Abstract
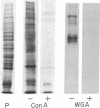
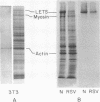
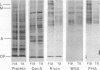
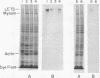
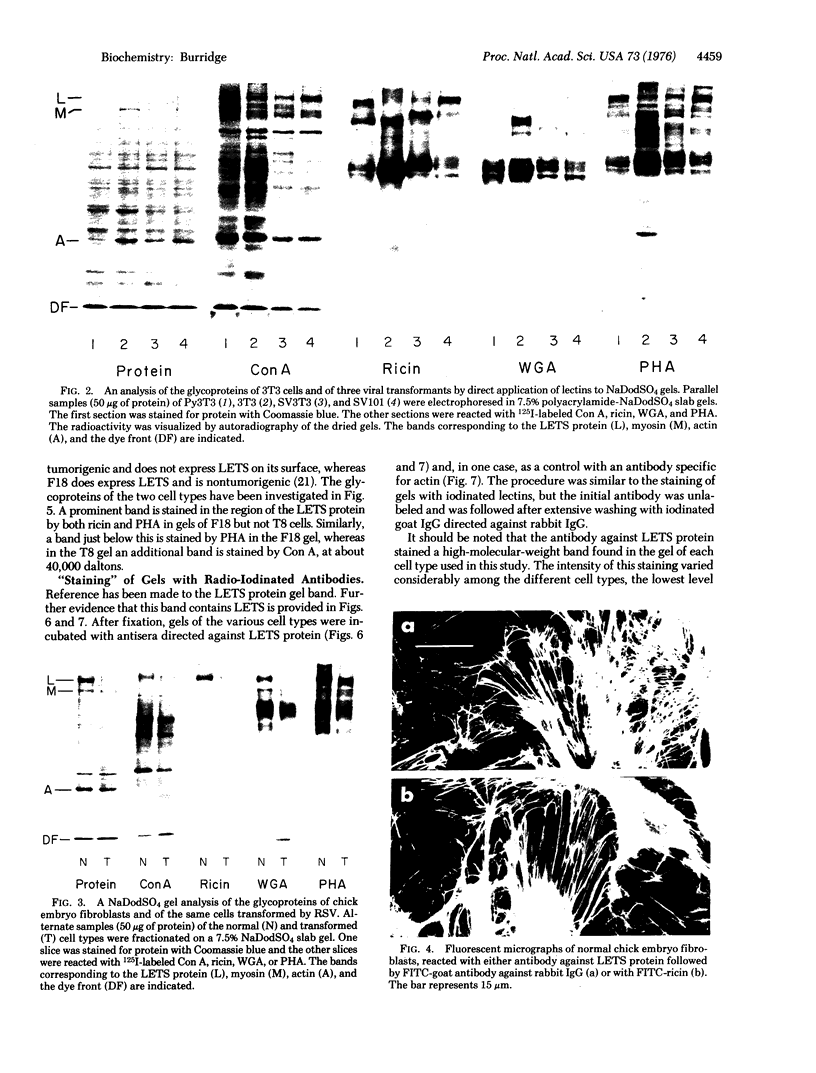
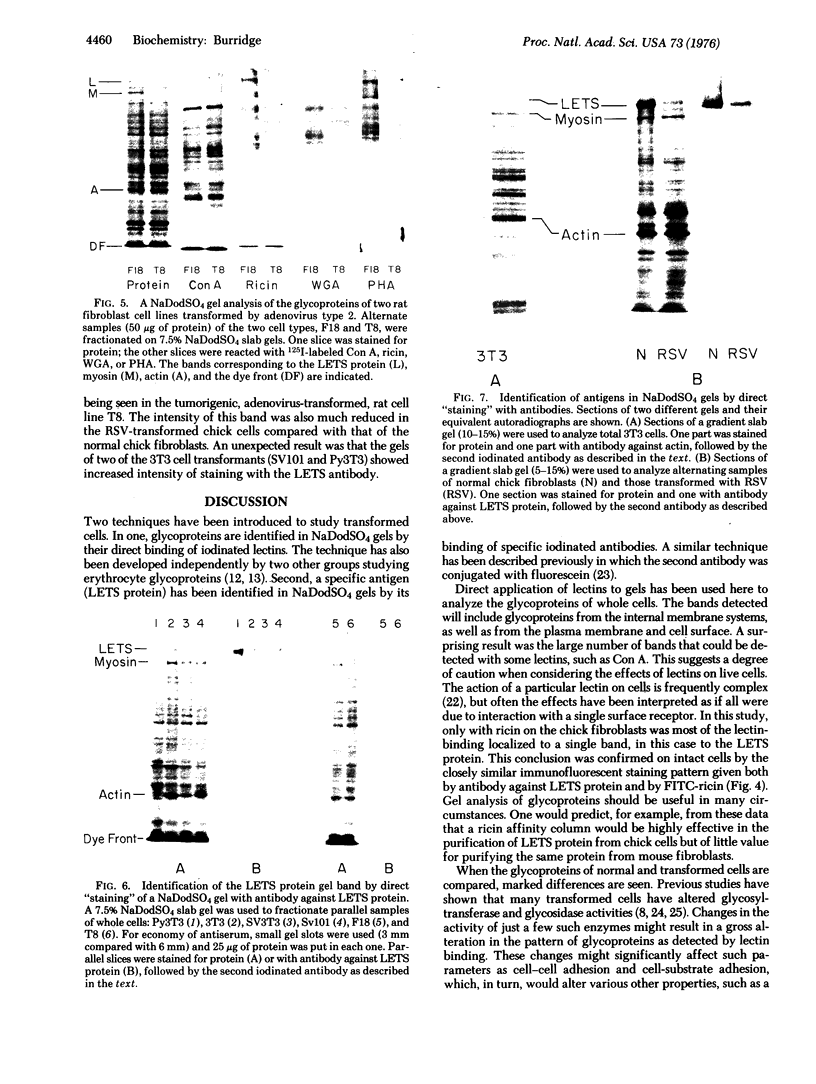
The glycoproteins of whole cells have been analyzed by direct application of radio-iodinated lectins to sodium dodecyl sulfate gels, followed by autoradiography. By use of lectins with different carbohydrate specificities, different sets of glycoproteins have been visualized. The most prominent lectin-binding band in many gels is thelarge, external, transformation-sensitive (LETS) protein. Major glycoprotein differences are revealed when normal and virus-transformed cells are compared. Certain differences, however, are also seen when the glycoproteins are compared from two separately derived simian virus 40 transformants of 3T3 cells, suggesting a degree of clonal variation between these lines that may not relate to transformation. A complementary technique is used to detect specific antigens in sodium dodecyl sulfate gels by direct application of iodinated antibodies. An antiserum specific for the LETS protein is used to identify this antigen in the gels of both normal and transformed cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosmann H. B., Lockwood T., Morgan H. R. Surface biochemical changes accompanying primary infection with Rous sarcoma virus. II. Proteolytic and glycosidase activity and sublethal autolysis. Exp Cell Res. 1974 Jan;83(1):25–30. doi: 10.1016/0014-4827(74)90683-1. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Gallimore P. H., McDougall J. K. Correlation between tumor induction and the large external transformation sensitive protein on the cell surface. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3570–3574. doi: 10.1073/pnas.73.10.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley D. R. Cell surface proteins of NIL1 hamster fibroblasts labeled by a galactose oxidase, tritiated borohydride method. Cell. 1974 Oct;3(2):121–125. doi: 10.1016/0092-8674(74)90115-9. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J. Sialic acid transferases and sialic acid levels in normal and transformed cells. Biochemistry. 1970 Dec 22;9(26):5083–5092. doi: 10.1021/bi00828a007. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I., Anfinsen C. B. Purification of synthetic ribonuclease S-peptide derivatives by specific complex formation on columns of ribonuclease S-protein bound to agarose. J Biol Chem. 1969 Nov 10;244(21):5849–5855. [PubMed] [Google Scholar]
- Kijimoto S., Hakomori S. Enhanced glycolipid: -galactosyltransferase activity in contact-inhibited hamster cells, and loss of this response in polyoma transformants. Biochem Biophys Res Commun. 1971 Aug 6;44(3):557–563. doi: 10.1016/s0006-291x(71)80119-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Ohta N., Pardee A. B., McAuslan B. R., Burger M. M. Sialic acid contents and controls of normal and malignant cells. Biochim Biophys Acta. 1968 Apr 16;158(1):98–102. doi: 10.1016/0304-4165(68)90076-7. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Robinson P. J., Bull F. G., Anderton B. H., Roitt I. M. Direct autoradiographic visualisation in SDS-gels of lectin-binding components of the human erythrocyte membrane. FEBS Lett. 1975 Oct 15;58(1):330–333. doi: 10.1016/0014-5793(75)80291-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama H., Burge B. W. Comparative studies of the carbohydrate-containing components of 3T3 and simian virus 40 transformed 3T3 mouse fibroblasts. Biochemistry. 1972 Apr 11;11(8):1366–1377. doi: 10.1021/bi00758a007. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- Stumph W. E., Elgin S. C., Hood L. Antibodies to proteins dissolved in sodium dodecyl sulfate. J Immunol. 1974 Dec;113(6):1752–1756. [PubMed] [Google Scholar]
- Tanner M. J., Anstee D. J. A method for the direct demonstration of the lectin-binding components of the human erythrocyte membrane. Biochem J. 1976 Feb 1;153(2):265–270. doi: 10.1042/bj1530265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Critchley D., Macpherson I. Surface glycoproteins and glycolipids of chicken embryo cells transformed by a temperature-sensitive mutant of Rous sarcoma virus. Nature. 1972 Feb 4;235(5336):275–278. doi: 10.1038/235275a0. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]