Abstract
Background
The relation between the source of cognitive complaint and objective cognitive performance is not well understood.
Objective
Examine self and informant cognitive complaint as predictors of objective cognitive and functional trajectory in non-demented elders.
Methods
Participants from the National Alzheimer’s Coordinating Center had a baseline diagnosis of normal cognition (NC; n=6133, 72±8 years, 68% female) or mild cognitive impairment (MCI; n=3010, 74±8 years, 55% female). Four independent groups defined cognitive complaint: no complaint, self-only complaint, informant-only complaint, or mutual complaint (both self and informant complaint). Linear mixed model regression analyses related complaint status (referent was no complaint) to cognitive and functional trajectories, adjusting for age, sex, race, education, and follow-up period.
Results
Among NC participants, mutual complaint related to faster decline in global cognition (p<0.0001), language (all p-values<0.0001), processing speed (p=0.0002), and executive functioning (p=0.0006). Informant-only complaint related to faster decline in global cognition (p=0.0001) and processing speed (p=0.0001). Self-only complaint related to greater decline in immediate (p<0.0001) and delayed (p=0.0005) episodic memory. In MCI, mutual complaint related to faster decline in global cognition (p<0.0001), verbal episodic memory (all p-values<0.0001), language (all p-values<0.0001), and processing speed (all p-values<0.0006). Informant-only or self-only complaint associations with cognitive trajectory did not survive correction factor for multiple comparisons.
Conclusion
Cognitive complaint appears to have clinical significance, as it is related to declines in objective cognitive performance over time. Mutual complaint was associated with the worst cognitive trajectory in both NC and MCI elders, highlighting the importance of incorporating an informant into evaluation of elders whenever feasible.
Keywords: mild cognitive impairment, cognitive complaint, dementia, cognition, Alzheimer’s disease
Introduction
Cognitive complaint, or a concern regarding a change in cognition, is a diagnostic criterion for mild cognitive impairment (MCI) [1], a prodromal phase of Alzheimer’s disease (AD), because complaints purportedly represent a clinically relevant change in cognitive health [2]. More recent work in non-demented older adults supports the inclusion of cognitive complaint as a diagnostic criterion for MCI by linking cognitive complaint with AD pathology quantified in vivo with amyloid imaging [3] or ex vivo with post-mortem markers [4]. Furthermore, among adults without dementia, cognitive complaint is associated with structural imaging evidence of smaller medial temporal lobe volumes [5], a region profoundly affected by AD neuropathology [6].
Despite evidence that cognitive complaint may be an early manifestation of unhealthy brain aging, it remains unclear how cognitive complaint aligns with objective cognitive performance in non-demented adults. Individuals with cognitive complaint experience global cognitive decline as compared to individuals with no complaint [7], and cognitive complaint correlates with declines in information processing speed [8] and episodic memory [8,9]. However, not all studies to date report associations between complaint and cognitive performance among non-demented older adults [10].
One complicating factor in understanding how complaint relates to objective cognitive performance may be an overreliance on assessing self-only complaint [7,11]. Rather, the presence of an informant report, either alone or in conjunction with a self complaint, has been shown to be more prognostically useful in predicting diagnostic conversion [12,13]. However, there has been limited empirical consideration of how informant complaint alone or in combination with self complaint relates to cognitive and functional trajectories. This gap in the literature is noteworthy, as the MCI diagnostic criterion for cognitive complaint allows for multiple sources of complaint, including the patient (self), someone close to the patient (informant), or the clinician treating the patient [1]. Understanding the clinical implications of different cognitive complaint sources may aid in clarifying the ambiguity of complaint and help clinicians better identify individuals at risk for cognitive decline.
The current study reconciles the prognostic properties of cognitive complaint in relation to longitudinal cognitive and functional trajectories of individuals with normal cognition (NC) and MCI. We consider how different sources of complaint relate to changes in cognition and instrumental activities of daily living (IADLs) over time. Leveraging the National Alzheimer’s Coordinating Center (NACC) database, we hypothesize that NC and MCI individuals with mutual complaint (i.e., a combination of self complaint and informant complaint) will experience more cognitive and functional decline than peers with no cognitive complaint or only one type of complaint (i.e., self-only complaint or informant-only complaint). Because episodic memory loss is often the first clinical symptom of AD [14], we hypothesize relations will be stronger between cognitive complaint and episodic memory declines compared to other cognitive domains.
Materials and Methods
Participants
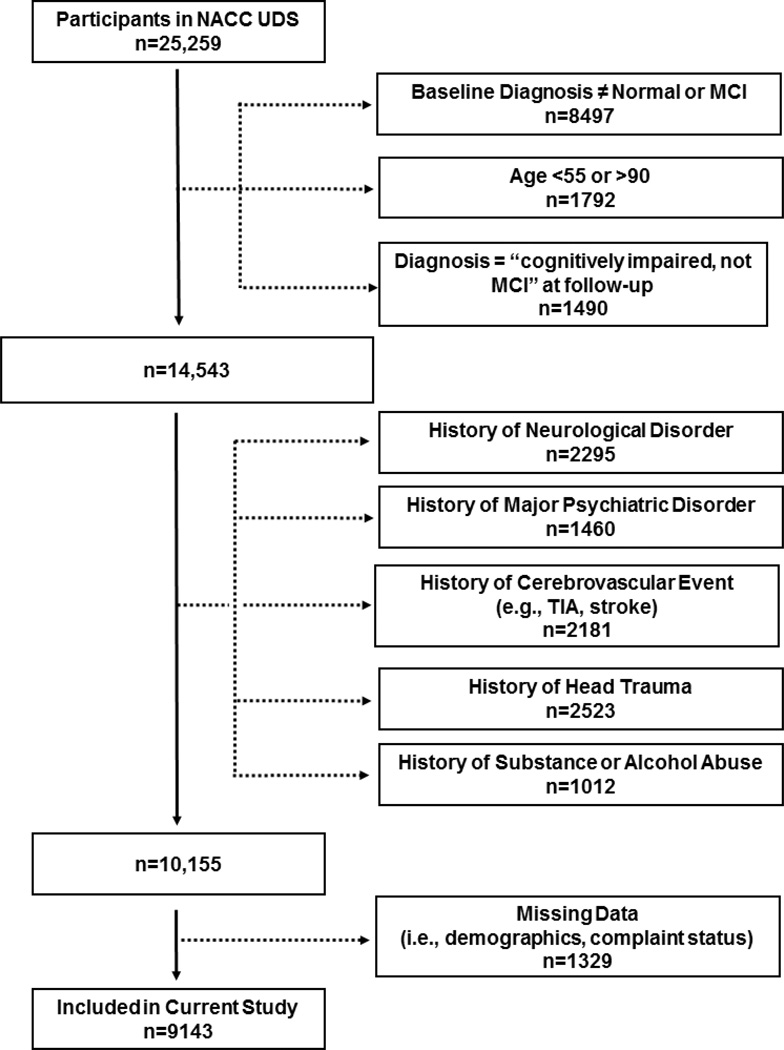
As previously described [15], NACC electronically stores participant information collected from 34 past and present National Institute on Aging-funded Alzheimer’s Disease Centers. Participant recruitment methods vary across sites but mostly include clinician-referrals or self-referrals from community outreach efforts. Participants complete annual visits involving collection of demographic information, medical history, neurological examination, and neuropsychological assessment [16]. Participants evaluated between 9/01/2005 and 9/01/2013 as part of the Uniform Data Set with a baseline diagnosis of NC or MCI were included (diagnostic details are below). Figure 1 summarizes inclusion and exclusion details, resulting in a total sample of 8827 participants. The study received Institutional Review Board approval prior to data access or analysis.
Figure 1. Participant Inclusion & Exclusion Details.
Note: The exclusion numbers provided are not mutually exclusive; missing data includes demographic variables at baseline (i.e., race, education) and complaint status at baseline; cognitively impaired-not MCI were excluded due to non-standardized classification criteria. Within the missing data category (n=10,155), 470 individuals (<5%) were excluded due to missing informant report of complaint.
Cognitive Diagnostic Classification
Cognitive diagnosis was based upon clinician judgment or consensus at baseline using neuropsychological performance, neurological examination results, and medical history details. Classifications included:
NC was defined as Clinical Dementia Rating (CDR) [17]=0 (no dementia), no deficits in activities of daily living attributable to cognitive impairment, and no evidence of objective cognitive impairment defined as standard scores falling 1.5 standard deviations within the age-adjusted normative mean on all cognitive tests [18].
MCI was based upon Petersen criteria [19] and defined as a CDR=0 (no dementia) to 1.0 (mild severity of impairment); spared IADLs; report of cognitive change by the participant, informant, or clinician; objective cognitive impairment in at least one domain (i.e., performances falling greater than 1.5 standard deviations outside the age-adjusted normative mean) or a significant cognitive decline over time; and absence of dementia.
Cognitive Complaint
In the NACC Uniform Dataset, cognitive complaint is reported by the clinician dichotomously as present or absent for the participant (self) and the informant based on a clinical interview with both parties [13]. There are no specific methods for querying cognitive complaint, but examples for capturing such information during clinician-facilitated interviews include asking CDR questions about memory status (i.e., “Do you have problems with your memory or thinking?” or “Does s/he have problems with his/her memory or thinking?”) or general questions about memory and thinking abilities (e.g., “Do you feel that you have been having a significant problem with your short term memory?” or “Are you worried about the participant’s short-term memory?”). Based on the dichotomous classification (present/absent) made by the clinician, four mutually exclusive groups were created for the current study: (1) no complaint, (2) self-only complaint, (3) informant-only complaint, or (4) mutual complaint (both self and informant complaint).
Neuropsychological Assessment
As part of the Uniform Data Set, all participants completed a common neuropsychological protocol assessing multiple cognitive systems [18]. See Table 1 for details on this protocol.
Table 1.
Neuropsychological & Functional Protocol
| Domain | Test | Description | Range |
|---|---|---|---|
| Global Cognition | Mini-Mental State Examination [41] | Measures global cognitive status | 0–30 |
| Learning and Memory | Logical Memory Immediate Recall [42] | Assesses immediate verbal episodic learning for details of short story | 0–25 |
| Logical Memory Delayed Recall [42] | Assesses delayed verbal episodic retrieval for details of short story after a 20-minute delay | 0–25 | |
| Language | Animal Naming [43] | Measures ability to rapidly generate words from a specified category | n/a |
| Vegetable Naming[44] | Measures ability to rapidly generate words from a specified category | n/a | |
| Boston Naming Test-30 Item [45] | Assesses confrontation naming and lexical retrieval abilities | 0–30 | |
| Attention | Digit Span Forward [42] | Measures attention and concentration | 0–12 |
| Information Processing | Digit Symbol Coding [42] | Measures speed of visuo-motor processing | 0–93 |
| Trail Making Test Part A [46] | Measures visual-scanning and attention in a number sequencing task | 0–150 | |
| Executive Functioning | Trail Making Test Part B[46] | Measures sequencing and mental flexibility in a number and letter set-shifting task | 0–300 |
| Digit Span Backward [42] | Measures working memory | 0–12 | |
| Instrumental Activities of Daily Living | Functional Activities Questionnaire [20] | Measures capacity to complete independent activities of daily living | 0–30 |
IADLs Assessment
Information about IADLs was collected via the clinician-rated Functional Activities Questionnaire (FAQ) [20]. Each FAQ item is rated by the clinician based on information gathered from the participant and informant during the clinical interview. This tool allows for gradations in functioning where individuals can complete a task normally (0), complete a task with difficulty but independently (1), complete a task with assistance (2), or be completely dependent for the task (3). Higher scores represent worse functional abilities. See Table 1 for more details.
Statistical Analyses
Baseline cognitive and functional performances were compared across the four complaint categories separately by diagnostic group (i.e., NC, MCI) using Kruskal-Wallis tests for continuous variables or Pearson’s chi-square tests for categorical variables. To account for repeated measurements within the same participant, generalized linear mixed models compared the complaint categories to longitudinal cognitive and functional trajectories measured at each annual time point. To determine the appropriate link functions for each outcome, histograms for the baseline cognitive and functional measures were plotted separately for each diagnostic group. Based on results, the identity link function was used for all cognitive measures, and the log link function was applied to the FAQ data. For all regression models, fixed effects included baseline clinical characteristics (i.e., age, sex, education, and self-declared race defined as White or non-White), and cognitive complaint category. For all models, random effects included follow-up period (defined as time from first visit to the most recent visit) for each observation. No complaint was used as the referent for comparisons between the complaint categories. Interaction between follow-up period and cognitive complaint was included to capture different trajectories between cognitive complaint categories. Random intercept and random slope for years of follow-up were considered to allow individual-specific trajectories. Beta-coefficients for the interaction terms characterized the difference in slope between the complaint subgroups with no complaint as the referent. Beta-coefficient interpretations for FAQ were based on log-scale due to the use of the log link function, and higher scores on Trail Making Test Part A, Trail Making Test Part B, and FAQ indicated worse performance. Positive beta-coefficients for the length of follow-up correspond to worse performance for these three tests. For demographic and clinical comparisons, significance was set a priori at p<0.0015 based on a Bonferroni correction factor (i.e., α=0.05/34 comparisons). For hypothesis testing, significance was set a priori at p<0.00069 based on a Bonferroni correction factor (i.e., α=0.05/72 comparisons). Analyses were conducted using R 2.14.1 with the lmer function from lme4 package.
Results
Participant Characteristics
At baseline, participants included 6133 NC and 3010 MCI individuals. Among NC participants, the four cognitive complaint subgroups did not differ on age (p=0.04), race (p=0.13), or education (p=0.03) but differed with respect to sex and follow-up period (p-values<0.001). The complaint subgroups differed on many baseline cognitive tests and the FAQ. See Table 2 for details.
Table 2.
Baseline Clinical Characteristics and Cognitive and Functional Performances
| NC Participants | |||||
|
No Complaint |
Self-only Complaint |
Informant- only complaint |
Mutual complaint |
p-value | |
| Sample size, n | 4498 | 965 | 203 | 467 | n/a |
| Age, y | 72±8 | 72±9 | 73±8 | 73±8 | 0.05 |
| Sex, % Female | 70 | 69 | 54 | 59 | <0.001*,§, ¶,**,†† |
| Race, % White | 79 | 78 | 85 | 80 | 0.14 |
| Education, y | 15.6±3.0 | 15.7±3.0 | 15.4±3.4 | 15.1±3.4 | 0.025 |
| Follow-up Period,† y | 2.9±2.4 | 2.5±2.3 | 2.6±2.3 | 2.4±2±2 | <0.001*,¶,# |
| Mini-Mental State Examination | 29.0±1.4 | 28.8±1.5 | 28.7±1.6 | 28.5±1.7 | <0.001*,¶,†† |
| Logical Memory Immediate Recall | 13.6±3.8 | 13.4±4.0 | 12.9±3.9 | 12.2±4.3 | <0.001*,¶,†† |
| Logical Memory Delayed Recall | 12.4±4.1 | 12.0±4.3 | 11.5±4.2 | 10.6±4.5 | <0.001*,¶,†† |
| Boston Naming Test | 27.0±3.3 | 26.6±3.8 | 26.6±3.6 | 26.4±3.8 | <0.001*,¶ |
| Animal Naming | 20.2±5.7 | 19.9±5.6 | 19.9±5.5 | 19.0±5.6 | <0.001*,¶ |
| Vegetable Naming | 15.0±4.2 | 14.6±4.3 | 13.5±4.5 | 13.5±4.3 | <0.001*,§,¶,†† |
| Digit Span Forward | 8.6±2.0 | 8.5±2.1 | 8.3±2.1 | 8.2±2.2 | 0.017 |
| Digit Symbol Coding | 48.0±12.0 | 47.0±12.0 | 45.0±14.0 | 43.0±13.0 | <0.001*,¶,†† |
| Trail Making Test Part A‡ | 34.0±16.0 | 34.0±14.0 | 38.0±18.0 | 39.0±22.0 | <0.001*,‡‡ |
| Trail Making Test Part B‡ | 89.0±49.0 | 92.0±48.0 | 98.0±52.0 | 105.0±63.0 | <0.001*,‡‡ |
| Digit Span Backward | 6.8±2.2 | 6.7±2.2 | 6.5±2.4 | 6.5±2.3 | 0.002 |
| Functional Activities Questionnaire‡ | 0.1±0.9 | 0.2±0.9 | 0.8±2.0 | 1.4±3.0 | <0.001*,‡‡,§§,¶¶,##,*** |
| MCI Participants | |||||
|
No Complaint |
Self-only complaint |
Informant- only complaint |
Mutual complaint |
p-value | |
| Sample size, n | 428 | 339 | 254 | 1989 | n/a |
| Age, y | 74±8 | 74±8 | 76±7 | 74±8 | 0.002 |
| Sex, % Female | 61 | 67 | 48 | 53 | <0.001*,**,†† |
| Race, % White | 66 | 64 | 77 | 82 | <0.001*,‡‡,§§ |
| Education, y | 14.5±3.6 | 14.8±3.5 | 14.8±3.7 | 15.1±3.5 | 0.004 |
| Follow-up Period,† y | 2.2±2.3 | 2.0±2.2 | 2.1±2.1 | 2.0±2.0 | 0.83 |
| Mini-Mental State Examination | 27.4±2.6 | 27.2±2.3 | 27.1±2.5 | 26.7±2.6 | <0.001*,¶ |
| Logical Memory Immediate Recall | 10.2±4.2 | 9.8±3.9 | 8.9±4.3 | 8.4±4.1 | <0.001*,§,¶,†† |
| Logical Memory Delayed Recall | 8.4±4.5 | 8.0±4.2 | 6.3±4.9 | 5.7±4.5 | <0.001*,§,¶,**,†† |
| Boston Naming Test | 23.5±5.4 | 23.4±5.2 | 24.7±4.6 | 24.3±5.1 | <0.001* |
| Animal Naming | 15.6±4.9 | 15.5±4.8 | 16.1±5.2 | 15.5±4.8 | 0.44 |
| Vegetable Naming | 11.9±3.8 | 11.6±3.6 | 11.0±3.9 | 10.7±3.9 | <0.001*,¶,†† |
| Digit Span Forward | 7.7±2.2 | 7.7±2.0 | 7.6±2.2 | 7.8±2.1 | 0.44 |
| Digit Symbol Coding | 37.0±13.0 | 36.0±13.0 | 37.0±13.0 | 37.0±12.0 | 0.15 |
| Trail Making Test Part A‡ | 47.0±25.0 | 47.0±25.0 | 46.0±26.0 | 45.0±22.0 | 0.63 |
| Trail Making Test Part B‡ | 142.0±80.0 | 150.0±80.0 | 152.0±82.0 | 139.0±76.0 | 0.02 |
| Digit Span Backward | 5.5±2.2 | 5.6±2.1 | 5.7±2.2 | 5.8±2.1 | 0.03 |
| Functional Activities Questionnaire‡ | 1.0±2.9 | 0.7±2.0 | 3.9±5.6 | 4.0±4.8 | <0.001*,‡‡,§§,¶¶,## |
Note: Data presented as mean±standard deviation;
=significant Bonferroni-corrected p-value <0.05/34 (i.e., 0.0015);
Follow-up period is time from the first visit until last visit;
=higher score=worse performance; Post-hoc group comparisons-Bonferroni corrected:
no complaint>informant-only complaint;
no complaint>mutual complaint;
no complaint>self-only complaint;
self-only complaint>informant-only complaint;
self-only complaint>mutual complaint;
no complaint<mutual complaint;
self-only complaint<mutual complaint;
no complaint<informant-only complaint;
self-only complaint<informant-only complaint,
no complaint<self-only complaint
Among MCI participants, the complaint subgroups did not differ for age (0.003), education (p=0.003), or follow-up period (p=0.87) but did differ with respect to sex and race (p-values<0.001). The complaint subgroups differed on many baseline cognitive tests and the FAQ. See Table 2 for details.
Informant Characteristics
Informants were primarily spouses (NC=48%, MCI=58%) but also included adult children (NC=24%, MCI=24%), other relatives (NC=9%, MCI=6%) or close friends (NC=19%, MCI=12%). A majority of informants saw or spoke to the participant at least weekly (NC=81%, MCI=90%).
Cognitive Complaint & Longitudinal Cognitive & Functional Performances Among NC Participants
Compared to NC participants with no complaint, NC participants with a mutual complaint had greater decline on Mini-Mental State Examination (MMSE, p<0.0001), Boston Naming Test (p=0.0001), Vegetable Naming (p<0.0001), Trail Making Test Part A (p=0.0002), and Trail Making Test Part B (p=0.0006). No between-group differences in trajectory were seen on Logical Memory Immediate Recall (p=0.02), Logical Memory Delayed Recall (p=0.09), Animal Naming (p=0.02), Digit Span Forward (p=0.19), Digit Symbol Coding (p=0.003), or Digit Span Backward (p=0.003), even when a less stringent correction factor (i.e., Hochberg method, p-value<0.001) was considered.
Compared to NC participants with no complaint, NC participants with an informant-only complaint declined faster on MMSE (p=0.0001) and Digit Symbol Coding (p=0.0001). No between-group differences were detected on Logical Memory Immediate Recall (p=0.03), Logical Memory Delayed Recall (p=0.004), Boston Naming Test (p=0.009), Animal Naming (p=0.004), Vegetable Naming (p=0.004), Digit Span Forward (p=0.74), Trail Making Test Part A (p=0.001), Trail Making Test Part B (p=0.03), or Digit Span Backward (p=0.03), even when a less stringent correction factor was applied (p-value<0.001).
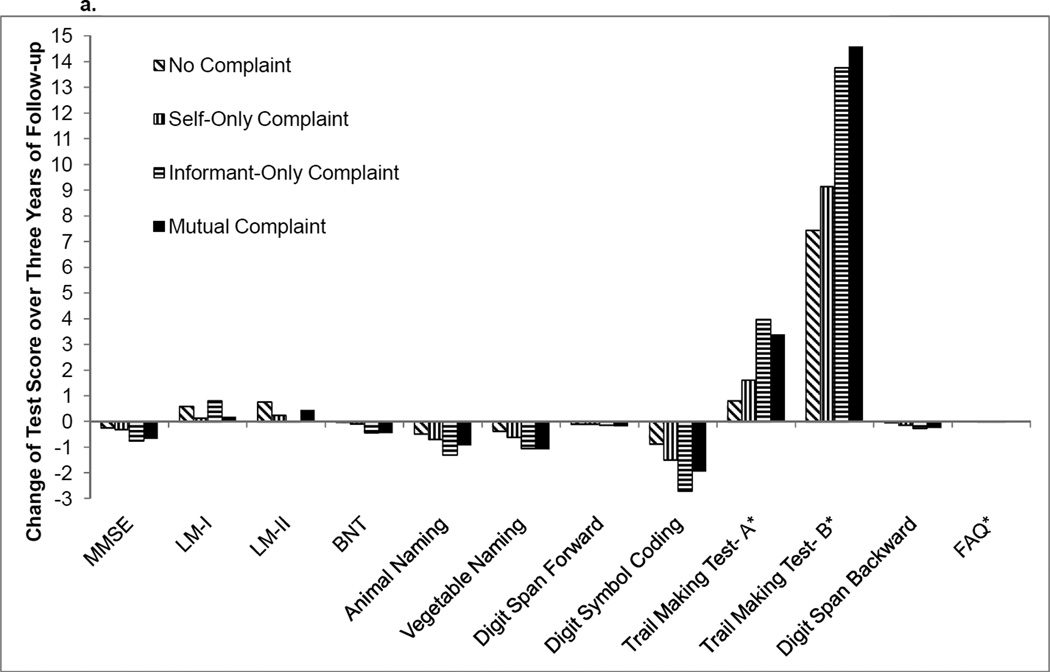
Compared to no complaint, NC participants with a self-only complaint declined more rapidly on Logical Memory Immediate Recall (p<0.0001) and Logical Memory Delayed Recall (p=0.0005). No differences between no complaint and self-only complaint were found for the remaining cognitive tasks (all p-values>0.009) even when a less stringent correction factor was applied (p-value<0.001). Also, there was no difference between no complaint and any of the other complaint subgroups for functional trajectory (all p-values>0.65). See Table 3 and Figure 2a for details. When models were recalculated to include data collection site as a random effect, findings were essentially unchanged (data not shown).
Table 3.
Cognitive Complaint Categories and Cognitive and Functional Trajectories
| NC Participants | |||||||||
| Self-only complaintŧ | Informant-only complaintŧ | Mutual complaintŧ | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| Mini-Mental State Examination | −0.02 | −0.06,0.02 | 0.36 | −0.17 | −0.25,−0.08 | 0.0001* | −0.14 | −0.21,−0.08 | <0.0001* |
| Logical Memory Immediate Recall | −0.15 | −0.23,−0.08 | <0.0001* | −0.17 | −0.32,−0.02 | 0.03 | −0.13 | −0.24,−0.03 | 0.02 |
| Logical Memory Delayed Recall | −0.17 | −0.26,−0.09 | <0.0001* | −0.25 | −0.41,−0.08 | 0.004 | −0.10 | −0.22,0.01 | 0.09 |
| Boston Naming Test | −0.03 | −0.08,0.03 | 0.30 | −0.14 | −0.25,−0.04 | 0.009 | −0.15 | −0.23,−0.08 | 0.0001* |
| Animal Naming | −0.07 | −0.16,0.02 | 0.14 | −0.27 | −0.46,−0.09 | 0.004 | −0.16 | −0.29,−0.02 | 0.02 |
| Vegetable Naming | −0.08 | −0.16,−0.01 | 0.03 | −0.23 | −0.38,−0.07 | 0.004 | −0.24 | −0.35,−0.14 | <0.0001* |
| Digit Span Forward | 0.00 | −0.03,0.03 | 0.96 | −0.01 | −0.07,0.05 | 0.74 | −0.03 | −0.08,0.02 | 0.19 |
| Digit Symbol Coding | −0.20 | −0.36,−0.05 | 0.009 | −0.61 | −0.92,−0.30 | 0.0001* | −0.36 | −0.59,−0.14 | 0.003 |
| Trail Making Test Part A** | 0.27 | −0.05,0.58 | 0.10 | 1.05 | 0.42,1.69 | 0.001 | 0.86 | 0.40,1.31 | 0.0002* |
| Trail Making Test Part B** | 0.57 | −0.38,1.51 | 0.24 | 2.11 | 0.19,4.02 | 0.03 | 2.38 | 1.02,3.74 | 0.0006* |
| Digit Span Backward | −0.04 | −0.07,−0.00 | 0.04 | −0.08 | −0.14,−0.01 | 0.03 | −0.07 | −0.12,−0.03 | 0.003 |
| Functional Activities Questionnaire** | 0.02 | −0.12,0.17 | 0.78 | 0.05 | −0.17,0.27 | 0.65 | −0.01 | −0.17,0.14 | 0.86 |
| MCI Participants | |||||||||
| Self-only complaintŧ | Informant-only complaintŧ | Mutual complaintŧ | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| Mini-Mental State Examination | 0.15 | −0.12,0.42 | 0.27 | −0.45 | −0.73,−0.16 | 0.002 | −0.60 | −0.79,−0.40 | <0.0001* |
| Logical Memory Immediate Recall | −0.03 | −0.24,0.19 | 0.81 | −0.33 | −0.56,−0.10 | 0.005 | −0.51 | −0.66,−0.35 | <0.0001* |
| Logical Memory Delayed Recall | −0.03 | −0.24,0.18 | 0.78 | −0.35 | −0.57,−0.13 | 0.002 | −0.40 | −0.55,−0.25 | <0.0001* |
| Boston Naming Test | −0.07 | −0.35,0.21 | 0.64 | −0.26 | −0.56,0.03 | 0.08 | −0.48 | −0.68,−0.28 | <0.0001* |
| Animal Naming | −0.11 | −0.38,0.15 | 0.40 | −0.47 | −0.75,−0.19 | 0.0009 | −0.54 | −0.73,−0.35 | <0.0001* |
| Vegetable Naming | 0.05 | −0.16,0.26 | 0.67 | −0.24 | −0.46,−0.02 | 0.03 | −0.38 | −0.53,−0.23 | <0.0001* |
| Digit Span Forward | −0.01 | −0.10,0.08 | 0.76 | −0.03 | −0.12,0.07 | 0.61 | −0.08 | −0.15,−0.02 | 0.01 |
| Digit Symbol Coding | 0.24 | −0.35,0.83 | 0.42 | −0.70 | −1.32,−0.07 | 0.03 | −0.94 | −1.37,−0.51 | 0.00002* |
| Trail Making Test Part A** | −0.74 | −2.48,1.01 | 0.41 | 1.33 | −0.53,3.19 | 0.16 | 2.20 | 0.94,3.46 | 0.0006* |
| Trail Making Test Part B** | −2.77 | −6.93,1.39 | 0.19 | 2.27 | −2.21,6.74 | 0.32 | 5.15 | 2.12,8.18 | 0.0009 |
| Digit Span Backward | 0.05 | −0.04,0.15 | 0.29 | −0.07 | −0.17,0.04 | 0.21 | −0.12 | −0.18,−0.05 | 0.001 |
| Functional Activities Questionnaire** | 0.09 | −0.02,0.21 | 0.11 | 0.08 | −0.02,0.19 | 0.12 | 0.08 | 0.00,0.16 | 0.04 |
Note: Beta-coefficient represents the interaction of follow-up time and complaint subtype;
predictor referent=no complaint within each diagnostic group;
=significant Bonferroni-corrected p-value =p<0.05/72(0.00069);
=higher score=worse performance
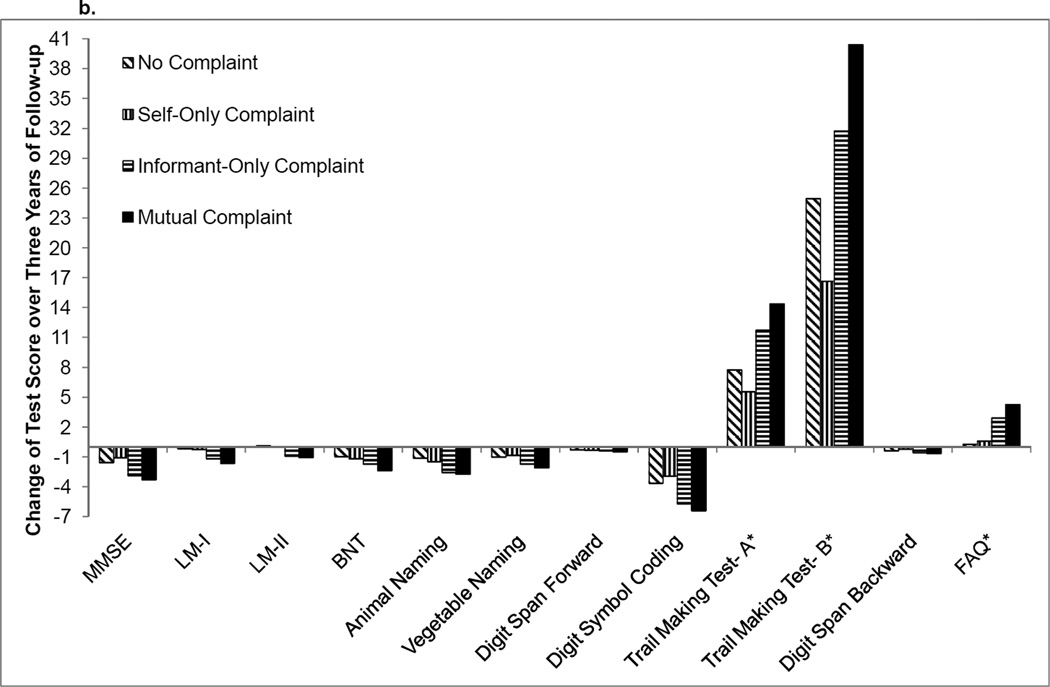
Figure 2.
a. Mean change in raw cognitive test performance over 3 years for NC participants
b. Mean change in raw cognitive test performance over 3 years for MCI participants
Note: MMSE=Mini-Mental State Examination, LM-I=Logical Memory Immediate Recall, LM-II=Logical Memory Delayed Recall, BNT=Boston Naming Test; FAQ=Functional Activities Questionnaire. *=higher scores reflect worse performance
Cognitive Complaint & Longitudinal Cognitive & Functional Performances Among MCI Participants
Compared to the no complaint referent, MCI participants with a mutual complaint declined faster on the MMSE (p<0.0001), Logical Memory Immediate Recall (p<0.0001), Logical Memory Delayed Recall (p<0.0001), Boston Naming Test (p<0.0001), Animal Naming (p<0.0001), Vegetable Naming (p<0.0001), Digit Symbol Coding (p<0.0001), and Trail Making Test Part A (p=0.0006). When a less stringent correction factor was applied (p-value<0.001), participants with mutual complaint declined faster than the referent on Trail Making Test Part B (p=0.0009) and Digit Span Backward (p=0.001). No difference was seen on Digit Span Forward (p=0.01).
Compared to the no complaint referent, MCI individuals with an informant-only complaint did not differ with respect to trajectory on any cognitive measure. When a less stringent correction factor was applied (p-value<0.001), faster decline was observed for Animal Naming (p=0.0009). No difference was detected on the remaining cognitive tests (all p-values>0.002). Self-only complaint was not associated with faster decline on any of the cognitive measures as compared to the no complaint referent (all p-values>0.19). Also, there was no difference in functional trajectory between no complaint and the other complaint subgroups (all p-values>0.04). See Table 3 and Figure 2b for details. When models were recalculated to include data collection site as a random effect, findings were unchanged (data not shown).
Discussion
Leveraging a large, multicenter cohort, we related different sources of cognitive complaint at baseline (i.e., no complaint, self-only, informant-only, and mutual complaint) to cognitive and functional outcomes over an approximate two-year follow-up period. When applying a stringent correction factor for multiple comparisons, results suggest that in NC participants, relative to the no complaint referent, a mutual (i.e., both self and informant) complaint was related to more rapid decline in global cognition (MMSE), lexical retrieval (Boston Naming Test), rapid word generation (Vegetable Naming), information processing speed (Trail Making Test Part A) and executive functioning (Trail Making Test Part B). Similarly, in MCI, a mutual complaint relative to no complaint was related to faster decline in global cognition (MMSE), lexical retrieval (Boston Naming Test), rapid word generation (Animal Naming, Vegetable Naming), information processing speed (Trail Making Test Part A), and episodic memory (Logical Memory Immediate Recall, Logical Memory Delayed Recall).
When considering participants with only one source of complaint, informant-only complaint in NC was related to faster decline in global cognition (MMSE) and information processing speed (Digit Symbol Coding). In contrast, informant-only complaint was not associated with trajectory in any cognitive domain after correction for multiple comparisons in MCI. NC participants with self-only complaint compared to no complaint evidenced faster decline in episodic memory (Logical Memory Immediate Recall, Logical Memory Delayed Recall); however, in MCI, self-only complaint was unrelated to any cognitive decline. In both NC and MCI diagnostic groups, none of the complaint categories (self-only, informant-only, or mutual) were associated with functional changes over the follow-up period in comparison to the no complaint referent.
The present results suggest that cognitive complaint is related to poorer cognitive trajectory in both NC and MCI elders (though there are variations in the pattern of associations between complaint categories and diagnostic groups). Findings highlight the clinical significance of complaint as a potential clinical marker of early neurodegenerative disease, especially among the NC participants, who have been determined by a clinician or consensus team to have normal cognition at baseline. Nevertheless, NC participants with any form of complaint (i.e., mutual, informant-only, or self-only) relative to their peers without any complaint evidenced more rapid cognitive decline over the follow-up period. Furthermore, these declines occurred in cognitive domains preferentially affected in the early clinical phase of AD, including episodic memory [21], executive functioning [22], language [23], and processing speed [24].
Another notable observation of the current study is the strength of a mutual complaint in predicting cognitive decline over the relatively short follow-up period. Regardless of diagnostic status (NC or MCI), a mutual complaint as compared to no complaint was related to cognitive performance decline across multiple domains, such as language, information processing speed, and executive functioning. Our prior work relating cognitive complaint categories to diagnostic conversion supports the notion that when there is concordant concern from a patient and their loved one, the risk for future cognitive worsening is significantly greater [13].
In the clinic, patients and their loved ones often ascribe memory impairments to a diverse range of cognitive changes (e.g., older adults most often complain about problems with poor memory for names or lapses in concentration [25]). However, it is unclear whether self-only or informant-only complaints are unique to episodic memory changes (or related to more diverse cognitive functions) and whether such associations vary by diagnostic status. Among NC participants, our results suggest that informant-only complaint and mutual complaint are associated with diffuse cognitive changes, such as global cognition, language, or information processing speed, but unrelated to episodic memory. While these latter findings differ from prior work in non-demented older adults where informant-only complaint is associated with memory decline over time [12], many prior studies suggest that informant-only complaint measures tap global cognition [26] or multiple areas of cognition [27]. It is plausible that informants who endorse the presence of diffuse cognitive changes do so only after observing a higher threshold of cognitive changes, reflecting a more advanced stage of neuropathological changes. For example, Tierney et al., [28] found that informants of memory clinic patients who develop clinical AD reported greater cognitive problems two years prior to the diagnosis than informants of patients who did not develop AD.
There are several null findings that warrant brief discussion. First, in contrast to the diffuse associations seen between informant-only complaint and cognitive decline in NC participants, informant-only complaint was unrelated to any cognitive decline in the MCI group. If we applied a more lenient significance threshold (p<0.001 versus p<0.00069), several associations would have emerged for the informant-only complaint group, including faster decline in cognitive processes commonly affected by AD (i.e., global cognition, delayed episodic memory, lexical retrieval). However, a more lenient threshold increases the probability of a false positive observation. Another null result worth mentioning is that among MCI participants, self-only complaint was unrelated to decline across all eleven cognitive measures tested. The absence of an association, especially with episodic memory performance, may be attributable to anosognosia or compromised meta-memory seen in MCI [29] and early AD [30]. Third, regardless of diagnostic status, all three complaint categories, relative to no complaint, were unrelated to decline on Digit Span Forward. This attention measure may be less sensitive to early cognitive changes associated with AD [31]. Finally, there was no association between complaint status and change in FAQ score over time in either diagnostic group, potentially because meaningful functional changes rarely occur in cognitively normal elders and are very mild in MCI [32]. Alternatively, FAQ may be insufficiently sensitive to the earliest forms of change. More sophisticated assessment of early functional errors may offer a sensitive approach to modest functional changes in MCI relative to NC elders [32]. Future studies may explore relating complaints to error-based functional assessment tools [33] rather than more global IADLs measures [20].
The current study has a number of strengths. The extensive NACC UDS neuropsychological protocol allowed for comparison of complaint in relation to general cognition as well as specific cognitive systems strongly implicated in AD (i.e., language, episodic memory, executive functioning). The large NACC sample size offered power to detect even modest effects across cognitive measures. Additionally, the diagnostic criteria for NC and MCI are standard across participating sites. The longitudinal follow-up and inclusion of self and informant complaint alone and in combination (mutual complaint) represent important methodological strengths, allowing us to better characterize relations between complaint and cognitive and functional trajectory.
Despite these strengths, there are some essential limitations of the current study. Participants comprising the NACC dataset represent a convenience sample, including clinician-referrals and community-based volunteers who are predominantly White and well-educated. Exclusion criteria eliminated participants with confounding medical histories (e.g., psychiatric disorder, head trauma, stroke, transient ischemic attack). These factors collectively limit the generalizability of our findings. Additionally, the two-year follow-up period was relatively short, potentially limiting our detection of meaningful cognitive decline in the cognitively normal participants. Ideally, the large sample size offsets this limitation by providing more power to detect subtle cognitive changes within the short follow-up timeframe; however, we cannot rule out the possibility that additional significant observations might have emerged had the timeframe been more robust. Another limitation is that the dichotomous coding of complaint does not allow for gradations of complaint severity. Finally, methods for determining cognitive complaint likely vary across sites, contributing unwanted variance in our statistical models and limiting the ability to detect a statistically significant association that might otherwise exist. For example, complaint can be assessed by one question [34] or a multi-item questionnaire [35]. There are various ways to query about complaint, including comparing cognition to one’s own past abilities [36], comparing cognition to one’s peers [37], judging cognition based upon the ability to perform a functional task [38], or using various assessment tools (i.e., measures of memory abilities, everyday functioning, dysexecutive questionnaire). Different complaint questions may have varying degrees of association with objective cognitive performance [39]. Moving forward, standardization of cognitive complaint definitions and assessment methods will be important to minimize or eliminate this problem.
This study enhances existing literature on the clinical relevance of cognitive complaints by methodologically integrating the informant’s perspective of cognitive changes in relation to cognitive and functional trajectories in older adults. Findings suggest that a mutual complaint, regardless of baseline diagnostic status, is associated with the greatest decline across a wide range of cognitive abilities. These results, which are most notable in MCI, highlight that the inclusion of informant report may increase sensitivity for predicting cognitive outcomes in comparison to information gathered exclusively from the identified patient [40]. Findings stress the importance of incorporating an informant whenever possible into memory loss work-ups for older adults.
Acknowledgements
U01- AG016976 (National Alzheimer’s Coordinating Center); Alzheimer’s Association NIRG-13-283276 (KAG); NACC Junior Investigator Award #2011-JI-08 (KAG); T32-AG036697 (KAG); K23-AG030962 (Paul B. Beeson Career Development Award in Aging; ALJ); K24-AG046373 (ALJ); Alzheimer’s Association IIRG-08-88733 (ALJ); R01-AG034962 (ALJ); P30-AG013846 (Boston University Alzheimer’s Disease Core Center); M01-RR00533 (General Clinical Research Centers Program of the National Center for Research Resources, NIH)
References
- 1.Albert MS, Dekosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kolsch H, Luck T, Mosch E, van den Bussche H, Wagner M, Wollny A, Zimmermann T, Pentzek M, Riedel-Heller SG, Romberg HP, Weyerer S, Kaduszkiewicz H, Maier W, Bickel H. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Archives of General Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 3.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiology of Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- 7.Dufouil C, Fuhrer R, Alperovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 8.Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, van Kamp GJ, Deeg DJ. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57:2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- 9.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 10.Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. Journal of the American Geriatrics Society. 2006;54:335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 11.Crowe M, Andel R, Wadley V, Cook S, Unverzagt F, Marsiske M, Ball K. Subjective cognitive function and decline among older adults with psychometrically defined amnestic MCI. Int J Geriatr Psychiatry. 2006;21:1187–1192. doi: 10.1002/gps.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual's complaints of memory impairment in early dementia. Neurology. 2000;55:1724–1726. doi: 10.1212/wnl.55.11.1724. [DOI] [PubMed] [Google Scholar]
- 13.Gifford K, Liu D, Tripodis Y, Palmisano J, Lu Z, Cantwell N, Kowall N, Jefferson AL. The source of cognitive complaints predict diagnostic conversion among non-demented older adults. Alzheimer's and Dementia. doi: 10.1016/j.jalz.2013.02.007. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert MS. Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93:13547–13551. doi: 10.1073/pnas.93.24.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 16.Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Smith GE, Ivnik RJ, Kokmen E, Tangalos EG. Memory function in very early Alzheimer's disease. Neurology. 1994;44:867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- 22.Albert MS, Moss M, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 23.Mickes L, Wixted JT, Fennema-Notestine C, Galasko D, Bondi MW, Thal LJ, Salmon DP. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer's disease. Neuropsychology. 2007;21:696–705. doi: 10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- 24.Twamley EW, Ropacki SA, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2006;12:707–735. doi: 10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cargin J, Collie A, Masters C, P M. The nature of cognitive complaints in healthy older adults with and without objective memory decline. Journal of Clinical and Experimental Neuropsychology. 2008;30:245–257. doi: 10.1080/13803390701377829. [DOI] [PubMed] [Google Scholar]
- 26.Jorm AF. Assessment of cognitive impairment and dementia using informant reports. Clinical Psychology Review. 1996;16:51–73. [Google Scholar]
- 27.Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, Sachdev PS. Prevalence and predictors of "subjective cognitive complaints" in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 28.Tierney MC, Szalai JP, Snow WG, Fisher RH. The prediction of Alzheimer disease. The role of patient and informant perceptions of cognitive deficits. Archives of Neurology. 1996;53:423–427. doi: 10.1001/archneur.1996.00550050053023. [DOI] [PubMed] [Google Scholar]
- 29.Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer's disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17:181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 30.Cosentino S, Metcalfe J, Butterfield B, Stern Y. Objective metamemory testing captures awareness of deficit in Alzheimer's disease. Cortex. 2007;43:1004–1019. doi: 10.1016/s0010-9452(08)70697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris JC, Storandt M, Miller JP, McKeel DWJ, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer's disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 32.Jefferson AL, Byerly LK, Vanderhill S, Lambe S, Wong S, Ozonoff A, Karlawish JH. Characterization of activities of daily living in individuals with mild cognitive impairment. American Journal of Geriatric Psychiatry. 2008;16:375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glosser G, Gallo J, Duda N, de Vries JJ, Clark CM, Grossman M. Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2002;15:198–206. [PubMed] [Google Scholar]
- 34.Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, West JD, Flashman LA, Wishart HA, Santulli RB, Rabin LA, Pare N, Arfanakis K, Saykin AJ. Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta. 2011;1822:423–430. doi: 10.1016/j.bbadis.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jessen F, Wiese B, Cvetanovska G, Fuchs A, Kaduszkiewicz H, Kolsch H, Luck T, Mosch E, Pentzek M, Riedel-Heller SG, Werle J, Weyerer S, Zimmermann T, Maier W, Bickel H. Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med. 2007;37:1753–1762. doi: 10.1017/S0033291707001122. [DOI] [PubMed] [Google Scholar]
- 37.Lam LC, Lui VW, Tam CW, Chiu HF. Subjective memory complaints in Chinese subjects with mild cognitive impairment and early Alzheimer's disease. Int J Geriatr Psychiatry. 2005;20:876–882. doi: 10.1002/gps.1370. [DOI] [PubMed] [Google Scholar]
- 38.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, Decarli C. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59:1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavett R, Dunn JE, Stoddard A, Harty B, Weintraub S. The Cognitive Change in Women study (CCW): informant ratings of cognitive change but not self-ratings are associated with neuropsychological performance over 3 years. Alzheimer Dis Assoc Disord. 2011;25:305–311. doi: 10.1097/WAD.0b013e31820d8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Wechsler D. Wechsler Memory Scale-Revised. San Antonio, Texas: Psychological Corporation; 1987. [Google Scholar]
- 43.Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 44.Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 46.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]