Abstract
Deep brain stimulation was introduced as a treatment for patients with Parkinsonism and other movement disorders in the early 1990s. The technique rapidly became the treatment of choice for these conditions, and is now also being explored for other diseases, including Tourette syndrome, gait disorders, epilepsy, obsessive-compulsive disorder, and depression. Although the mechanism of action of DBS remains unclear, it is recognized that DBS works through focal modulation of functionally specific circuits. The fact that that the same DBS parameters and targets can be used in multiple diseases suggests that DBS does not counteract the pathophysiology of any specific disorder, but acts to replace pathologic activities in disease-affected brain circuits with activity that is more easily tolerated. Despite the progress made in the use of DBS, much remains to be done to fully realize the potential of this therapy. We describe some of the most active areas of research in this field, both in terms of exploration of new targets and stimulation parameters, and in terms of new electrode or stimulator designs.
Keywords: Parkinson’s disease, dystonia, subthalamic nucleus, internal pallidal segment, pedunculopontine nucleus, segregated circuits
Introduction
Over the past decade, electrical high-frequency deep brain stimulation (DBS) has emerged as a major therapeutic modality for movement disorders such as Parkinson’s disease (PD), tremor, and dystonia, and is being studied for other neurologic disorders, including Tourette syndrome, epilepsy, eating disorders, and headache. The use of DBS is also being explored for several neuropsychiatric disorders, including obsessive compulsive disorder and treatment resistant depression. DBS, a form of neuromodulation, is adjustable and reversible, and less invasive than lesioning approaches, making it useful not only for therapeutic but also for investigative purposes, when combined with neuroimaging, neurophysiologic and other techniques [1, 2]. In this review we focus on DBS for movement disorders (specifically, PD), discussing its historical roots, possible mechanism of action, current challenges and technological advances.
Development of DBS
The current use of DBS for movement disorders arose out of prior experience with ablative procedures such as thalamotomy and pallidotomy for the treatment of PD and tremor. These interventions, which were empirically developed, were widely performed in the 1950s, at a time when medical treatments of PD were not available [3, 4]. With the exception of thalamotomy for treatment of tremor, the use of ablative procedures rapidly declined following the introduction of levodopa for PD in the mid-1960s. However, over the next 20 years, it became clear that levodopa and other dopaminergic drugs have significant side effects and limitations, including drug-induced motor fluctuations and dyskinesias. For this reason, surgical treatment options for PD were revisited in the early 1990s. During the preceding decade, basic science research had indicated that increased and abnormal activity in basal ganglia output from the internal pallidum (GPi) contributes to the development of parkinsonian motor signs, thus providing a clear rational for pallidotomy [5]. Lesions of the posterior (sensorimotor) portion of GPi in patients with PD, placed with the help of newly developed neuroimaging techniques, better electrophysiologic recording and improved neurosurgical techniques, were subsequently demonstrated to abolish all of the cardinal features of the disorder (tremor, rigidity and akinesia), as well as drug-induced dyskinesias and motor fluctuations [e.g., ref. 6, 7]. The success with pallidotomy for PD subsequently also led to a revival of this procedure for dystonia, and laid the groundwork for the renewed acceptance of neurosurgical treatments for advanced PD and dystonia, which paved the way for DBS.
Although the specific method of chronic DBS was newly introduced in the early 1990s, the therapeutic effects of electrical stimulation of the brain were already known to neurosurgeons in the 1950s and 1960s [4]. Prior to making a lesion in the thalamus to treat tremor, these surgeons would observe the response to stimulation through the lesioning probe. Enhancement of tremor with low frequency stimulation (5–10 Hz), or its attenuation with high frequency stimulation (50–100 Hz), was taken as a favorable sign for placing a radiofrequency lesion in the same area. However, stimulation received little attention as a therapeutic technique for movement disorders until a report in 1987 described the successful use of chronic high frequency thalamic stimulation for tremor [8]. Following the demonstration of the antiparkinsonian effects of inactivation of the STN in MPTP-treated monkeys [9–11], studies demonstrating beneficial effects of STN-DBS were carried out in the same animal model [12]. These encouraging results lead to clinical trials of STN DBS in patients with PD, which was shown to be safe and highly effective [13–15]. Similarly positive results were also described for DBS of the GPi [16]. Because DBS has the advantage of being less invasive than lesioning, and DBS effects are adjustable, it did not take long for DBS to replace pallidotomy and other ablative procedures as the neurosurgical treatment of choice for PD patients (at least in developed countries).
DBS was also found to be effective against primary generalized and focal dystonia and is currently under study for other hyperkinetic disorders, specifically Tourette syndrome, in which DBS of the nodes of the basal ganglia circuits and caudal intralaminar nuclei of the thalamus (i.e., the centromedian/parafascicular nucleus (CM/Pf)) are being studied [e.g., ref. 17]
Circuit disorders and DBS targets
One of the reasons why focal high-frequency stimulation of specific brain regions via macroelectrodes is successful is that the stimulated brain networks are anatomically and functionally segregated [figure 1 and ref. 18]. DBS is primarily applied to the basal ganglia, where it acts upon highly segregated circuits that also engage the ventrolateral and ventral anterior nucleus of the thalamus (VL, VA, respectively), as well as large areas of the cerebral cortex: Topographic projections from different cortical areas reach spatially separate domains of the basal ganglia, which, in turn, send their output to similarly specialized areas of the thalamus. Projections from the thalamus to discrete areas of the frontal cortical areas of origin complete the cortico-basal ganglia-thalamocortical loops [18, 19]. Reflecting the functions of the cortical region involved, a family of different cortico-basal ganglia-thalamocortical circuits were labeled as “motor,” “oculomotor,” “prefrontal,” (often referred to as “executive” or “associative”) and “limbic” circuits.
Figure 1.
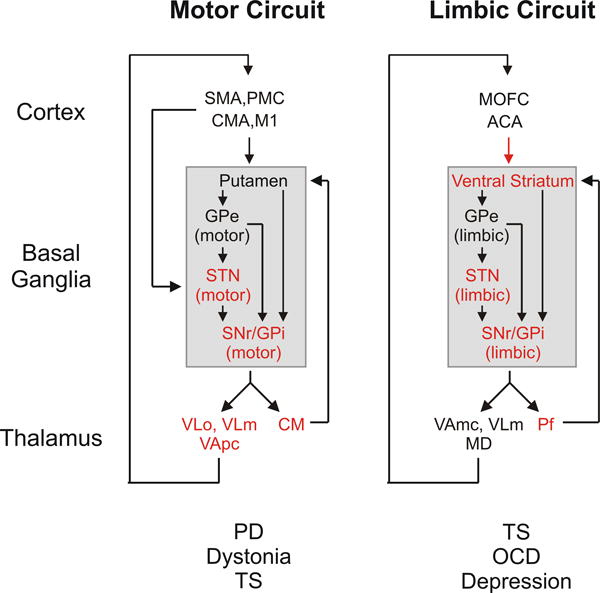
Motor and limbic cortex-basal ganglia-thalamocortical circuits. The targets of current DBS treatments are shown in red, and movement disorders and neuropsychiatric disorders caused by dysfunction of these circuits are listed below the circuit diagrams. Abbreviations: ACA, anterior cingulate area; CMA, cingulate motor area; M1, primary motor cortex; MD, mediodorsal nucleus of thalamus; MOFC, medial orbitofrontal cortex; OCD, obsessive-compulsive disorder; PMC, premotor cortex; SMA, supplementary motor area; TS, Tourette syndrome; VApc, ventral anterior nucleus of thalamus, pars parvocellularis; VAmc, ventral anterior nucleus of thalamus, pars magnocellularis; VLm, ventrolateral nucleus of thalamus, pars medialis; VLo, ventrolateral nucleus of thalamus, pars oralis. See text for other abbreviations. Modified from a figure in ref. [66], used with permission.
DBS is considered to be circuit-, rather than disease-specific: Diseases that result from dysfunction within specific stimulated circuits will respond to the stimulation, while functions that involve other brain areas will not be affected. This is exemplified by the fact that neurosurgeons use the same DBS targets in the basal ganglia for movement disorders as clinically diverse as PD, dystonia, and hemiballismus, because these disorders all arise from specific functional abnormalities of the basal ganglia-thalamocortical motor circuit [20]. This circuit originates from pre- and post-central sensorimotor areas and related post-central sensory cortical areas, and engages motor portions of the basal ganglia and thalamus. By contrast, DBS for neuropsychiatric disorders, such as OCD or Tourette syndrome, is typically directed at nodes of the limbic circuit, such as the ventral striatum, or the fiber tracts connecting them.
Although DBS is also applied outside of the basal ganglia, the chosen DBS targets are usually in areas that are closely related to these nuclei. One such target is the CM/Pf nuclear complex which is currently used as (experimental) treatment for Tourette syndrome. CM/Pf is topographically organized, receiving afferents from functionally specific areas of the basal ganglia, and sending similarly specific projections back to the basal ganglia [21]. Another more recent target for DBS is the pedunculopontine nucleus (PPN), a structure that also has very tight links to the basal ganglia output nuclei [22]. This nucleus is targeted for treatment of gait and balance disturbances in PD [see below, and ref. 23, 24].
DBS in Parkinson’s disease
Functional effects of DBS
As discussed, early models of basal ganglia dysfunction in movement disorders focused on brain activity changes in basal ganglia output, postulating that increased (inhibitory) GPi output would result in greater inhibition of thalamic and downstream targets, eventually leading to rigidity and bradykinesia, while reduced basal ganglia output was thought to contribute to the development of dyskinesias [25, 26]. Pallidotomy in PD was thought to lower basal ganglia output, and ameliorate akinesia and bradykinesia by releasing thalamocortical neurons from inhibition. The finding that DBS and ablative procedures had remarkably similar effects, suggested that DBS might, in fact, work like ablation, by reducing basal ganglia output. However, the view that either of these procedures works through a simple reduction of basal ganglia output has several shortcomings, most obviously the fact that ablation of GPi does not result in dyskinesias. Furthermore, studies in animal models of PD and movement disorder patients treated with DBS have demonstrated that DBS of the STN results in short-latency monosynaptic excitatory responses in the GPi, followed by polysynaptic periods of inhibition and excitation [27], and that DBS also alters firing patterns of neurons in thalamus, cortex, and brainstem. The task of explaining how DBS works is further complicated by the fact that DBS stimulation parameters that are currently used strongly bias the stimulation effects towards fiber pathways rather than cell bodies, so that the effects of DBS of a specific basal ganglia nucleus like the STN are not limited to cells, afferents or efferents within the stimulated nucleus itself, but may prominently involve passing fibers within the range of stimulation [28].
Studies of DBS effects have significantly contributed to ongoing revisions of models of the pathophysiology of PD and movement disorders. According to recent models, these diseases are no longer viewed as the result of global rate changes, but consequent to changes in activity patterns of basal ganglia neurons, including the development of oscillations, bursting and excessive synchrony of discharge which may be propagated throughout the entire basal ganglia-thalamocortical network of connections [29]. DBS appears to override and replace these abnormal activity patterns in the basal ganglia-thalamocortical circuitry with patterns that are less disturbing [1, 30, 31]. For instance, DBS may specifically disrupt pathologically synchronized ‘anti-kinetic’ beta-band oscillations in the basal ganglia, and may allow thalamocortical relay neurons to more faithfully respond to cortical inputs [30].
STN-DBS has been shown to normalize intracortical inhibitory mechanisms in transcranial magnetic stimulation studies [32, 33], and functional imaging studies have shown that STN-DBS induces widespread ‘normalization’ of activity in frontal motor areas both at rest and with movement tasks [e.g., 34, 35]. In terms of STN-DBS, such effects may not only involve the aforementioned trans-thalamic route of transmission, but may also result from antidromically mediated effects, via activation of corticosubthalamic fibers [reviewed in ref. 36]. There is, indeed, little doubt that DBS causes stimulus-coupled changes of cortical activities with very short latencies. However, the link between such changes and the behavioral effect(s) of DBS remain unclear. It should be noted that DBS of GPi is much less likely to invoke such antidromic effects on cortical circuits, given the distance of the stimulation from adjacent corticofugal fibers and the absence of a direct innervation of GPi by cortical inputs [reviewed in ref. 36].
Unsolved problems associated with currently used DBS methods
It is widely accepted that DBS is an effective treatment for advanced PD and other conditions and that it is superior to medical therapy in improving measures of the quality of life. Nonetheless, there remain a number of unsettled clinical issues [37]. One of these is the question of target selection. While the STN has been the most commonly targeted node of the motor circuit in the treatment of PD, GPi-DBS may offer comparable benefit, perhaps even with a lower rate of side-effects for certain subgroups of patients [37, 38]. Based on other differences between these procedures, such as the fact that GPi-DBS is particularly effective for dyskinesias, or the experience that patients treated with GPi DBS cannot lower their medications to the same extent as patients with STN-DBS, it is recommended that the choice of the stimulation target be individualized to the patient’s specific condition and needs.
Another unsettled question for DBS treatment of patients with advanced PD is whether DBS leads should be implanted bilaterally in a single session, or whether the lead implantation should be performed first on one side, and only if needed on the other. Our experience, and that of others, is that unilateral procedures, contralateral to the most affected side, have fewer side effects than bilateral procedures, and often provide symptom relief (even on the ipsilateral side) sufficient to obviate the need for the second procedure [39, 40].
Because the ultimate measure of success with DBS is not simply the reduction of parkinsonism, but improved quality of life, many factors other than the motor signs and symptoms of the disease are important in selecting patients for DBS procedures. The importance of factors such as patient age, response to levodopa therapy, and the presence of certain clinical features, such as levodopa-unresponsive balance and gait impairments, impulsivity, and cognitive and psychiatric disturbances, remain topics for discussion and further study.
Future directions for DBS
DBS has been in routine clinical use for nearly two decades, but the technology has remained largely unchanged. Since its introduction, the stimulating leads have provided four equally spaced contacts along the shaft which can be used for stimulation in multiple mono- and bipolar combinations, and implanted pulse generators generate continuous electrical impulses which can be adjusted in terms of voltage, pulse duration and frequency [41] The stimulation parameters in use have been derived largely by trial and error, based on their observed clinical effects. These vary depending on the targeted structure and the particular disorder.
The apparent lack of technical progress or change may reflect, to some extent, the overall success of the currently available technique. However, there continues to be a need for greater efficacy, reduced side-effects, and benefit for currently non-responsive symptoms. The field is ripe for innovation in the use of alternative programming algorithms, more accurate procedures for placing the electrodes, on-demand stimulation, alternative electrode designs, and alternative stimulation regimes.
Alternative targets for treatment of PD
The nodes and fiber connections of the cortico-basal ganglia-thalamo cortical circuits are all possible targets for DBS. Thus far, clinicians have largely limited themselves to DBS at GPi, STN and thalamic targets. As mentioned above one of the other targets that are currently under study is the PPN, a region of the brainstem locomotor region, which appears to provide benefits for refractory gait and balance problems (so-called axial symptoms) in PD, following encouraging studies of DBS of the PPN on gait in MPTP-treated monkeys [42]. The PPN is a major target of descending GPi output as well as providing inputs to the basal ganglia and thalamus. Moderately positive effects of low-frequency (10–25 Hz) stimulation of the PPN on balance problems and falls have been described in several studies [for instance, ref. 24, 43, 44], but questions remain as to the exact position of the electrodes and the degree and durability of improvement.
DBS of the caudal zona incerta (ZI) region of the thalamus has also been shown to have beneficial effects, specifically in patients with prominent proximal limb tremor, patients with PD and, possibly, also patients with dystonia [e.g., 45, 46]. The experience with this target remains relatively limited at this time, but stimulation at this site is reported to have fewer cognitive or emotional side effects than STN- or GPi-DBS [e.g., 47]. As in the case of the PPN, the exact location of the most effective target remains uncertain. Bilateral simultaneous ZI and PPN stimulation has been reported to be beneficial for untreatable ‘on’ state axial symptoms of PD [23].
For decades researchers have viewed the basal ganglia-thalamocortical and the cerebellar-thalamocortical circuits as separate systems and viewed movement disorders as either basal ganglia or cerebellar in origin. There is now evidence that the cerebellum and the basal ganglia are, in fact, anatomically interconnected [48, 49], and that some forms of dystonia, including specifically primary generailized dystonia, may involve dysfunction within the cerebellar thalamo-cortical circuitry [50–52]. If this is the case, the use of DBS of the cerebellar cortex or cerebellar outflow pathways should be considered as a possible treatment for these patients. It is noteworthy that stimulation of the cerebellar cortex was, in fact, used in the 1970’s for spasticity and ‘athetoid’ forms of cerebral palsy with favorable results, but then fell out of favor in part because of shortcomings with the pacemaker technology available at that time [53].
Engineering and technologic advances
Several important technical changes are currently being evaluated which should lead to greater efficacy and fewer side effects of DBS treatment in the future. One area of innovation is the development of strategies to ‘sculpt’ the electrical field around the electrode in order to adjust it better to the patient’s needs, and to limit the speech, cognitive and behavioral side effects of DBS. The currently available DBS devices already allow programmers to assign positive and negative polarities to any combination of electrodes along the lead, permitting considerable flexibility to shape the electrical field. This readily available method of shaping the electrical field is under-utilized. Modeling studies suggest that more extensive and detailed sculpting of the electrical field induced by DBS may be possible through the use of electrodes with more contacts than the currently used ones [54, 55].
For years, it has been discussed whether novel stimulation regimes might lead to improved clinical outcomes. The commercially available stimulators are open-loop constant-frequency, constant-voltage devices. Changes in these stimulator characteristics are currently being investigated, specifically with regard to the treatment of PD. For instance, constant current devices have recently been introduced by different manufactures, as an alternative to constant voltage devices. Although available for implantation in patients with movement disorders, there is no known advantage of constant-current stimulation over constant-voltage stimulation. Modeling has suggested the possibility of greater activation of targeted neuronal populations, while sparing nearby fiber pathways by current steering with multiple independent sources than with a single current source [56].
Another heavily researched area is the possibility that the recording of feedback signals could help to tailor the stimulation to the patient’s needs. Recent animal experiments involving MPTP-treated primates have shown that feedback-triggered (closed-loop) stimulation may be advantageous compared to conventional open-loop stimulation [57]. These studies indicate, moreover, that closed-loop paradigms that utilize electrical feedback signals recorded in the basal ganglia or cortex (local field potentials or single-neuron spiking) may act to modulate oscillatory discharge in these areas, suggesting that the observed amelioration of parkinsonism with these stimulation methods may involve disruption of synchronized cortico-basal ganglia oscillations, and that outcomes are better than continuous stimulation [see also, ref. 58]
Researchers have shown that continuous high-frequency stimulation with irregular stimuli is less effective than continuous high-frequency stimulation with constant rates [59]. However, continuous stimulation may not be necessary, as recent experiments utilizing trains of high-frequency stimuli (rather than constant stimulation) have shown significant promise [see figure 2, and ref. 57, 60]. One of the basic benefits of the use of trains of stimuli is that they would utilize less battery charge than conventional DBS methods.
Figure 2.
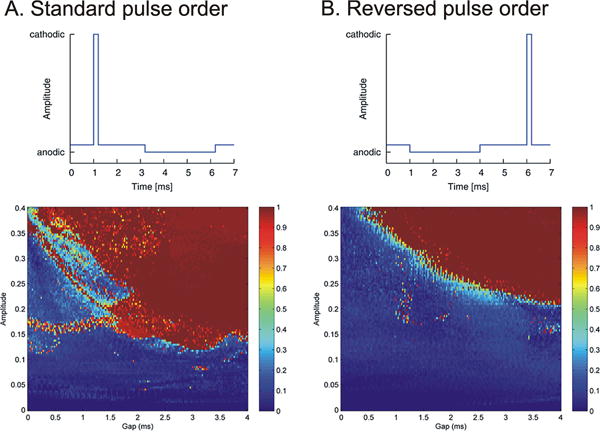
Example of the possible use of alternative stimulation regimes [60]. Conventional DBS pulses consist of an initial cathodal stimulation pulse, followed immediately by an anodal (recharge) pulse. This study modeled the impact of introducing a gap between the two pulses, and of reversing the order of cathodal and anodal pulse steps. The two pulse shapes are shown in the top row, where the figure under A. shows the conventional order of stimulation, while the figure under B shows the reversed order. The diagrams on the bottom show the color-coded results of such stimulation. Introduction of a gap within the standard pulse order (A) resulted in a more effective stimulation than conventional stimulation (gap = 0). As shown under (B), reversing the order of stimulation resulted in a similar outcome, although the necessary stimulation strengths were higher than for the conventional order of stimulation pulses. Figure reproduced from figures in [60], used with permission.
A more radical departure from currently used stimulation methods is the use of coordinated reset stimulation, a method designed through neural network modeling studies that aims to alter aberrant plasticity in basal ganglia circuits and to break up abnormally synchronized network oscillations [61]. Coordinated reset stimulation can be used in open-loop configurations with regular DBS electrodes, and may consist of the application of short trains of pulses to different regions of the (hyper-synchronized) target nucleus. These pulse trains act to systematically reset activities of sub-populations of neurons within the stimulated nucleus, thereby reducing synchrony with other, not stimulated, populations of neurons within the same nucleus [62].
Future improvements may also be expected with the use of better techniques of optimizing electrode placement, such as the use of high-field strength MRI for pre-operative localization [63] and improved electrophysiological recording techniques [64], or the use of intraoperative MRI [65].
Conclusions
The renaissance in functional neurosurgery triggered by the introduction of DBS has brought profound benefits for patients afflicted with a number of severe neuropsychiatric disorders, and shows promise for several other. A primary action of DBS is the modulation of dysfunctional neuronal networks underlying these disorders. Simultaneously it has proven, when combined with neuroimaging, neurophysiology and other investigative approaches, to be a novel investigative tool for understanding the functions of these networks and the abnormalities underlying neuropsychiatric disorders. The field is ripe for technologic innovation and testing of novel stimulation paradigms.
Acknowledgments
The preparation of this article was supported through grants from the NIH/NINDS (R01-NS054976, R01-NS071074 and P50-NS071669 (TW)), and by NIH/NCRR grant RR-000165 (Yerkes National Primate Center).
References
- 1.McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38:329–337. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kringelbach ML, et al. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 3.Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus. 2010;29:E1. doi: 10.3171/2010.4.FOCUS10106. [DOI] [PubMed] [Google Scholar]
- 4.Blomstedt P, Hariz MI. Deep brain stimulation for movement disorders before DBS for movement disorders. Parkinsonism Relat Disord. 2010;16:429–433. doi: 10.1016/j.parkreldis.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Wichmann T, et al. Milestones in research on the pathophysiology of Parkinson’s disease. Mov Disord. 2011;26:1032–1041. doi: 10.1002/mds.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76:53–61. doi: 10.3171/jns.1992.76.1.0053. [DOI] [PubMed] [Google Scholar]
- 7.Baron MS, et al. Treatment of advanced Parkinson’s disease by posterior GPi pallidotomy: 1-year results of a pilot study. Ann Neurol. 1996;40:355–366. doi: 10.1002/ana.410400305. [DOI] [PubMed] [Google Scholar]
- 8.Benabid AL, et al. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 9.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 10.Aziz TZ, et al. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 11.Guridi J, et al. Subthalamotomy in parkinsonian monkeys. Behavioural and biochemical analysis. Brain. 1996;119:1717–1727. doi: 10.1093/brain/119.5.1717. [DOI] [PubMed] [Google Scholar]
- 12.Benazzouz A, et al. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 13.Limousin P, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 14.Benabid AL, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- 15.Deuschl G, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 16.Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35:1126–1129. doi: 10.1227/00006123-199412000-00016. discussion 1129–1130. [DOI] [PubMed] [Google Scholar]
- 17.Porta M, et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375–1380. doi: 10.1212/WNL.0b013e3181bd809b. [DOI] [PubMed] [Google Scholar]
- 18.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 19.Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- 20.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 21.Smith Y, et al. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Khan S, et al. Outcomes from stimulation of the caudal zona incerta and pedunculopontine nucleus in patients with Parkinson’s disease. Br J Neurosurg. 2011;25:273–280. doi: 10.3109/02688697.2010.544790. [DOI] [PubMed] [Google Scholar]
- 24.Stefani A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 25.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 26.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T, et al. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIntyre CC, et al. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 29.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, et al. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- 31.Brown P, et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol. 2004;188:480–490. doi: 10.1016/j.expneurol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Cunic D, et al. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Neurology. 2002;58:1665–1672. doi: 10.1212/wnl.58.11.1665. [DOI] [PubMed] [Google Scholar]
- 33.Tisch S, et al. Pallidal stimulation modifies after-effects of paired associative stimulation on motor cortex excitability in primary generalised dystonia. Exp Neurol. 2007;206:80–85. doi: 10.1016/j.expneurol.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Grafton ST, et al. Normalizing motor-related brain activity: Subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–1199. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- 35.Trost M, et al. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devergnas A, Wichmann T. Cortical potentials evoked by deep brain stimulation in the subthalamic area. Front Syst Neurosci. 2011;5:30. doi: 10.3389/fnsys.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okun MS, Foote KD. Parkinson’s disease DBS: what, when, who and why? The time has come to tailor DBS targets. Expert Rev Neurother. 2010;10:1847–1857. doi: 10.1586/ern.10.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bronstein JM, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberts JL, et al. Are two leads always better than one: An emerging case for unilateral subthalamic deep brain stimulation in Parkinson’s disease. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taba HA, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113:1224–1229. doi: 10.3171/2010.8.JNS10312. [DOI] [PubMed] [Google Scholar]
- 41.Volkmann J. Deep brain stimulation for the treatment of Parkinson’s disease. J Clin Neurophysiol. 2004;21:6–17. doi: 10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport. 2004;15:2621–2624. doi: 10.1097/00001756-200412030-00012. [DOI] [PubMed] [Google Scholar]
- 43.Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- 44.Moro E, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215–224. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- 45.Blomstedt P, et al. The posterior subthalamic area in the treatment of movement disorders: past, present, and future. Neurosurgery. 2009;64:1029–1038. doi: 10.1227/01.NEU.0000345643.69486.BC. discussion 1038–1042. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa M, et al. Two-year follow-up of chronic stimulation of the posterior subthalamic white matter for tremor-dominant Parkinson’s disease. Neurosurgery. 2005;56:281–289. doi: 10.1227/01.neu.0000148167.49105.a3. discussion 281–289. [DOI] [PubMed] [Google Scholar]
- 47.Fytagoridis A, Blomstedt P. Complications and side effects of deep brain stimulation in the posterior subthalamic area. Stereotact Funct Neurosurg. 2010;88:88–93. doi: 10.1159/000271824. [DOI] [PubMed] [Google Scholar]
- 48.Hoshi E, et al. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 49.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neychev VK, et al. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niethammer M, et al. Hereditary dystonia as a neurodevelopmental circuit disorder: Evidence from neuroimaging. Neurobiol Dis. 2011;42:202–209. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calderon DP, et al. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat Neurosci. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis R. Cerebellar stimulation for cerebral palsy spasticity, function, and seizures. Arch Med Res. 2000;31:290–299. doi: 10.1016/s0188-4409(00)00065-5. [DOI] [PubMed] [Google Scholar]
- 54.Buhlmann J, et al. Modeling of a segmented electrode for desynchronizing deep brain stimulation. Frontiers in neuroengineering. 2011;4:15. doi: 10.3389/fneng.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martens HC, et al. Spatial steering of deep brain stimulation volumes using a novel lead design. Clin Neurophysiol. 2011;122:558–566. doi: 10.1016/j.clinph.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 56.Chaturvedi A, Foutz TJ, McIntyre CC. Current steering to activate targeted neural pathways during deep brain stimulation of the subthalamic region. Brain stimulation. 2011 doi: 10.1016/j.brs.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosin B, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 58.Rosa M, et al. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson’s disease in hyperacute and chronic phases. Neuro-Signals. 2011;19:151–162. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- 59.Birdno MJ, et al. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. J Neurophysiol. 2012;107:364–383. doi: 10.1152/jn.00906.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hofmann L, et al. Modified pulse shapes for effective neural stimulation. Frontiers in neuroengineering. 2011;4:9. doi: 10.3389/fneng.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hauptmann C, Popovych O, Tass PA. Desynchronizing the abnormally synchronized neural activity in the subthalamic nucleus: a modeling study. Expert Rev Med Devices. 2007;4:633–650. doi: 10.1586/17434440.4.5.633. [DOI] [PubMed] [Google Scholar]
- 62.Lysyansky B, Popovych OV, Tass PA. Desynchronizing anti-resonance effect of m: n ON-OFF coordinated reset stimulation. J Neural Eng. 2011;8:036019. doi: 10.1088/1741-2560/8/3/036019. [DOI] [PubMed] [Google Scholar]
- 63.Cho ZH, et al. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg. 2010;113:639–647. doi: 10.3171/2010.3.JNS091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaidel A, et al. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease. Brain. 2010;133:2007–2021. doi: 10.1093/brain/awq144. [DOI] [PubMed] [Google Scholar]
- 65.Lee MW, et al. Deep brain stimulation in intraoperative MRI environment – comparison of imaging techniques and electrode fixation methods. Minim Invasive Neurosurg. 2005;48:1–6. doi: 10.1055/s-2004-830169. [DOI] [PubMed] [Google Scholar]
- 66.Wichmann T, DeLong MR. Deep-Brain Stimulation for Neurologic and Psychiatric Disorders. In: Steiner H, Tseng K, editors. Handbook of Basal Ganglia Structure and Function. Academic Press (Elsevier); London: 2010. pp. 659–681. [Google Scholar]


