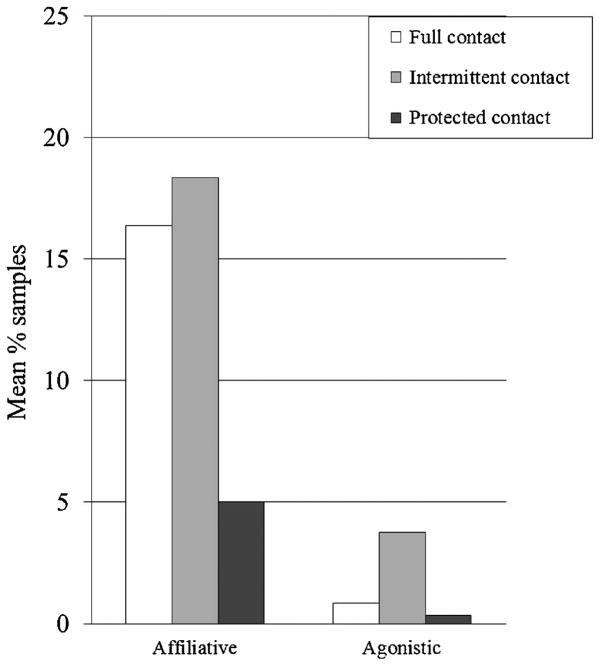
In males, as with females, levels of affiliative behavior were lower in protected contact than either of the other forms of pair housing, but no difference was detected between full and intermittent contact. Agonistic behavior was seen more often in intermittent contact than in protected contact (Table V and Fig. 4).
Fig. 4.
Social behaviors among male subjects (backtransformed arcsin square root means).
DISCUSSION
Consistent with previous research, this study clearly documented improved behavioral indicators of well-being among singly housed rhesus macaques when they were provided via full contact a compatible same-sex social partner, supporting Hypothesis 1. Full contact reduced levels of abnormal behavior among females. In both sexes, it increased locomotion and reduced anxiety-related behavior and inactivity; these are all widely accepted as indicators of improved welfare. It is interesting to note that levels of abnormal behavior decreased dramatically after pairing into full or intermittent contact among females but not among males. A previous study of adult male rhesus macaques found that significant reductions in abnormal behavior occurred soon after social introduction, but were no longer significant 5–9 months after introduction [Doyle et al., 2008]. Improvements in abnormal behavior may be relatively ephemeral in males. Alternatively, potential sex differences in the types of abnormal behavior displayed may bear future research to explore whether abnormal behaviors particularly resistant to treatment or long-term improvement predominate in males. In the current study, the level of abnormal behavior in singly housed males was relatively low (3.6% of samples, lower than what was achieved among female subjects in social housing), and the failure to respond to changes in housing may relate to the relative rarity of abnormal behaviors. Nonetheless, when a primary goal is to substantially reduce abnormal behavior over the long term, intervention beyond social housing may be required for male rhesus macaques.
The findings of the current study demonstrate that the frequent separation and reunion associated with intermittent contact housing preserved the reductions in undesirable behaviors seen with continuous pair housing, contradicting Hypothesis 2. In contrast to predictions based on the literature, which indicates that repeated separations and reunions may be stressful, the current study found that intermittent contact did not result in increases in abnormal behavior or stress-related behaviors. Perhaps this is because there was a routine of repeated separation and reunion for the current study subjects when housed in intermittent contact, whereas most other published literature assessed one-time events. However, it must be noted that behavior during the separation period was not assessed, so levels of behavior in temporary single housing, in comparison to protected contact, or, for that matter, long-term single housing, are not known. A dramatic diminution in wellbeing would warrant avoiding the use of intermittent contact social housing. Anecdotal but very frequent observation of behavior during very short-term separation does not support the idea that it is met with distress among adults [personal observation]. Absent such a response, the need for frequent separations would not be an appropriate justification for single housing, and adult rhesus macaques involved in research requiring frequent separations should be socially housed in the absence of scientific, clinical or behavioral justifications for single housing. However, because behavior during separation was not assessed in the current study, employing intermittent contact when it is not strictly required (e.g., to “give monkeys a break from each other”) cannot be recommended based upon the current study.
The only potentially negative contrast between full and intermittent contact pertained to male agonism. Unlike females, males showed more agonistic behavior in intermittent contact than in protected contact. While this finding may be in keeping with male response to reintegration into a group setting [Gust et al., 1993], it is important to note that levels of agonism in intermittent contact were equivalent to those in full contact, so there was no problematic increase in agonism associated with intermittent contact housing. Also, in none of the male pairs and in none of the social housing conditions did the observed agonism include contact aggression. And the relatively high level of agonism was not associated with a higher level of anxiety-related behavior in comparison to full contact, suggesting that the agonism experienced did not result in distress to the animals. It may, in fact, be a manifestation of ritualized rank-related behavior which can serve as a social cohesive mechanism. Nonetheless, when frequent separation is necessitated by research or other needs, managers may want to consider moving male rhesus macaques with worrisome levels of agonism to protected contact rather than resorting to separation into single housing. Additional research using data sampling methodology better designed to capture behaviors of short duration would also be prudent.
In contrast, placing partial barriers between caged rhesus macaque pairs comes at a price, contrary to Hypothesis 3. Protected contact housing did not improve activity levels as did the other forms of social housing. One potential implication of this finding is that the increased locomotion and decreased inactivity associated with the other forms of social housing can be attributed simply to the increased available space for each animal, rather than the opportunity for social interaction, which remained possible in protected contact. It is possible that if additional space had been provided to each animal housed in protected contact, the same reduction in inactivity would have occurred. However, this result would be somewhat surprising given the prior literature on increases in cage space [Crockett et al., 1994, 1995, 2000; Kaufman et al., 2004; Line et al., 1990]. It is also possible that the control over proximity provided in protected contact obviated the need for shifts in location or avoidance of dominant animals, which is in fact one rationale for using this form of housing. Regardless of the reason, protected contact was not able to elicit increased locomotion and decreased activity, and also reversed the improvements in anxiety-related behavior seen in both full and intermittent contact housing. This is an important finding given the above rationale for using this form of housing, in addition to the amelioration of possible stress brought on by repeated reunions with pair mates. Most importantly, protected contact dramatically reduced affiliative contact, despite the fact that the panels enabled these interactions. In other words, while protected contact provides the opportunity for affiliative contact, it is not fully exploited by the animals. Nonetheless, descriptively, for females, protected contact reduced by over 30% the levels of abnormal behavior seen in single housing. By this measure, and because it did allow and result in social contact that is impossible in single housing, protected contact housing is superior to single housing, but not as beneficial as full contact or intermittent contact housing.
In the current study, 20% of female introductions and 11% of male introductions were unsuccessful. It is important to point out that in the implementation of social housing programs, failures can occur at similar or even greater rates. This does not of course, suggest that these proportions of individuals are unpairable. In practice, alternative social partners are sometimes available, but often the pool of potential candidates is limited so that not all individuals can be socially housed. Many environmental enhancements can be implemented and evaluated broadly across a population of animals with relatively little concern for adverse responses in some individuals. In contrast, the results of social introductions must be considered for each individual. Pairing all eligible animals regardless of the nature of the interactions between pair mates would be detrimental to some animals’ welfare and safety. An evaluation of pair housing is therefore different from studies of other behavioral management techniques because humane care requires that the collection of behavior data in “poor responders” should be terminated and animals separated. Results of the current study reflect the behavioral consequences of successful social introductions rather than the indiscriminate application of pair housing.
It also would not be appropriate to generalize to other species the apparent inferiority of protected contact in comparison to full or intermittent contact. A study of female long-tailed macaques found no benefits of full contact over protected contact [Lee et al., 2012]. A carefully controlled comparison of female long-tailed and rhesus macaques found that the relatively high levels of abnormal and tension-related behavior in protected contact in comparison to full contact, was seen only in the rhesus macaques [Baker et al., 2012b]. Given that these two closely related species seem to show different responses to protected contact housing, it is likely that other species will also differ in the response to this form of housing. In addition, it will be important to evaluate partition styles, both those employed in the current study but additional styles as well. Creating holes in solid panels is a relatively simple and inexpensive retrofit, but the use of widely spaced bars may better accommodate grooming actions and other forms of contact as well as facilitate visual access during interactions. This type of partition was employed with the long-tailed macaques studied by Lee et al. [2012] and Baker et al. [2012b], and may have contributed to the apparent species difference. Further research on alternative partition styles should be conducted before drawing wide-scale conclusions regarding the relative unfavorability of protected contact housing as a tool for providing social contact to caged nonhuman primates.
Several potential confounds were present in the current study. Aside from the initial phase of single housing, the order of the social housing phases was deliberately varied. If it had not been, then there would have been a consistent relationship between housing styles and prior familiarity and social experience with the partner. However, altering the order increased the variation in prior familiarity within a particular social housing style, which could not be controlled for and may have introduced its own confounds. In addition, practical constraints created variation in phase duration, and changes in behavior within phases was not assessed. While none of these potential confounds were systematic or related to behavior of the animals, they may reduce confidence in the findings among some readers and bear mentioning.
The contrasts between housing conditions were broadly similar in female and male rhesus macaques. This finding has particular practical significance given the fairly common skepticism regarding the suitability of adult male rhesus macaques as candidates for pairing. In both sexes, full and intermittent contact housing resulted in decreases in anxiety-related behavior, and protected contact was inferior as measured by these behaviors. Also, full contact increased locomotion over single housing and over protected contact as well. Lastly, levels of affiliative behavior were seen at high levels in full and intermittent contact housing and were significantly lower in protected contact. Broadly speaking, decisions about the style of pair housing to use for rhesus macaques do not need to take sex into account.
Another key finding of this study is that pairing female rhesus macaques with a history of SIB produced no undesirable effects in any of the three forms of social housing, contrary to Hypothesis 4. No self-injury occurred in any of the three forms of pair housing nor during the social introduction process, which suggests that individuals with a history of SIB are not a more vulnerable or less appropriate population for pairing. However, a complete absence of social contact history can sometimes underlie the expression of SIB, although it did not among the study subjects. It may be that the onset of social contact could incite SIB in animals with no prior social experience, and introduction success rates and the benefits of pair housing for individuals reared and housed in this manner cannot be assumed from results of the current study.
Also, while not assessed, it is possible that among subjects of this study, there were short-term negative responses (e.g., elevations in abnormal behavior or anxiety-related behavior) among females with a history of SIB during introduction and short-term adjustment to social housing or the transition to different forms of social housing. This suggestion remains to be explored. Nonetheless, in the long term, there was no evidence of increases in undesirable behaviors in any social housing style. Since social housing shows promise as an intervention for treating SIB [Weed et al., 2003], these results are encouraging. However, in the current study, despite the sometimes short duration of self-biting and possible undersampling, it must be noted that pair housing did not extinguish self-biting. Across both sexes, self-biting without wounding was recorded at least once in four subjects during data collection in single housing, two subjects during the full-contact phase, four in the intermittent contact phase, and five in the protected contact phase (no subjects self-bit in all four phases). Pair housing simply cannot be considered a cure for SIB [Reinhardt & Rossell, 2001]. Results did not suggest that protected contact housing was superior to full or intermittent contact with regard to treating SIB as we had hypothesized. Among this small set of subjects, the reductions in abnormal and anxiety-related behavior in full and intermittent contact across all subjects lost statistical significance. Descriptively, however, the reduction in abnormal behavior was more dramatic in females with a history of SIB (57%) than among females without a history of SIB (44%), particularly with reference to appetitive abnormal behaviors (data not shown). Concerns over the use of social housing for macaques displaying SIB appear unfounded, and in fact it may be, particularly effective for these animals.
Frequent separation of pairs does not appear to detract from the behavioral benefits of pair housing adult rhesus macaques. The ability to interact through a barrier is inferior to free access but showed modest improvements over single housing nonetheless. While the effects of the form of protected contact housing used in the current study did not mirror the benefits of full contact, based upon the benefits documented here we believe that protected contact should be considered a restricted type of social housing for rhesus macaques rather than a form of single housing. However, because protected contact confers fewer benefits than full contact housing, it is appropriate for the proposed use of protected contact to receive review by animal care and use committees as a restricted form of social housing. This recommendation contradicts the Guide for the Care and Use of Laboratory Animals [National Research Council, 2011] which seems to categorize protected contact housing as a form of single housing (as described in the Introduction section of this paper), and certainly has been interpreted to do so in policy statements (e.g., the Association of Primate Veterinarians (2012) Socialization Guidelines for Nonhuman Primates in Biomedical Research). While the findings of the current study suggest that this categorization may not be an appropriate stance, it does support the statement made by Association for the Assessment and Accreditation of Laboratory Animal Care, International on their website that “Full time social housing is the optimum manner to provide social experience. However, when full time housing with conspecifics is not possible, whether due to social incompatibility, veterinary concerns or scientific necessity, other social experiences should be considered such as part time access (e.g., overnight, when the animals are between studies, defined periods of time during the day, etc.) to full contact with conspecifics or protected contact that allows interaction through a mesh panel, grooming bars or other type of perforated barrier on either a part or full time basis.” (2012).
This study adds to the scientific literature used to guide the implementation of pair housing to improve the psychological well-being of captive rhesus macaques. This type of research is particularly valuable given the increased emphasis on social housing for laboratory primates [National Research Council, 2011].
Acknowledgments
Contract grant sponsor: NIH; contract grant number: RR0164 (TNPRC), RR0165 (YNPRC); contract grant sponsor: Office of Research Infrastructure Programs; contract grant number: OD011104 (TNPRC), P51OD011132 (YNPRC)
All research complied with the US Department of Agriculture Animal Welfare Regulations. The authors thank Maddalena Baker for proofreading and a thoughtful critique. The authors also thank the husbandry and behavioral management staff involved in the care of the monkeys at both centers.
Footnotes
Conflict of interest: None.
References
- Association for Assessment and Accreditation of Laboratory Animal Care International. Frequently asked questions, Section D. [Accessed December 1, 2012];Animal environment, housing and management. 2012 Available online at: http://www.aaalac.org/accreditation/faq_landing.cfm#C6.
- Association of Primate Veterinarians. [Accessed December 1, 2012];Socialization Guidelines for Nonhuman Primates in Biomedical Research. 2012 Available online at: http://www.primatevets.org/education.
- Baker KC, Weed JL, Crockett CM, Bloomsmith MA. Survey of environmental enhancement programs for laboratory primates. Am J Primatol. 2007;69:377–394. doi: 10.1002/ajp.20347. [DOI] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, et al. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Appl Anim Behav Sci. 2012a;137:148–156. doi: 10.1016/j.applanim.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Crockett CM, Lee GH, et al. Pair housing for caged female longtailed and rhesus macaques: behavior in protected contact versus full contact. J Appl Animal Welf Sci. 2012b;15:126–143. doi: 10.1080/10888705.2012.658330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne KA, Dexter S, Suomi S. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Anim. 1992;21:38–46. [Google Scholar]
- Bayne KA, Haines M, Dexter S, Woodman D, Evans C. Non-human primate wounding prevalence: a retrospective analysis. Lab Anim. 1995;24:40–44. [Google Scholar]
- Crockett CC, Bowers CL, Bowden DM, Sackett GP. Sex differences in compatibility of pair-housed adult longtailed macaques. Am J Primatol. 1994;32:73–94. doi: 10.1002/ajp.1350320202. [DOI] [PubMed] [Google Scholar]
- Crockett CC, Bowers CL, Shimoji M, et al. Behavioral responses of longtailed macaques to different cage sizes and common laboratory experiences. J Comp Psychol. 1995;109:368–383. doi: 10.1037/0735-7036.109.4.368. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Bellanca RU, Bowers CL, Bowden DM. Grooming-contact bars provide social contact for individually caged laboratory macaques. Contemp Top Lab Anim Sci. 1997;36:53–60. [PubMed] [Google Scholar]
- Crockett C, Shimoji M, Bowden DM. Behavior, appetite, and urinary cortisol responses by adult female pigtailedmacaques to cage size, cage level, room change, and ketamine sedation. Am J Primatol. 2000;52:63–80. doi: 10.1002/1098-2345(200010)52:2<63::AID-AJP1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol Psychiatry. 2008;63:990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LA, Baker KC, Cox LD. Physiological and behavioral effects of social introduction on adult male rhesus macaques. Am J Primatol. 2008;70:542–550. doi: 10.1002/ajp.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. Psychological well-being in paired adult female rhesus (Macaca mulatta) Am J Primatol. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Fernström AL, Sutian W, Royo F, et al. Stress in cynomolgus monkeys (Macaca fascicularis) subjected to long-distance transport and simulated transport housing conditions. Stress. 2008;11:467–476. doi: 10.1080/10253890801903359. [DOI] [PubMed] [Google Scholar]
- Gilbert MH, Baker KC. Social buffering in adult male rhesus macaques (Macaca mulatta): effects of stressful events in single vs. pair housing. J Med Primatol. 2011;40:71–78. doi: 10.1111/j.1600-0684.2010.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon TP, Gust DA. Return of juvenile rhesus monkeys (Macaca mulatta) to the natal social group following an 18-week separation. Aggr Behav. 1993;19:231–239. [Google Scholar]
- Gordon TP, Gust DA, Wilson ME, et al. Social separation and reunion affects immune system in juvenile rhesus monkeys. Physiol Behav. 1992;51:467–472. doi: 10.1016/0031-9384(92)90166-y. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK. Response to removal from and return to a social group in adult male rhesus monkeys. Physiol Behav. 1993;53:599–602. doi: 10.1016/0031-9384(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiol Behav. 1994;55:681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Gust DA, Wilson ME, Stocker T, et al. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocrin Metab. 2000;85:2556–2563. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- Kaufman BM, Pouliot AL, Tiefenbacher S, Novak MA. Short and long-term effects of a substantial change in cage size on individually housed, adult male rhesus monkeys (Macaca mulatta) Appl Anim Behav Sci. 2004;88:319–330. [Google Scholar]
- Lee GN, Thom JP, Chu KL, Crockett CM. Comparing the relative benefits of grooming-contact and full-contact pairing for laboratory-housed adult female Macaca fascicularis. Appl Anim Behav Sci. 2012;137:157–165. doi: 10.1016/j.applanim.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line SW, Morgan KN, Markowitz H, Roberts JA, Riddle M. Increased cage size does not alter heart rate or behavior in female rhesus monkeys. Am J Primatol. 1990;20:107–113. [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Mineka S, Suomi SJ, DeLizio R. Multiple separations in adolescent monkeys: an opponent-process interpretation. J Exp Psychol. 1981;110:56–85. doi: 10.1037//0096-3445.110.1.56. [DOI] [PubMed] [Google Scholar]
- National Research Council (Institute of Laboratory Animal Resources) Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- National Research Council (Institute of Laboratory Animal Resources) Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 2011. [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Survey of environmental enhancement for research macaques. Lab Primate Newsl. 1994;33:1–2. [Google Scholar]
- Reinhardt V, Rossell M. Self-biting in caged macaques: cause, effect, and treatment. J Appl Anim Welf Sci. 2001;4:285–294. [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Suarez A. Effects of social and inanimate enrichment on the behavior of yearling rhesus monkeys. Am J Primatol. 1996;40:247–260. doi: 10.1002/(SICI)1098-2345(1996)40:3<247::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Thom J, Crockett CM. Managing environmental enhancement plans for individual research projects at a National Primate Research Center. J Am Assoc Lab Anim Sci. 2004;47:51–57. [PMC free article] [PubMed] [Google Scholar]
- Tiefenbacher S, Novak MA, Jorgensen MJ, Meyer JS. Physiological correlates of self-injurious behavior in captive, socially-reared rhesus monkeys. Psychoneuroendocrinology. 2000;25:799–817. doi: 10.1016/s0306-4530(00)00027-5. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture. Final rules: code of federal regulations, title 9, part 3. Fed Regist. 1991;55:6426–6505. [Google Scholar]
- Weed JL, Wagner PO, Byrum R, et al. Treatment of persistent self-injurious behavior in rhesus monkeys through socialization: preliminary report. Contemp Top Lab Anim Sci. 2003;42:21–23. [PubMed] [Google Scholar]