Abstract
Background
Perception of alcohol intoxication presumably plays an important role in guiding behavior during a current drinking episode. Yet, there has been surprisingly little investigation of what aspects associated with intoxication are used by individuals to attribute their level of intoxication.
Methods
Building on recent laboratory-based findings, the current study employed a complex field-based design to explore the relative contributions of motor performance versus cognitive performance – specifically executive control – on self-attributions of intoxication. Individuals recruited outside of bars (N = 280; mean age = 22; range: 18–32) completed a structured interview, self-report questionnaire, and neuropsychological testing battery, and provided a breath alcohol concentration (BrAC) sample.
Results
Results of a multiple linear regression analysis demonstrated that current level of subjective intoxication was associated with current alcohol-related stimulant effects, current sedative effects, and current BrAC. After controlling for the unique variance accounted for by these factors, subjective intoxication was better predicted by simple motor speed, as indexed by performance on the Finger Tapping Test, than by executive control, as indexed by performance on the Trail Making Test.
Conclusions
These results – generated from data collected in a naturally occurring setting – support previous findings from a more traditional laboratory-based investigation, thus illustrating the iterative process of linking field methodology and controlled laboratory experimentation.
Keywords: alcohol, subjective intoxication, motor, executive control, field methodology
Introduction
Ethanol exerts a variety of effects ranging from euphoria, social facilitation and stimulation, to motor impairment, cognitive disruptions, sedation and other aversive outcomes. Production of specific alcohol effects is dependent on a number of factors, including the amount and timing of alcohol exposure. For instance, euphoric and stimulating effects are more prevalent as alcohol levels rise shortly after administration, and sedative and motor impairing effects predominate as alcohol levels decline during the descending limb of the alcohol time-concentration curve (e.g., see Newlin and Thomson, 1990). Differential sensitivity to these and other alcohol effects, revealed in studies of humans and laboratory animals, is the result of an array of critical factors. These include, but are not limited to, the organism’s history of alcohol use (King et al., 2002; Varlinskaya and Spear, 2010), the impact of stressors (Grzywacz and Almeida, 2008), and the organism’s ontogeny (Varlinskaya et al., 2010). Indeed, adolescents often display greater sensitivity than do adults to the social facilitatory, rewarding, and cognitive impairing effects of alcohol, but attenuated sensitivity to its sedative, motor impairing and aversive effects (Achesonet al., 1998; see Spear, 2011; Spear and Varlinskaya, 2005; White and Swartzwelder, 2005, for reviews). Neural substrates underlying these various ethanol effects also differ (e.g., see Eckardt et al., 1998).
Abundant evidence exists that sensitivity to these separable effects of alcohol may influence the propensity to continue drinking or terminate a drinking episode by affecting the experience of intoxication and the self-perception of drunkenness. Indeed, individuals with a history of high levels of alcohol use often display accentuated responses to the stimulating and euphoric effects evident during the rising phase, while being less sensitive than light drinkers to various intoxicating effects of ethanol during the descending phase (King et al., 2002; Quinn and Fromme, 2011). However, the extent to which these differences pre-date extensive alcohol use or are a consequence of that use remain unclear. Studies in both heavy drinkers and individuals with a family history of alcoholism have found that these groups at high risk for development of alcohol use disorders are characterized by a general insensitivity to the intoxicating effects of alcohol, at least during the often extended period of time following the initial rapid rise in alcohol levels (Quinn and Fromme, 2011; Schuckit and Gold, 1988; Schuckit et al., 2010).
Despite such evidence of specific effects, self-perceptions of alcohol intoxication and level of drunkenness are often described more globally in the vernacular (e.g., “drunk,” “wasted,” and “wrecked”). Such global self-perceptions likely serve to guide behavior during intoxication – including, for example, decisions to continue drinking or to drive an automobile. Yet, there has been surprisingly little investigation of what specific aspects associated with ethanol intoxication are used by individuals to attribute their level of intoxication. According to Bem’s (1967) self-perception theory, individuals look at their own behavior when deriving attributions about how they feel. From this perspective, one might expect that readily detectable behavioral concomitants of drinking (such as basic motor functions and level of sedation) might be more strongly related to self-attributions of intoxication than less-readily perceived effects of ethanol (such as intoxication-related disruptions in cognition). Indeed, in a recent laboratory study comparing perceived levels of intoxication with cognitive functioning following alcohol challenge, measures of visuomotor speed and perceived levels of intoxication both showed evidence of acute tolerance (i.e., more subjective intoxication and slower visuomotor speed on the rising than descending limb of the curve of alcohol concentration over time), whereas no acute tolerance emerged on executive function and memory tasks (Cromer et al., 2010). As a result, during the course of the intoxication period there became progressively less correspondence between levels of subjective intoxication and impairment in executive function. This escalating dissociation between subjective intoxication and impaired executive function during the elimination phase led the authors to suggest that alcohol-induced disruptions in executive function may have less impact on self-perceived intoxication than impairment in basic visuomotor performance. Whether these discrepancies in the relevance of motor versus cognitive cues to self-perceived intoxication would hold in a more naturalistic setting was unknown and was the focus of this investigation.
Using a field-based approach, the specific aim of the present study was to explore the relative contributions of performance on a simple motor task (the Finger Tapping Test) versus an executive control task (the Trail Making Test) on self-attributions of intoxication in a sample of individuals recruited outside of bars. The influence of alcohol on these measures of motor and cognitive performance was examined in conjunction with breath alcohol concentration (BrAC), as well as self-reported past year drinking pattern, and ratings of alcohol-related stimulatory and sedative effects. Our predictions reflect both the work of Cromer, et al., noted above, as well as other findings that acute tolerance has been observed for speed of cognitive processing (e.g., reaction time), but not for cognitive errors (see Schweizer & Vogel-Sprott, 2008, for a review). Thus, we hypothesized that self-rated intoxication would be more strongly associated with performance on the simple motor speed task than performance on the executive control task.
Materials and Methods
Setting and Participants
Data collection took place within a bar district of a small metropolitan area, consisting of eight bars within a city block. All procedures were approved by the university IRB. As part of a larger study, 1433 participants were recruited outside of bars to complete a brief interview and survey. From this larger sample, 312 randomly selected participants (of approximately 450 invited) agreed to complete an additional testing component. Random selection was accomplished by pre-labeling 33% of the survey packets with a cover sheet that prompted research staff to invite that participant for additional testing. Nine cases (i.e., 3%) were removed due to invalid responding, defined as a clear pattern of gross deviation from the response choices provided on the standardized survey. Twenty-three additional cases were removed due to missing data, resulting in a final sample of 280 participants.
On each of 31 nights (15 Thursdays and 16 Fridays), recruitment began at 11:00pm and concluded by 2:30am. The research team comprised 8 to12 trained research assistants supervised by the project director. Recruitment procedures were conducted by groups of three to four research assistants positioned at varying points within the identified area (see Figure 1). Research assistants were trained to approach potential participants and deliver a brief informational statement about the purpose of and procedures involved in the investigation. During the consenting process, research assistants were instructed to evaluate whether individuals displayed overt symptoms of severe intoxication (e.g., grossly incoherent speech, inability to stand). Such individuals were not invited to participate, not only due to concerns about ability to provide informed consent, but also because our previous recruitment efforts demonstrated that such individuals were unable to complete the basic elements of the protocol (e.g., answering questions in interview format, completing a paper and pencil survey while standing). Those who agreed to participate were asked to complete a series of self-report questionnaires that took approximately six minutes. A random subset of participants was invited to continue to the testing station after completing the survey phase.
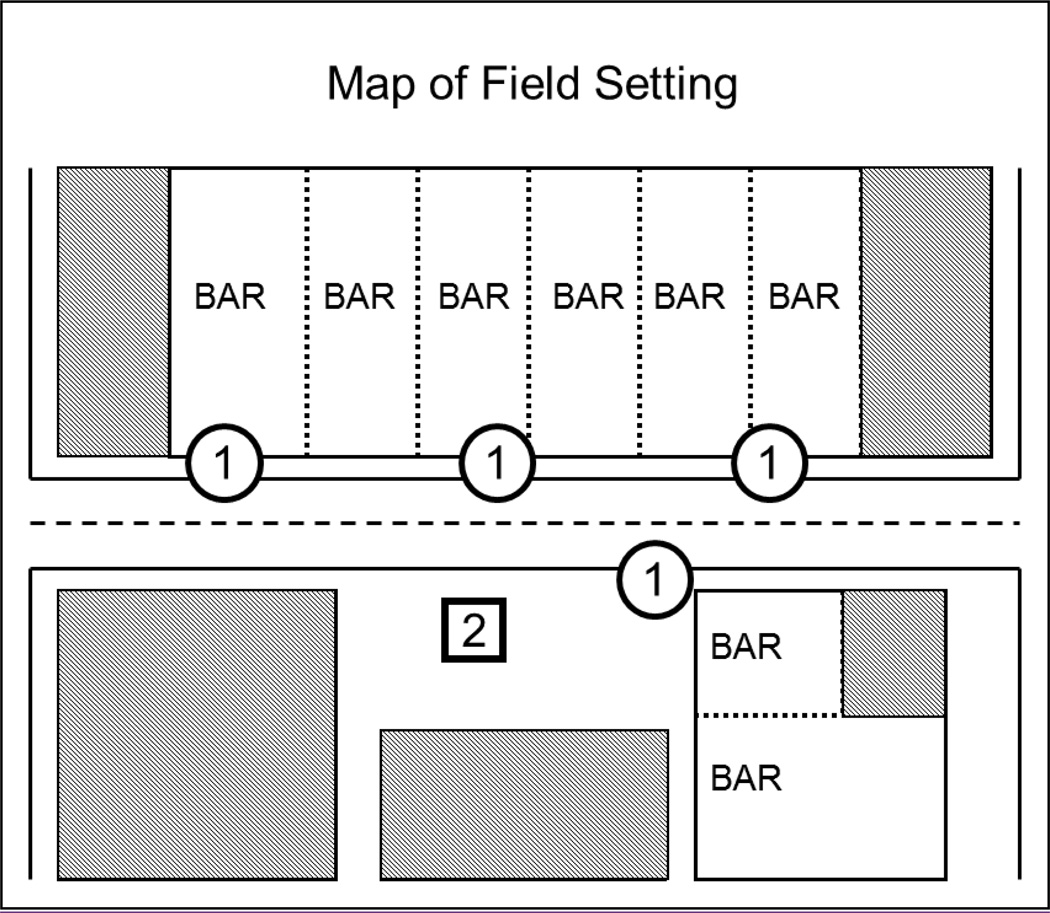
Figure 1.
Overhead map of recruitment area
Note. Circles labeled “1” represent the sidewalk areas where research assistants recruited participants and conducted the consent, interview, and survey. The square labeled “2” represents the research station where the testing battery was conducted.
The testing phase of the protocol was administered within a 10-by-10 foot enclosed testing station sub-divided into two separate, well-lit work areas. Participants completed one cognitive and one motor test (see measures section below) and provided two breath samples; one at the start and again at the end of the approximately eight-minute testing protocol. Collectively, the consent, survey, and testing procedures took at least 15 minutes, thereby ensuring that adequate time had passed for residual alcohol in the oral cavity to dissipate between the participant’s last drink and their final BrAC measurement (Caddy et al., 1978).
Upon completion of the protocol, participants were provided with feedback regarding their current BrAC using a graphic that displayed three risk levels labeled “safe” (less than .02), “caution” (.02 to .07), and “danger” (.08 or higher). Participants were informed that their actual BrAC would be available online the next day, and were provided with a wallet-sized card with their unique identification number and passcode for anonymous access to the dedicated study webpage. These cards also included contact information for the University Counseling Center and local addiction services.
Measures
The questionnaire packet included an assessment of relevant demographics. Participants were asked to indicate the amount of time (in minutes) since their last drink. In addition, participants were asked to rate their current level of intoxication using a single, 11-point item anchored by 0 indicating “not at all intoxicated” and 10 indicating “extremely intoxicated.” Other subjective effects of current alcohol use were assessed using a modified version of the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993). The BAES is a 14-item self-report scale designed to assess stimulant and sedative effects of alcohol. Due to the time limitations inherent in field research, we selectively administered three stimulant items (“excited,” “talkative,” and “up”) and three sedative items (“inactive,” “sedated,” and “sluggish”). These items were derived through statistical analysis of pilot data using the 14-item BAES. Specifically we ran a principle component factor analysis on the full BAES and selected the three stimulant and three sedative items with the highest factor loadings. Four out of the six resulting items overlap with those items included in the Brief-BAES (Rueger et al., 2009). Participants were asked “to what extent has drinking produced each of these feelings right now?” Each item was scored on an 11-point item anchored by 0 indicating “not at all” and 10 indicating “extremely.” A mean score was calculated separately for stimulant and sedative effects.
The Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001) was included in the survey both because of its brevity and because it is a global measure of one’s alcohol-related risk and associated problems over the past 12 months. The AUDIT yields a score ranging from 0 (no alcohol-related risk) to 40 (maximum alcohol-related risk), with a score of 8 or greater suggesting a hazardous drinking pattern and possible alcohol use disorder. The reliability and construct validity of the AUDIT have been demonstrated in laboratory and field settings (Celio et al., 2011; Reinert and Allen, 2007).
The testing phase consisted of two brief neuropsychological tests administered in the following order: The Trail Making Test (TMT; Army Individual Test Battery, 1944; Reitan, 1958) and the Finger Tapping Test (FTT; Halstead, 1947; Reitan and Wolfson, 1993). The TMT is a measure of executive control, which includes elements of visuospatial scanning, divided attention, working memory, and overall cognitive flexibility (Lezak et al., 2004). The TMT has been found to be sensitive to the acute effects of alcohol intoxication (Guillot et al., 2010). In accordance with standardized administration procedures (Reitan, 1958), we administered the Part A sample and timed trial, followed by the Part B sample and timed trial. Completion time was recorded for both Part A and Part B. The TMT Composite Score (Part B completion time minus Part A completion time; see Lezak et al., 2004) was used to remove the motor speed element from the test evaluation, resulting in a more refined index of executive control and cognitive performance (Arbuthnott and Frank, 2000, Corrigan and Hinkeldey, 1987). Higher scores indicate worse performance.
The FTT is a measure of simple motor performance. The FTT has been shown to be sensitive to the effects of alcohol abuse, and improvement in test performance has been observed in alcoholics following cessation of drinking (Goldman et al., 1983). In an effort to reduce the time of the overall testing battery, we administered three 10-second trials for dominant hand only instead of five 10-second trials for both hands. Handedness was determined based on self-report. Participants had approximately 10 seconds between trials to recover from potential fatigue. Total number of taps was recorded for each trial, and the overall FTT score was computed as the average number of taps across three trials. Higher scores indicate better performance.
Breath samples were collected using two hand-held Breath Alcohol Concentration test units (CMI Intoxilyzer 400PA; CMI, Inc., Owensboro, KY; manufactured in 2009), which were calibrated monthly according to manufacturer specifications. As previously indicated, two BrAC measures were collected for each participant: one at the onset of the testing protocol and another at the termination of the testing protocol. The second BrAC measure was used in all cases (although it should be noted that the two measures were correlated highly, r = .92, p < .001).
Statistical Analyses
Descriptive statistics and graphics were used to examine each variable of interest and determine whether it was appropriate for parametric analyses. As none of these variables demonstrated gross violations of normality, a bivariate correlation examining the associations among the variables of interest was completed. Multiple linear regression with simultaneous entry was employed to determine the extent to which each variable of interest was uniquely associated with subjective intoxication. Age and gender were explored as a priori covariates. However, the inclusion of these demographic variables neither changed the observed pattern of results, nor did they add to the total variance accounted for by the regression model. Consequently, these variables were excluded from the analyses presented below. AUDIT total score was included in the model as a “historical predictor.” Stimulant and sedative effects were included in the model as “event-level subjective predictors.” Finally, BrAC, TMT, and FTT were included as “event-level objective predictors.” Collinearity diagnostics and residual plots were examined to assess for model violations (e.g., multicollinearity, nonlinearity, and heteroscedasticity). A Bonferroni correction was applied to adjust for multiple comparisons within the bivariate correlation; a p-value of less than .0083 was interpreted as statistically significant. Because regression analysis is sufficiently conservative without correction for multiple comparisons, a p-value of less than .05 was interpreted as statistically significant.
Results
The current sample of 280 participants was predominantly male (67%), Caucasian (79%), and college students (78%), with a mean age of 21.59 years. The descriptive statistics presented in Table 1 suggest that this is a heavy drinking sample, with the average participant having a BrAC of .09 (i.e., 90 mg/dl). The mean AUDIT score for the sample was 13.58, which is 5.58 points above the recommended cut-point for hazardous drinking and suggests possible alcohol use disorder; approximately 83% of this sample scored in the hazardous drinking range. On average, participants reported that 15.45 minutes had passed between their last drink and the onset of the consenting process (SD = 16.53; range: 0 – 120).
Table 1.
Descriptive statistics (N = 280)
| Variables | Mean (SD); Min - Max |
|---|---|
| 1. Subjective Intoxication | 4.81 (2.14); 0 – 10 |
| 2. BrAC a | .09 (.06); .00 – .32 |
| 3. AUDIT b | 13.58 (6.61); 2 – 32 |
| 4. Stimulant c | 7.23 (2.21); 0 – 10 |
| 5. Sedative d | 2.36 (2.35); 0 – 10 |
| 6. Trail Making Test – Part A e | 25.03 (10.04); 9 – 100 |
| 7. Trail Making Test – Part B e | 63.81 (27.66); 21 – 224 |
| 8. Trail Making Test - Composite e | 38.61 (22.78); 6 – 145 |
| 9. Finger Tapping Test – Trial 1 f | 46.18 (9.58); 14 – 69 |
| 10. Finger Tapping Test – Trial 2 f | 44.95 (9.35); 9 – 76 |
| 11. Finger Tapping Test – Trial 3 f | 43.28 (8.66); 16 – 68 |
| 12. Finger Tapping Test – Mean f | 44.80 (8.17); 15 – 67 |
BrAC = Breath Alcohol Concentration;
AUDIT = Alcohol Use Disorders Identification Test total score;
Stimulant = Subjective rating of stimulant alcohol effects;
Sedative = Subjective rating of sedative alcohol effects;
Higher scores on the Trail Making Test indicate worse performance;
Higher scores on the Finger Tapping Test indicate better performance.
In comparison to the 1153 individuals in the larger survey sample who were not included in the current analyses, the 280 participants of the present investigation were similar with regard to age despite the observed statistically significant difference, t (1428) = 3.37, p = .001, with a mean age of 21.59 years among the current sample (SD = 2.90; range: 18 – 32) and 21.02 years (SD = 2.42; range 17 – 35) in the larger project sample. These participants reported significantly higher subjective intoxication than the larger project sample, t (1426) = 3.31, p = .001, with a mean of 4.81 out of 10 (SD = 2.14) among the current sample and a mean of 4.27 out of 10 (SD = 2.52) among the larger project sample. These participants were comparable to the larger project sample with regard to gender, AUDIT score, stimulant effects and sedative effects.
Compared to normative data from a nonclinical sample of adults (age 20 – 39; Bornstein, 1985), the current sample means for both FTT and TMT (presented in Table 1) are within one standard deviation of the normative means. Furthermore, the observed TMT performance is equivalent to mean scores obtained from acutely intoxicated individuals tested under laboratory-based conditions (Guillot et al., 2010). Taken together, our performance-based measures appear to yield data comparable to previous investigations despite adaptation for field administration.
The results of the bivariate correlation analysis examining the associations between current intoxication and other variables of interest (see Table 2) demonstrated that subjective rating of intoxication was significantly correlated with BrAC, r = .30, p < .001. In addition, subjective intoxication was associated with subjective alcohol-related stimulant effects, r = .39, p < .001, and sedative effects, r = .16, p = .007. Significant correlations were observed between subjective intoxication and TMT performance, r = .19, p = .002, and FTT performance, r = −.24, p < .001. The observed correlation between subjective intoxication and AUDIT score (r = .10) was not significant. Other relevant first-order correlations of interest include the associations between BrAC and TMT, r = .49, p < .001, and between BrAC and FTT, r = −.24, p < .001, suggesting a dose-effect relationship between current BrAC level and task performance.
Table 2.
Zero-order correlations among the variables of interest
| Variables | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Intoxication a | --- | |||||
| 2. AUDIT b | .10 | --- | ||||
| 3. Stimulant c | .39* | .28* | --- | |||
| 4. Sedative d | .16* | .12 | .05 | --- | ||
| 5. BrAC e | .30* | .20* | .09 | −.02 | --- | |
| 6. TMT f | .19* | .09 | −.00 | .04 | .49* | --- |
| 7. FTT g | −.24* | −.04 | −.13 | −.12 | −.24* | −.16* |
Intoxication = Subjective rating of current intoxication;
AUDIT = Alcohol Use Disorders Identification Test total score;
Stimulant = Subjective rating of stimulant alcohol effects;
Sedative = Subjective rating of sedative alcohol effects;
BrAC = Breath Alcohol Concentration;
TMT = Trail Making Test (Composite Score) with higher scores indicating worse performance;
FTT = Finger Tapping Test (Dominant Hand) with higher scores indicating better performance.
p < .0083.
Finally, multiple regression analysis was used to test the unique contribution of event-level predictors of subjective intoxication. Note that all variables were entered simultaneously. The results (presented in Table 3) indicated that the six predictors explained approximately 26 percent of the variance, R2 = .264, F(6, 273) =16.30, p < .001. It was found that subjective intoxication was significantly predicted by BrAC (β = .22, p < .001), stimulant effects (β = .37, p < .001), sedative effects (β = .14, p = .01), and FTT performance (β = −.12, p = .03), whereas AUDIT score (β = −.07) and TMT performance (β = .06) were not significant predictors. Collinearity diagnostics and residual plots found no evidence of model violations.
Table 3.
Multiple linear regression of current intoxication
| Variables | B | SE | β | t | p |
|---|---|---|---|---|---|
| AUDIT | −.02 | .02 | −.07 | −1.28 | .20 |
| Stimulant | .35 | .05 | .37 | 6.71 | <.001 |
| Sedative | .12 | .05 | .14 | 2.58 | .01 |
| BrAC | 7.51 | 2.10 | .22 | 3.57 | <.001 |
| TMT | .01 | .01 | .06 | 1.03 | .31 |
| FTT | −.03 | .01 | −.12 | −2.20 | .03 |
Note. R2 = .26. All independent variables were entered simultaneously.
Discussion
Recent laboratory-based findings suggest that self-perceived intoxication may be more influenced by disruptions in basic motor responses than by alterations in higher-order cognitive functioning (Cromer et al., 2010). The aim of the current field-based investigation was to explore the relative contributions of performance on a simple motor task versus an executive control task on self-ratings of intoxication, with the hypothesis that self-rated intoxication would be better predicted by motor performance than by performance on the cognitive task. To this end, we employed a multi-phase design that included survey and neuropsychological testing components administered sequentially within a naturally occurring setting where drinking takes place. The results demonstrated that subjective intoxication was significantly predicted by motor speed, as indexed by performance on the FTT, but not by executive control, as indexed by the TMT composite score. In the context of self-perception theory (Bem, 1967), it is possible that the observed association between motor performance and subjective intoxication is at least in part due to the likelihood that motor disruptions (e.g., sluggishness and impaired dexterity) are more salient and therefore more easily detectible compared to disruptions in executive functions (e.g., organization, inhibitory control and decision making). This explanation is akin to the phenomenon in which individuals who are drinking while seated (e.g., in an alcohol challenge) frequently report an increase in their sensation of intoxication upon standing (N. E. Noel, personal communication, June 26, 2012).
As expected, current BrAC – a direct reflection of alcohol consumption – was a significant predictor of subjective intoxication. It is worth noting that the results of the zero-order correlation analysis demonstrated that TMT performance was more strongly related to BrAC than was FTT performance. Specifically, BrAC explains 24 percent of the variance in TMT performance (r2 = .24), versus 6 percent for FTT performance (r2 = .06). This observation is consistent with the suggestion that executive control is more closely tied to objective intoxication (i.e., BrAC), both of which are – in theory – less salient to the participant. In contrast, motor functioning appears to be closely tied to subjective intoxication, perhaps in part due to the increased salience of motor disruption. It is also notable that – despite the relatively small amount of variance accounted for (r2 = .03) – performance on the TMT and FTT are significantly correlated with each other. Hence, even though TMT was not found to be a statistically significant predictor of subjective intoxication, TMT performance may have exerted some influence on the current regression model.
In this study, much of the variance in subjective intoxication rating was associated with other event-level subjective ratings, specifically current stimulant effects (i.e., feeling energetic, talkative, and up) and current sedative effects (i.e., feeling inactive, sedated, and sluggish). While both of these subjective states were statistically significant predictors, stimulant effects accounted for a larger proportion of overall variance. It is important to note that stimulant effects were reported at a much higher level than sedative effects (7.23 out of 10.00 versus 2.36 out of 10.00, respectively). The observed difference in self-ratings of biphasic effects suggests that a large majority of the sample may have been in an ascending phase of the alcohol time-concentration curve at the time of participation, which offers some explanation for the relative strength of association between stimulant effects and subjective intoxication. In contrast, the potential floor effect in sedative effects, which is likely a product of having fewer individuals in advanced stages of the descending limb of the alcohol time-concentration curve, may serve to explain the significant yet relatively weak (r2 = .03) association between sedative effects and subjective intoxication. Another alternative could be that the natural context may in part drive heightened ratings of stimulant effects. In line with this point, King, de Wit, McNamara, and Cao (2011) observed stronger stimulant (versus sedative) effects even on the descending limb of the alcohol curve in an investigation conducted in a quasi-social context.
With regard to our historical predictor, alcohol-related risk pattern – as indexed by the AUDIT score– was not a significant predictor of subjective intoxication. Although the effect did not reach statistical significance, we observed the expected trend between AUDIT and subjective intoxication such that individuals with higher AUDIT scores reported lower levels of subjective intoxication. It may be noteworthy that much of the current sample was characterized by high-risk drinking, with 83% of individuals reporting a hazardous drinking pattern (i.e., AUDIT total score ≥ 8) over the past 12 months. It is possible that the association between AUDIT score and subjective intoxication might have been significant if there were more low-risk drinkers represented in the sample.
The main strength of the current study is the employment of a complex field-based design allowing for multi-method data collection through self-report questionnaire, standardized neuropsychological assessment procedures, and BrAC measurement. In general, field methodology offers the potential to access unique samples (e.g., underage individuals in intoxicated states), observe naturally occurring phenomena, and investigate events not easily replicated in laboratory settings. The current study featured a relatively large sample (N = 280) with 38% of individuals under age 21, and 50% of individuals currently engaged in an alcohol binge (i.e., BrAC ≥ 0.08; NIAAA National Advisory Council, 2004). While access to intoxicated populations in naturally occurring settings clearly provides creative research opportunities, it is also the case that increases in ecological validity are accompanied by sacrifices in experimental control.
Indeed, not only the strengths but also the limitations of the current study largely lie in the employment of a field-based design. For example, the correlational design of the current study does not allow for baseline testing (i.e., prior to the onset of drinking – when BrAC = 0); therefore the performance measures employed do not reflect a pure measure of alcohol-induced impairment, nor can we rule out the possible role of a third variable. For example, the reciprocal association between alcohol-induced restriction of attention and concomitant reduction in emotional responsiveness (“alcohol myopia”; see review by Giancola et al., 2010) could impact self-perceived intoxication. In addition, participants in the current sample differ in terms of their past exposure to alcohol (as indexed by the AUDIT), and, by extension, could differ in terms of acute alcohol tolerance. With specific regard to the observed positive association between Trail Making performance and BrAC, it is possible that individuals with lower cognitive functioning may be more likely to drink to higher BrACs. That being said, laboratories studies that have employed more rigid experimental control have documented similar dose-effect relationships between alcohol and brief neuropsychological test performance (e.g., Guillot et al., 2010).
The somewhat restricted time frame over which our target population was assessed during their current drinking episode provides an additional limitation. The data support a rich sampling of individuals who are still in the course of their drinking episode, making it difficult to accurately assess phase of the alcohol time-concentration curve. In line with this point, it is also notable that the most severely intoxicated individuals were not represented. The decision to exclude such individuals was a procedural necessity in this naturally occurring context, given that participation required that individuals maintain a coherent dialogue, complete a paper and pencil survey while standing, and complete two brief yet involved neuropsychological tests.
The current sample of 280 participants was found to differ statistically from those participants in the larger project sample who were not included (n = 1153), in that the current sample was slightly older (i.e., 21.59 years versus 21.02 years), reported more standard drinks consumed, and reported higher levels of subjective intoxication. These differences may reflect a form of selection bias, with participants who drink more and feel more acutely intoxicated showing greater interest when invited to participate in the testing phase of this project as a means of obtaining feedback on their current BrAC. This is consistent with the statistically derived observation that participants who chose to access the project web-site the next day to obtain feedback on their exact BrAC had significantly higher BrACs than those who did not seek out this information (Celio et al., 2011). The loss of 32 cases due to missing and invalid data is another source of potential bias, and future field investigations should employ rigorous data collection procedures to maximize the rate of complete and valid cases. In light of these observations, it is important to note that the current sample is relatively large and reflects the full range of subjective intoxication and objective alcohol consumption, with normally distributed subjective intoxication ratings ranging from 0 (not at all) to 10 (extreme), and BrAC measurements ranging from .00 to .32.
In closing, the current findings support the hypothesis that self-rated intoxication is predicted more by motor performance than by performance in cognitive functions. This study represents an important step in a line of research, in that the current findings – generated from data collected in a naturally occurring setting – support previous findings from a more traditional laboratory-based investigation (Cromer et al., 2010). We view our findings as illustrating the iterative process of linking field methodology and controlled laboratory experimentation, with future work returning to a laboratory setting where it is feasible to employ a more comprehensive battery of motor and cognitive performance measures to examine, for example, precisely what aspects of motor performance disruption predict subjective intoxication. Advancements achieved through laboratory-based work would in turn provide additional findings to be validated in a naturalistic setting, thus continuing the iterative process.
Acknowledgments
Funding for this study was provided by the Binghamton University Professional Employees Council (PEC) Individual Development Award and The Center for Development and Behavioral Neuroscience (CDBN).
References
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: Age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. Geneva, Swizterland: World Health Organization; 2001. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care World Health Organization (WHO Publication No. 01.6a) [Google Scholar]
- Bem DJ. Self-perception: An alternative interpretation of cognitive dissonance phenomena. Psychol Rev. 1967;74:183–200. doi: 10.1037/h0024835. [DOI] [PubMed] [Google Scholar]
- Bornstein RA. Normative data on selected neuropsychological measures from a nonclinical sample. J Clin Psychol. 1985;41:651–659. [Google Scholar]
- Caddy GR, Sobell MB, Sobell LC. Alcohol breath tests: Criterion times for avoiding contamination for “mouth alcohol”. Behav Res Meth. 1978;10:814–818. [Google Scholar]
- Celio MA, Vetter-O’Hagen CS, Lisman SA, Johansen GE, Spear LP. Integrating field methodology and web-based data collection to assess the reliability of the Alcohol Use Disorders Identification Test (AUDIT) Drug Alcohol Depend. 2011;119:142–144. doi: 10.1016/j.drugalcdep.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol. 1987;43:402–408. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cromer JR, Cromer JA, Maruff P, Snyder PJ. Perception of alcohol intoxication shows acute tolerance while executive functions remain impaired. Exp Clin Psychopharm. 2010;18:329–339. doi: 10.1037/a0019591. [DOI] [PubMed] [Google Scholar]
- DeMartini KS, Carey KB. Optimizing the use of the AUDIT for alcohol screening in college students. Psychol Assess. 2012;24:954–963. doi: 10.1037/a0028519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Josephs RA, Parrott DJ, Duke AA. Alcohol myopia: Aggression and other acts of disinhibition through a distorted lens. Perspect Psychol Sci. 2010;5:265–278. doi: 10.1177/1745691610369467. [DOI] [PubMed] [Google Scholar]
- Grzywacz JG, Almeida DM. Stress and binge drinking: a daily process examination of stressor pile-up and socioeconomic status in affect regulation. Int J Stress Manage. 2008;15:364–380. doi: 10.1037/a0013368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot CR, Fanning JR, Bullock JS, McCloskey MS, Berman ME. Effects of alcohol on tests of executive functioning in men and women: A dose response examination. Exp Clin Psychopharmacol. 2010;18:409–417. doi: 10.1037/a0021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead WC. Brain and Intelligence. 1st ed. Chicago: University of Chicago Press; 1947. [Google Scholar]
- King AC, De Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, De Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism [NIAAA Web site] National Institute of Alcohol Abuse and Alcoholism Council approves definition of binge drinking. NIAAA Newsletter. 3 Available at http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.htm. [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The alcohol use disorders identification test: An update of research findings. Alcohol Clin Exp Res. 2007;31:185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skill. 1958;8:271–276. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Applications. 2nd ed. Tuscon, Az: Neuropsychology Press; 1993. [Google Scholar]
- Rueger SY, McNamara PJ, King AC. Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES. Alcohol Clin Exp Res. 2009;33:916–924. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol placebo challenger in sons of alcoholics and controls. Arch Gen Psychiatry. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS. Letter to the Editor: University of California, San Diego. Alcohol Clin Exp Res. 2010;34:203–205. [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: A review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharm. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent neurobehavioral characteristics, alcohol sensitivities and intake: Setting the stage for alcohol use disorder? Child Dev Perspect. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. In: Adolescence: Alcohol sensitivity, tolerance, and intake, in Recent Developments in Alcoholism, Volume 17, Alcohol Problems in Adolescents and Young Adults. Galanter M, editor. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 143–159. [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Sensitization to social anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats following repeated ethanol exposure. Alcohol. 2010;44:99–110. doi: 10.1016/j.alcohol.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. In: Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults, in Recent Developments in Alcoholism, Volume 17, Alcohol Problems in Adolescents and Young Adults. Galanter M, editor. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 161–176. [DOI] [PubMed] [Google Scholar]