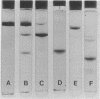
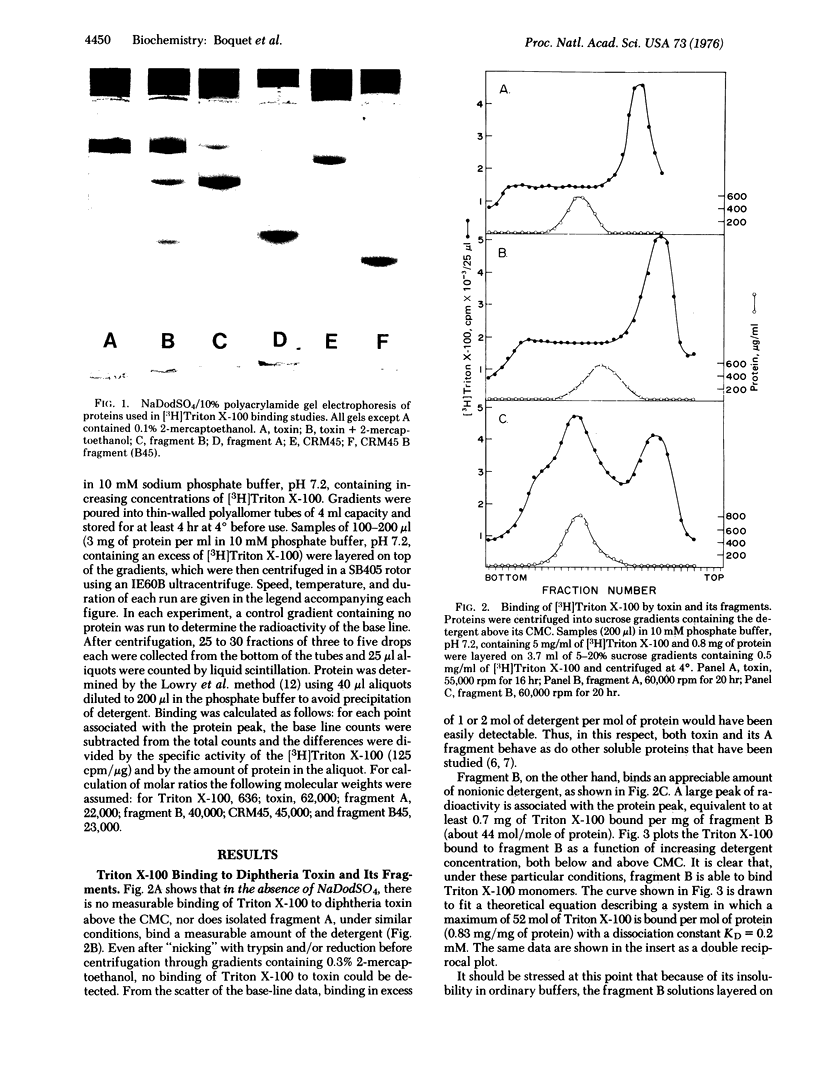
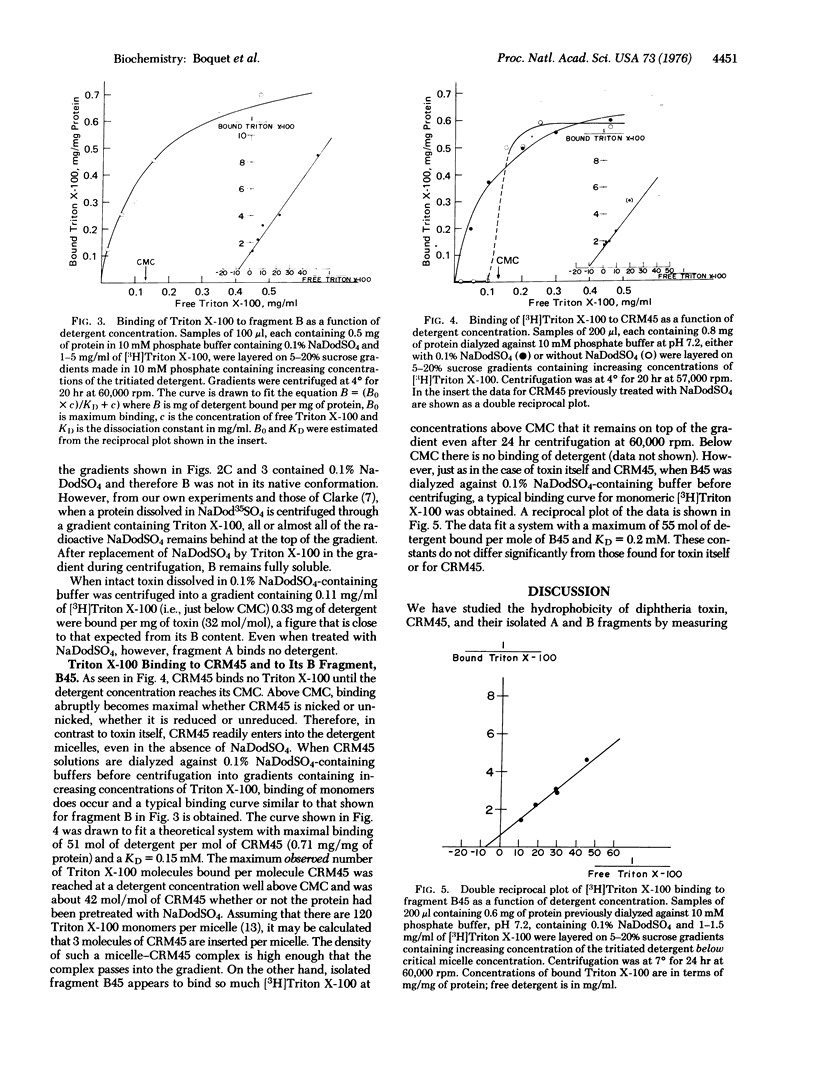
Abstract
Binding of the nonionic detergent [3H]Triton X-100 by diphtheria toxin, by the nontoxic serologically related protein crossreacting material (CRM) 45, and by their respective A and B fragments has been studied. If first denatured in 0.1% sodium dodecyl sulfate, all of the proteins with the exception of fragment A bind increasing amounts of Triton X-100, reaching a maximum of more than 40 mol bound per mol of protein when the detergent concentration exceeds its critical micelle concentration. No measurable amount of Triton X-100 is bound by native toxin or its A fragment of any concentration of the detergent. Undenatured CRM45 or its B45 fragment, on the other hand, readily became inserted into Triton X-100 micelles when the detergent reaches its critical micelle concentration. The results show that the toxin molecule contains a hydrophobic domain located on the portion of the B fragment that is linked to A. This region is masked in native toxin. Based on these findings, a model is proposed to describe how fragment B facilitates the transport of the enzymically active hydrophilic fragment A across the plasma membrane to reach the cytoplasm.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzonana G. Study of bile salts micelles: properties of mixed oleate-deoxycholate solutions at pH 9.0. Biochim Biophys Acta. 1969 Jun 10;176(4):836–848. doi: 10.1016/0005-2760(69)90265-3. [DOI] [PubMed] [Google Scholar]
- Boquet P., Pappenheimer A. M., Jr Interaction of diphtheria toxin with mammalian cell membranes. J Biol Chem. 1976 Sep 25;251(18):5770–5778. [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Drazin R. E., Collier R. J. Amino-acid sequence of fragment A, an enzymically active fragment from diphtheria toxin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):69–72. doi: 10.1073/pnas.73.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem. 1972 Jun 10;247(11):3656–3661. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Gill D. M., Pappenheimer A. M., Jr Mutation in the structural gene for diphtheria toxin carried by temperate phage . Nat New Biol. 1971 Sep 1;233(35):8–11. doi: 10.1038/newbio233008a0. [DOI] [PubMed] [Google Scholar]
- Visser L., Robinson N. C., Tanford C. The two-domain structure of cytochrome b5 in deoxycholate solution. Biochemistry. 1975 Mar 25;14(6):1194–1199. doi: 10.1021/bi00677a015. [DOI] [PubMed] [Google Scholar]