Discussion Leaders: Eberhart Zrenner and Bradley Greger
Scribe: Daniel Rathbun
Session Participants: David Birch, John Dowling, Erika Ellis, Fred Fitzke, Donald Hood, Alan Laties, Daniel Palanker, John Pezaris, Joseph Rizzo, Gary Rubin, Ronald Schuchard, Dirk Trauner, James Weiland, and Frank Werblin
Introduction
The timing of the Lasker/IRRF (International Retinal Research Foundation) Initiative on Restoring Vision to the Blind in March of 2014 was particularly opportune given that the first commercial sales of implanted visual prostheses (the Argus II) occurred in 2011 (Rizzo et al., 2014), and a second commercial device (the Alpha-IMS [Institut für Maschinelle Sprachverarbeitung]) entered the market in 2013 (Zrenner, 2013). We are therefore at a perfect point to look back on the successes of visual prostheses as well as to look forward to what the future may hold. The core question for the Implanted Visual Prostheses session, which was fine-tuned by the session members and provided a framework for our discussions was: How to provide useful visual information to patients blind from lesions in the afferent visual pathway by means of safe and efficient electronic implants?
Accomplishments to Date
Several strategies have been employed to electrically activate the neurons that remain after loss of vision. These can loosely be arranged according to which neuron along the visual pathway is being targeted (Fig. 1.1). For extensive reviews of these different strategies, the reader is directed to recent review articles: Chuang, Margo, & Greenberg, 2014; Guenther, Lovell, & Suaning, 2012; Luo and da Cruz, 2014; Matthaei et al., 2011; Maynard, 2001; Weiland, Cho, and Humayun, 2011; Zrenner, 2013. For a further history of how the principal research groups and concepts emerged, the reader is directed to Dowling, 2005.
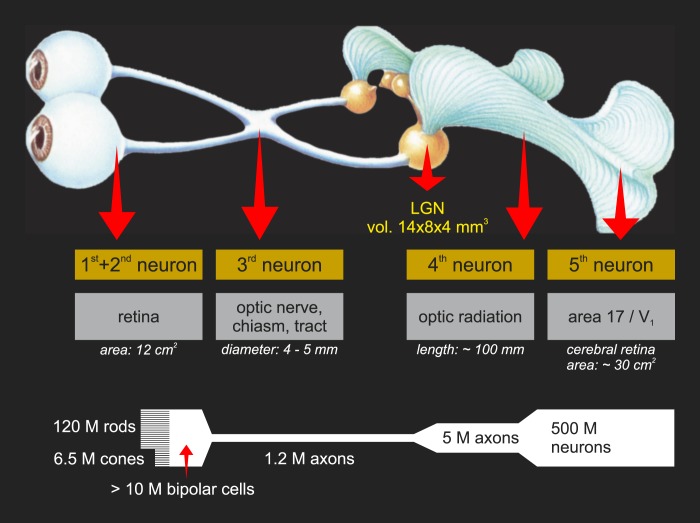
Figure 1.1.
Overview of the visual system as related to visual prostheses. In most retinal dystrophies, the first order photoreceptor neurons (rods and cones) are lost. Thus, second order neurons (bipolar cells) are the earliest viable target, typically for subretinal and suprachoroidal devices. Epiretinal devices typically target retinal ganglion cells (RGCs), the third order neurons that form the output of the retina. Likewise, optic nerve devices target these neurons either within the eye at the nerve head or outside of the eye. The fourth order neurons, relay cells of the thalamic lateral geniculate nucleus (LGN) are targeted with penetrating electrode arrays. Finally, cortical implants target the fifth and higher order neurons found in the primary visual cortices. (Modified from Krey, H.F., & Brauer, H. (1998). Chibret Augenatlas: Eine Repetition für Ärtze mit Zeigetafeln für Patienten. Munich: Chibret Med Serv.)
Given the clinical successes and the diversity of strategies employed by retinal implants, a brief overview of their merits relative to each other is appropriate. The most common retinal implants can be classified as epiretinal, subretinal, or suprachoroidal according to the placement of their electrode arrays (Fig. 1.2). Typically, epiretinal and suprachoroidal implants have employed extraocular light sensors, whereas subretinal implants couple light sensors with the stimulating electrodes at the position of lost photoreceptors to ensure that the sensors exploit natural eye movements. In subretinal and suprachoroidal implants, bipolar cells are targeted for stimulation in hopes that by activating the retinal network as early as possible residual neural processing of bipolar and amacrine cells can be exploited. Epiretinal implants employ a simpler surgical procedure than subretinal implants to target the ganglion cells for more direct control of the output signals of the retina. Even simpler and less invasive is suprachoroidal placement in which a scleral tunnel through the back of the eye is used to insert the array. To date, only preliminary clinical experiments with suprachoroidal implants have been conducted in which a small number of widely spaced electrodes were stimulated directly through a laboratory computer without the accompanying camera system.
Figure 1.2.
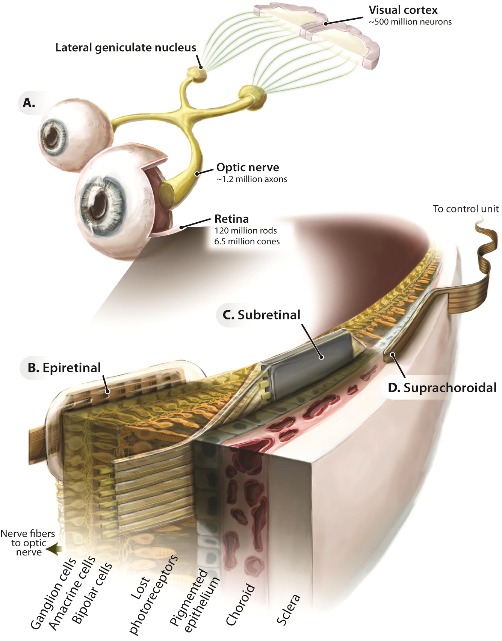
General approaches to stimulating the retina. (A) Schematic of the visual system highlighting the locations or retinal implants. (B) Epiretinal placement: imaging sensors are distant from the retina (typically external to the eye) and then delivered to an array of electrodes positioned on the vitreous-contacting side of the retina. (C) Subretinal placement: imaging sensors and electrodes are coupled and positioned on the retinal pigment epithelium–contacting side of the retina. (D) Suprachoroidal placement: imaging sensors are typically extraocular, and electrodes are positioned between the choroid and sclera. (From Zrenner, E. (2013). Fighting blindness with microelectronics. Science Translational Medicine, 5(210), 210ps16. Reprinted with permission from AAAS.)
Recent Advances in Retinal Stimulation: Clinical Applications
Of the many devices investigated, retina-based devices have shown the greatest success. These devices are limited, however, to eye diseases that destroy the photoreceptors but leave, at the very least, the ganglion cells intact for direct stimulation (Kellner, 2000). Of those diseases, retinitis pigmentosa (RP) has been the most attractive candidate because some affected individuals reach near-complete blindness at a relatively early age, and the inner (neural) retina is relatively spared. In comparison, age-related macular degeneration (AMD) has a late onset and does not cause total blindness for many years, typically leaving the retinal periphery functional.
At present, there are seven major ongoing retinal prosthesis projects that have either implanted test subjects or have concrete plans to do so in the near future. The Argus II developed by Second Sight (Second Sight Medical Products, Lausanne, Switzerland) has been implanted during clinical trials in over 30 patients in both Europe and the United States (Humayun et al., 2012) where it has also been approved for commercial sale (in 2011 and 2013, respectively [Rizzo et al., 2014]). Currently, approximately 45 commercial devices have been implanted. In comparison, the Alpha-IMS system of Retina Implant AG has been implanted during clinical trials in over 40 patients (Zrenner, 2013) and in 2013 received CE (Conformité Européenne) approval for commercial sale in the European Union with the first sales expected in 2014 (W. Wrobel, personal communication,). In addition to these two commercial devices, the IRIS device developed by Intelligent Medical Implants (IMI) was implanted in 20 patients in 2003 and 2004 (Hornig et al., 2007). This IMI (Innovative Medicines Initiative) device has been acquired by Pixium, which is conducting renewed clinical trials. Furthermore, devices developed by the Bionic Vision Australia consortium (Saunders et al., 2014) the Boston Retina Implant Project (Rizzo, 2011), Nidek Co., Ltd. (Nidek Co., Aichi, Japan) (Fujikado et al., 2011), and the Stanford-based photovoltaic retinal prosthesis (PRIMA) (Mandel et al., 2013; Mathieson et al., 2012) have announced plans to enter clinical trials in the next few years. Functional results from some of these retinal implants will be discussed below.
Recent Advances in Optic Nerve and Thalamic Stimulation
Optic nerve stimulation seeks to create action potentials in the axons of the ganglion cells. Whereas epiretinal stimulation targets these ganglion cells at or very near their cell bodies in order to preserve retinotopy (Fried, Lasker, Desai, Eddington, & Rizzo, 2009; Jensen, Rizzo, Ziv, Grumet, & Wyatt, 2003; Sekirnjak et al., 2008), optic nerve stimulation targets these axons at the bottleneck of the visual system where about one million axons from the entire retina are tightly packed into the 3.5-mm wide optic nerve. Regrettably, the greatest drawback to optic nerve stimulation, especially for surface stimulation, is that resultant light perceptions (phosphenes) are irregular in both shape and visual location, requiring sophisticated image processing algorithms to create a meaningful visual scene. To date, two patients have been implanted with optic nerve cuff devices in Belgium, and ongoing work is targeted at optimizing optic nerve electrode arrays and the related surgical techniques and stimulation paradigms (Brelen, Vince, Gerard, Veraart, & Delbeke, 2010; Lu et al., 2013; Sakaguchi et al., 2009).
A relatively new approach seeks to stimulate the targets of retinal ganglion cell axons in the lateral geniculate nucleus (LGN) of the thalamus (Pezaris & Reid, 2007). One potential advantage of this approach compared with retinal stimulation is that while the whole visual space is topographically represented in the compact LGN, the crucial foveal representation comprises a larger proportion of the neural tissue than the fovea in the retina. A potential advantage of LGN stimulation compared to the cortical approach is that the neural representation for vision in the LGN is less complex, although much work still remains in understanding the encoding of the space- and time-varying neuronal signals that pass through the LGN.
Recent Advances in Cortical Stimulation
The earliest multielectrode prosthesis for vision was on the surface of a patient's visual cortex in July of 1967, and was an array of flat electrodes, embedded in silicone, and activated wirelessly through transdermal radio transmission (Brindley & Lewin, 1968). This implant demonstrated that electrical stimulation of the visual system could produce light percepts (phosphenes) with reliable location, shape, and size; however, it was inconveniently bulky, required high currents, occasionally caused pain upon stimulation, and was unable to produce sufficient coverage of the visual space for useful perceptions. Building on this work another group continued to develop cortical stimulation devices for decades afterward (Dobelle, 2000; Dobelle & Mladejovsky, 1974). As semiconductor microfabrication developed, penetrating intracortical microstimulation (ICMS) was investigated by other groups to overcome some of the limitations of cortical surface stimulation. ICMS should theoretically allow for higher spatial resolution when penetrating implants finally enter clinical trials (Bak et al., 1990; Bradley et al., 2005; Davis et al., 2012).
It should be noted that many more devices are under development than could reasonably be presented here, and the details of each research project change on a monthly basis. For this reason, interested readers are encouraged to visit the continuously updated list at http://www.eye-tuebingen.de/zrenner/retimplantlist/ for the latest information.
How Good Is Restored Vision?
Visual function is notoriously difficult to quantify, especially in low-vision subjects (Geruschat, Bittner, & Dagnelie, 2012). Even the seemingly simple metric of visual acuity can be measured with a broad range of tests that only loosely correlate with each other. In addition to visual acuity, an important feature of visual function is the location and size of the visual field. In some cases, it makes more sense to assess restoration of the visually-guided behaviors restored to a patient by an implant in terms of improved quality of life (see also Chapter 8). Furthermore, given the psychological impact of blindness, psychological benefit should also be considered.
Of the nearly 100 patients implanted with either an Argus II or an Alpha-IMS, the three best visual acuities achieved to date are 20/1262 with an Argus II device using a grating orientation test (Humayun et al., 2012), 20/1000 with a prototype that preceded the Alpha-IMS device measured with Landolt C test (Zrenner et al., 2011), and most recently, 20/546 with an Alpha-IMS implant using the same Landolt C test (Stingl et al., 2013). With the Argus II device, patients receive stimulation from 60 electrodes over an area of approximately 10° × 20° of visual angle, whereas with the Alpha-IMS device, they receive input from 1500 electrodes over an area of 11° × 11°. Beyond visual acuity, implanted patients have demonstrated object localization, discrimination, and identification; motion detection and discrimination; letter identification; and limited reading where none of these tasks were possible either before implantation or afterward with the implants turned off. Furthermore, patients can perform simple orientation and mobility tasks using the devices. All of these tasks have been measured in the laboratory to enable comparison among test subjects but have also been reported in real-world use by a limited subset of patients.
What Is the Value of an Implant?
Recent clinical trials have established that visual prostheses can provide visual information that is useful in daily life. Looking forward, it can be anticipated that, with continuing development, these devices have a high probability of acceptance by the RP patient population. The risks associated with implanted visual prostheses in terms of serious adverse events (SAE) have been shown to be low (Humayun et al., 2012; Stingl et al., 2013). However, limited real-world experience currently precludes a complete assessment of the cost/benefit tradeoff that can be expected with such devices. In evaluating this tradeoff, the seemingly contradictory outcome goals of patient independence and social connectedness need to be carefully evaluated. Critically, such evaluation needs to consider the life situation of each individual patient. For example, while one person may value reading highly, a sports enthusiast may prefer faster signals with lower spatial resolution (although both normal reading and sporting activities lie beyond current visual prosthesis capabilities). Given this context-dependence of the utility of restored vision, it is important to identify the most important aspects of visual function. For most visually guided tasks such as reading and navigation, nonprosthetic solutions are well developed (see Chapter 7). Therefore, we propose that the two primary goals of prosthetic vision should be to improve the subject's independence and social connectedness. Developing meaningful measures for these two should, therefore, be a top priority for psychophysical testing of implanted prostheses in the future. Validated questionnaires for such assessments are discussed further in Chapter 8. Furthermore, in quantifying regained independence and connectedness and integrating them with more traditional measures of visual function, the goal should be to facilitate cost-benefit analyses such as the quality-adjusted life year (QALY) for use by individuals, clinicians, insurers, researchers, governments, and research funding agencies. For general information on how the usefulness of medical interventions is evaluated, see Fanshel & Bush, 1970; Pliskin, Shepard, & Weinstein, 1980; for specific evaluations of implantable visual prostheses, see Vaidya et al., 2014; Wrobel, 2010, as an example. The final monetary and societal value of an implant device will be a deciding factor in whether it can prove viable either as a commercial venture or as a humanitarian/societal endeavor.
Managing Expectations
The development of the Argus II and Alpha-IMS implants has been the culmination of decades of research and the investment of enormous capital resources from many governmental, charitable, and commercial entities. However, it is vital that we as a community are careful not to oversell the capabilities of our devices. As a general rule of thumb, these two devices have yielded extraordinary visual restoration in approximately one-quarter of the patients tested. Useful gains in daily function were seen in another one-quarter. The final one-half of patients realized only rudimentary functional gains, like simple light perception or localization of bright versus dark areas, which is although of limited use, appreciated by blind people. Only relatively few had no benefit at all, despite undergoing hours of surgery and weeks of recovery. Of the one-quarter of patients who have extraordinary gains, only a handful have come close to the theoretical limits of visual acuity possible based on the device limitations. Although we hope to see typical results continue to improve as the devices move into mainstream medicine and as manufacturing and surgical techniques improve further, such improvements have yet to be demonstrated.
A Goggle System for Image Preprocessing
One realm in which further improvements may be achieved with existing implanted devices is in more sophisticated preprocessing of the images prior to their conversion into electrical stimulation patterns. Since an external camera is implicit in its design, Second Sight and its collaborators have already made strides in image preprocessing, including face localization software to activate the electrodes corresponding to a face location, implementing zoom to resize the visual scene onto the electrode array, and the direct presentation of Braille letters (Dorn et al., 2013; Guerra et al., 2013; Lauritzen et al., 2012). With the Alpha-IMS, the camera is an integrated part of the subretinally implanted device and cannot easily be modified. However, an attractive option for this device is to incorporate Google Glass, Oculus Rift, or one of the other head-mounted displays currently being developed. This modification also simplifies the process of testing and updating image processing algorithms. Notably, goggles have always been an integral component of the Stanford photovoltaic system, which can use conventional liquid crystal display (LCD) or dot matrix display (DMD) displays, or a novel holographic projection for enhanced brightness (Goetz, Mandel, Manivanh, Palanker, & Cizmar, 2013). Integration of eye tracking into the system enables location-specific image processing, such as correction of the radial spread in the fovea (Asher, Segal, Baccus, Yaroslavsky, & Palanker, 2007). Given the broad applicability of such a goggle system for low-vision aids, optogenetics, and photopharmacology in addition to implantable prostheses, there is a strong case to be made for development of a standardized platform custom-built to meet the needs of the blind community. For additional discussion of this issue, see Chapter 7.
Consensus Statements Regarding Implantable Visual Prostheses:
• They can provide useful vision in daily life.
• They have a high probability of acceptance by blind RP patients.
• The associated risks are low.
• Evaluation of the cost-benefit tradeoff requires further investigation.
• Patient expectations should be carefully managed.
• Primary outcome measures should focus on improvement of both independence and social connectedness.
• Better image preprocessing will improve prosthetic vision.
Short-Term Research Goals
Improve Phosphene Reliability – Epiretinal Approach
Irregularity and inconsistency of phosphene perception across different electrodes during direct epiretinal stimulation of ganglion cells continues to limit the effectiveness of this strategy and requires significant training (published and public statements have ranged from several months to up to 3 years) (Cosendai, 2014; Cosendai et al., 2014; Humayun et al., 2012). One possible reason is the large variation in distance between the electrodes and target cells. Another is that it is difficult to stimulate only local ganglion cells without also activating axonal fibers of other cells that pass under the electrodes, producing arcuate percepts instead of a single dot. Multiple studies in animal models have shown that axonal stimulation can be avoided only by using longer stimuli, which activate inner retinal neurons rather than ganglion cells.
Increase Stimulation Frequency – Subretinal Approach
Although subretinal stimulation has yielded the best-restored visual acuity via visual prosthetics to this date, creating perceptions at a high stimulus frequency remains problematic. This is likely due to adaptation of the neural network to high frequency pulse trains. Experiments are currently underway in multiple labs to better understand the complex responses generated by subretinal stimulation and harness them to produce better visual perception.
Improve Spatial Resolution – Most Devices
It has been asserted that to be useful in daily life, retinal prostheses should include at least 500 pixels spread over an area of approximately 10° × 15° in the central visual field (Fornos, Sommerhalder, Rappaz, Safran, & Pelizzone, 2005; Perez Fornos, Sommerhalder, Pittard, Safran, & Pelizzone, 2008; Sommerhalder et al., 2003; Sommerhalder et al., 2004). Therefore, it is important that devices that do not currently meet these minimal requirements are either modified to increase the number of pixels and/or stimulation area or to compensate in some other way. Nevertheless, based on physical limitations, it will be difficult to go beyond a pitch of 50 μm for either epi- or subretinal stimulation without employing sophisticated methods like current focusing or current steering (Eiber, Lovell, & Suaning, 2013).
Improve Contrast
Electrical stimulation results in visual perceptions that differ from those occurring with natural retinal signaling originating in photoreceptors. Further research of the neural signaling might elucidate protocols to enhance the perceptual range and contrast of the image. Furthermore, contrast enhancement can improve the spatial resolution up to the limit set by electrode spacing.
Image Preprocessing
Prosthetic devices discussed in this chapter transform images from the visual world into electrical signals. Ideally, image processing should compensate for the missing signal processing in the lost part of the neural network, the altered state of existing retinal processing, where relevant, and the input-output relationship between electrical stimulation and neural response. For epiretinal devices, retinal ganglion cell (RGC) spike trains can be driven at rates in excess of 500 Hz, where each pulse drives a separate spike (Cai, Ren, Desai, Rizzo, & Fried, 2011). Subretinal devices stimulate nonspiking inner retinal neurons and rely on conversion of these signals into RGC spiking via the retinal network. A more detailed understanding of how stimulation is converted into spike patterns in various types of ganglion cells should help with optimization of the signal preprocessing. In the case of direct stimulation of the ganglion cells, a complete input/output model of retinal visual processing (encoder) is required to define the spike pattern that should be generated for a particular visual stimulus (Nirenberg & Pandarinath, 2012). With an extraocular camera, such “encoding” of the images should also include information about eye movements. The higher up in the visual system a prosthesis is situated, the more visual processing must be incorporated into such an encoder. Furthermore, beyond compensating for the substitution of retinal processing with an implant system, image preprocessing also holds the potential to enhance artificial percepts.
Improve and Standardize Assessment of Performance
Until recently, patient studies have been focused on demonstrating the safety and rudimentary effectiveness of prosthetic implants. For example, the Food and Drug Administration Investigational Device Exemption (IDE) guidance for retinal prostheses recommends testing letter acuity, grating acuity, spatial mapping, form vision, orientation/mobility, activities of daily living, and patient reported outcomes (Cohen EL, 2013). These recommended tests represent a good starting point but cannot provide a full picture of the utility of visual prostheses. To better characterize what is actually gained by the patients, we recommend the widespread engagement of psychophysicists with the appropriate expertise, as well as orientation and mobility specialists incorporating the following improvements to current assessment methods: (1) incorporating cognitive load testing and measuring response latency to contextualize current performance measures, (2) documenting device usage with embedded electronics and questionnaires to assess how much and for what purposes patients actually use the device at home, and (3) assessing the economic benefit of the device with standardized measures such as various formulations of the QALY as discussed above (also see Evaluating Visual Function, Endpoints, Chapter 8).
Long-Term Research Goals
How Can We Increase Both Visual Resolution and Visual Field Size of the Implants?
To date, the best-restored acuity in patients is 20/546, corresponding to a gap in the Landolt C of approximately 1.8 sensor units (126 μm) (Stingl et al., 2013). Recent results with subretinal stimulation in rats demonstrated that prosthetic acuity may reach the theoretical limit of the sampling density of the arrays, 65 μm (Palanker et al., 2014). Accordingly, it is reasonable to assume that even better visual acuity might be achieved by decreasing the pitch between electrodes further. In addition to visual acuity though, most definitions of legal blindness include a minimum allowable visual field (20° in the United States) specifically because of the importance of the field size in visual function. Therefore, we must also strive to increase the area of restored vision while at the same time improve acuity. This topic is the focus of a recent review (Eiber, Lovell, & Suaning, 2013). Notably, increasing field size may be achieved by implanting several autonomous implants (Mathieson et al., 2012), possibility also proposed for the Retina Implant Alpha-IMS.
Investigate Potential for Implantation During the Critical Period in Young Children
Although cochlear implants were first marketed as an aid for lip reading in adults, it was eventually realized that young children with congenital deafness benefit the most from implantation. Since the critical period for development of the auditory system, especially for language skills, ends well before adulthood, children are being implanted in the early years of life. It is reasonable to expect that a similar situation may exist for congenital forms of blindness like Usher's syndrome and Leber's congenital amaurosis (LCA). Indeed, the success of eye patching in amblyopia attests to the utility of early intervention in the visual system.
What Is the Role of Neural Plasticity in Processing Prosthetic Vision?
Nearly all patient studies to date have observed that training and motivation help maximize the benefits of an implant. Therefore, the role of training and experience should be enthusiastically investigated, including the potential role of retinal and cortical plasticity driven by prosthetic stimulation.
How and When Can We Expand Applicability of Prosthetic Vision Beyond RP Patients?
To date, the vast majority of patients with retinal implants have one of the dozens of forms of RP. Heterogeneity of RP may underlie the broad variability of functional results. However, AMD is a fast growing patient population, and therefore, a key question is under which circumstances they may benefit from visual prostheses. To date, few attempts have been made to adapt existing retinal implants for treatment of AMD out of fear of damaging residual peripheral vision.
Do Implants Slow Degeneration?
A surprising byproduct of prosthetic research was the discovery that electrical stimulation of the retina, even below levels necessary to elicit phosphenes may have neurotrophic effect and slow the progression of retinal degeneration (Morimoto et al., 2007; Pardue, Ciavatta, & Hetling, 2014; Schatz et al., 2011). It may therefore prove beneficial to implant retinal prostheses earlier to not only replace the vision that eventually will be lost, but to also delay retinal degeneration outside the implanted area. It is plausible that creating continuity between degenerating natural vision and prosthetic vision may improve the effectiveness of prosthetic vision alone by minimizing reorganization of the retinal circuit and degenerative plasticity in the visual cortex. In support of this hypothesis, a transcorneal electrical stimulation (TES) device (OkuStim; Okuvision GmbH, Reutlingen, Germany), which has received the CE mark for commercial sale in Europe has been shown to yield visual improvement in RP patients preceding complete vision loss (Schatz et al., 2011). That said, unpublished data from at least one of these same authors found that negative retinal plasticity and retinal remodeling was accelerated with the introduction of electrical stimulation. Additional parametric studies are required to look at current load, frequency, and other parameters of stimulation.
When Will There Be a Commercial Cortical Implant?
Given that the earliest visual implants were cortical devices, it is perhaps surprising that the first two commercial visual implants are both situated in the retina. Despite the lack of success in realizing a clinical device through cortical stimulation, many groups remain committed to developing cortical devices and work to ensure that a cortical prosthesis for restoration of vision will one day achieve clinical use.
Final Remarks
Retinal implants have recently been approved for clinical use with acceptable risk/benefit tradeoffs. Still, major improvements are both necessary and possible. Given the ongoing interest in brain–machine interfaces for both clinical and research applications, we expect that improvement of retinal implants will also continue in the coming years. These improvements are certain to translate to enhanced benefits for blind patients. Further development of cortical implants may allow restoration of sight to patients who cannot benefit from retinal approaches due to complete loss of their retinal neurons or even the whole eye.
This chapter is part of the Restoring Vision to the Blind report by the Lasker/IRRF Initiative for Innovation in Vision Science. The full report, Restoring Vision to the Blind, including a complete list of contributors, is available in the Supplementary Material (1.8MB, pdf) .
References
- Asher A, Segal W, Baccus S, Yaroslavsky L, Palanker D. Image processing for a high-resolution optoelectronic retinal prosthesis. IEEE Transactions on Bio-Medical Engineering. (2007);54:993–1004. doi: 10.1109/TBME.2007.894828. [DOI] [PubMed] [Google Scholar]
- Bak M, Girvin J.P, Hambrecht F.T, Kufta C.V, Loeb G.E, Schmidt E.M. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Medical & Biological Engineering & Computing. (1990);28:257–259. doi: 10.1007/BF02442682. [DOI] [PubMed] [Google Scholar]
- Bradley D.C, Troyk P.R, Berg J.A, Bak M, Cogan S, Erickson R, Kufta C. Visuotopic mapping through a multichannel stimulating implant in primate V1. Journal of Neurophysiology. (2005);93:1659–1670. doi: 10.1152/jn.01213.2003. [DOI] [PubMed] [Google Scholar]
- Brelen M.E, Vince V, Gerard B, Veraart C, Delbeke J. Measurement of evoked potentials after electrical stimulation of the human optic nerve. Investigative Ophthalmology & Visual Science. (2010);51:5351–5355. doi: 10.1167/iovs.09-4346. [DOI] [PubMed] [Google Scholar]
- Brindley G.S, Lewin W.S. The sensations produced by electrical stimulation of the visual cortex. Journal of Physiology. (1968);196:479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Ren Q, Desai N.J, Rizzo J.F, III, Fried S.I. Response variability to high rates of electric stimulation in retinal ganglion cells. Journal of Neurophysiology. (2011);106:153–62. doi: 10.1152/jn.00956.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EL, B. Investigational device exemption (IDE) guidance for retinal prostheses guidance for industry and food and drug administration staff. ed. USDoHaH Services, FaD Administration, CfDaR Health, OoSaE Laboratories, OoD Evaluation. (2013) Retrieved from http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm341954.htm. [Google Scholar]
- Cosendai G. Implantat Argus II: Wo Stehen wir. Concept Ophthalmologie. (2014):12–14. [Google Scholar]
- Cosendai G, da Cruz L, Sahel J.A, Stanga P.E, Hafezi F, Greenberg R.J. International Congress of German Ophthalmic Surgeons. Nürnberg, Germany: (2014). 2014. Group AIS. Clinical trials update from the Argus® II Retinal Prosthesis system. In. [Google Scholar]
- Chuang A.T, Margo C.E, Greenberg P.B. Retinal implants: A systematic review. British Journal of Ophthalmology. (2014);98:852–856. doi: 10.1136/bjophthalmol-2013-303708. [DOI] [PubMed] [Google Scholar]
- Davis T.S, Parker R.A, House P.A, Bagley E, Wendelken S, Normann R.A, Greger B. Spatial and temporal characteristics of V1 microstimulation during chronic implantation of a microelectrode array in a behaving macaque. Journal of Neural Engineering. (2012) doi: 10.1088/1741-2560/9/6/065003. 9, 065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobelle W.H. Artificial vision for the blind by connecting a television camera to the visual cortex. ASAIO Journal. (2000);46:3–9. doi: 10.1097/00002480-200001000-00002. [DOI] [PubMed] [Google Scholar]
- Dobelle W.H, Mladejovsky M.G. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. Journal of Physiology. (1974);243:553–576. doi: 10.1113/jphysiol.1974.sp010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn J.D, Ahuja A.K, Caspi A, da Cruz L, Dagnelie G, Sahel J.A, Greenberg R.J. The detection of motion by blind subjects with the epiretinal 60-electrode. Argus II, editor. Retinal Prosthesis. JAMA Ophthalmology. (2013);131:183–189. doi: 10.1001/2013.jamaophthalmol.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. Artificial human vision. Expert Review of Medical Devices. (2005);2:73–85. doi: 10.1586/17434440.2.1.73. [DOI] [PubMed] [Google Scholar]
- Eiber C.D, Lovell N.H, Suaning G.J. Attaining higher resolution visual prosthetics: a review of the factors and limitations. Journal of Neural Engineering. (2013) doi: 10.1088/1741-2560/10/1/011002. 10, 011002. [DOI] [PubMed] [Google Scholar]
- Fanshel S, Bush J.W.A. Health-Status Index and its Application to Health-Services Outcomes. (1970);18:1021–1066. Retrieved from http://dx.doi.org/10.1287/opre.18.6.1021. [Google Scholar]
- Fornos A.P, Sommerhalder J, Rappaz B, Safran A.B, Pelizzone M. Simulation of artificial vision, III: do the spatial or temporal characteristics of stimulus pixelization really matter? Investigative Ophthalmology & Visual Science. (2005);46:3906–3912. doi: 10.1167/iovs.04-1173. [DOI] [PubMed] [Google Scholar]
- Fried S.I, Lasker A.C, Desai N.J, Eddington D.K, Rizzo J.F., III Axonal sodium-channel bands shape the response to electric stimulation in retinal ganglion cells. Journal of Neurophysiology. (2009);101:1972–1987. doi: 10.1152/jn.91081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikado T, Kamei M, Sakaguchi H, Kanda H, Morimoto T, Ikuno Y, Nishida K. Testing of semichronically implanted retinal prosthesis by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Investigative Ophthalmology & Visual Science. (2011);52:4726–4733. doi: 10.1167/iovs.10-6836. [DOI] [PubMed] [Google Scholar]
- Geruschat D.R, Bittner A.K, Dagnelie G. Orientation and mobility assessment in retinal prosthetic clinical trials. Optometry and Visual Science. (2012);89:1308–1315. doi: 10.1097/OPX.0b013e3182686251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz G.A, Mandel Y, Manivanh R, Palanker D.V, Cizmar T. Holographic display system for restoration of sight to the blind. Journal of Neural Engineering. (2013) doi: 10.1088/1741-2560/10/5/056021. 10, 056021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P, Arosio D, Bregestovski P. Molecular probes and switches for functional analysis of receptors, ion channels and synaptic networks. Frontiers in Molecular Neuroscience. (2013) doi: 10.3389/fnmol.2013.00048. 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther T, Lovell N.H, Suaning G.J. Bionic vision: System architectures: A review. Expert Review of Medical Devices. (2012);9:33–48. doi: 10.1586/erd.11.58. [DOI] [PubMed] [Google Scholar]
- Guerra S, Stanga P, Merlini F, Sahel J, Mohand-Said S, daCruz L, Greenberg R. Presented at the Artificial Vision meeting. Aachen, Germany: (2013). Detection of human faces by blind patients implanted with the Argus® II Retinal Prosthesis System. August 8–9, 2013. [Google Scholar]
- Hornig R, Zehnder T, Velikay-Parel M, Laube T, Feucht M, Richard G. The IMI retinal implant system. In: Humayun M.S, Weiland J.D., Chader G, Greenbaum E, editors. Artificial sight: Basic research, biomedical engineering, and clinical advances. New York: Springer-Verlag; (2007). pp. 111–128). In. (Eds.) (pp. [Google Scholar]
- Humayun M.S, Dorn J.D, da Cruz L, Dagnelie G, Sahel J.A, Stanga P.E, Cideciyan A.V. Interim results from the international trial of Second Sight's visual prosthesis. Ophthalmololgy. (2012);119:779–788. doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.J, Rizzo J.F, III, Ziv O.R, Grumet A, Wyatt J. Thresholds for activation of rabbit retinal ganglion cells with an ultrafine, extracellular microelectrode. Investigative Ophthalmology & Visual Science. (2003);44:3533–3543. doi: 10.1167/iovs.02-1041. [DOI] [PubMed] [Google Scholar]
- Kellner U. Hereditary Retinal Dystrophies. Stuttgart Georg Thieme Verlag (2000) [Google Scholar]
- Krey H.F, Brauer H. Chibret Augenatlas: Eine Repetition für Ärtze mit Zeigetafeln für Patienten. Munich, Germany: Chibret Med Serv; (1998). [Google Scholar]
- Lauritzen T.Z, Harris J, Mohand-Said S, Sahel J.A, Dorn J.D, McClure K, Greenberg R.J. Reading visual braille with a retinal prosthesis. Frontiers in Neuroscience. (2012) doi: 10.3389/fnins.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.H, da Cruz L. A review and update on the current status of retinal prostheses (bionic eye) British Medical Bulletin. (2014);109:31–44. doi: 10.1093/bmb/ldu002. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yan Y, Chai X, Ren Q, Chen Y, Li L. Electrical stimulation with a penetrating optic nerve electrode array elicits visuotopic cortical responses in cats. Journal of Neural Engineering. (2013) doi: 10.1088/1741-2560/10/3/036022. 10, 036022. [DOI] [PubMed] [Google Scholar]
- Mandel Y, Goetz G, Lavinsky D, Huie P, Mathieson K, Wang L, Kamins T. Cortical responses elicited by photovoltaic subretinal prostheses exhibit similarities to visually evoked potentials. Nature Communications, (2013);1980;4 doi: 10.1038/ncomms2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson K, Loudin J, Goetz G, Huie P, Wang L, Kamins T.I, Galambos L. Photovoltaic retinal prosthesis with high pixel density. Nature Photonics. (2012);6:391–397. doi: 10.1038/nphoton.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei M, Zeitz O, Keserü M, Wagenfeld L, Hornig R, Post N, Richard G. Progress in the development of vision prostheses. Ophthalmologica. (2011);225:187–192. doi: 10.1159/000318042. [DOI] [PubMed] [Google Scholar]
- Maynard E.M. Visual prostheses. Annual Review of Biomedical Engineering. (2001);3:145–168. doi: 10.1146/annurev.bioeng.3.1.145. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Fujikado T, Choi J.S, Kanda H, Miyoshi T, Fukuda Y, Tano Y. Transcorneal electrical stimulation promotes the survival of photoreceptors and preserves retinal function in royal college of surgeons rats. Investigative Ophthalmology & Visual Science. (2007);48:4725–4732. doi: 10.1167/iovs.06-1404. [DOI] [PubMed] [Google Scholar]
- Nirenberg S, Pandarinath C. Retinal prosthetic strategy with the capacity to restore normal vision. Proceedings of the National Academy of Sciences of the United States of America. (2012);109:15012–15017. doi: 10.1073/pnas.1207035109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanker D, Lorach H, Goetz G, Mandel Y, Smith R, Boinagrov D, Lei X. Photovoltaic restoration of sight in rats with retinal degeneration: Assessment of spatial resolution and visual functions. Investigative Ophthalmology & Visual Science. (2014) 55, E-Abstract 5964. [Google Scholar]
- Pardue M.T, Ciavatta V.T, Hetling J.R. Neuroprotective effects of low level electrical stimulation therapy on retinal degeneration. Advances in Experimental Medicine and Biology. (2014);801:845–851. doi: 10.1007/978-1-4614-3209-8_106. [DOI] [PubMed] [Google Scholar]
- Perez Fornos A, Sommerhalder J, Pittard A, Safran A.B, Pelizzone M. Simulation of artificial vision: IV. Visual information required to achieve simple pointing and manipulation tasks. Vision Research. (2008);48:1705–1718. doi: 10.1016/j.visres.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Pezaris J.S, Reid R.C. Demonstration of artificial visual percepts generated through thalamic microstimulation. Proceedings of the National Academy of Sciences of the United States of America. (2007);104:7670–7675. doi: 10.1073/pnas.0608563104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliskin J.S, Shepard D.S, Weinstein M.C. Utility functions for life years and health status. Operations Research. (1980);28:206–224. [Google Scholar]
- Rizzo J.F., III Update on retinal prosthetic research: the Boston Retinal Implant Project. Journal of Neuro-ophthalmology. (2011);31:160–168. doi: 10.1097/WNO.0b013e31821eb79e. [DOI] [PubMed] [Google Scholar]
- Rizzo S, Belting C, Cinelli L, Allegrini L, Genovesi-Ebert F, Barca F, di Bartolo E. The Argus II Retinal Prosthesis: 12-Month Outcomes from a Single-Study Center. American Journal of Ophthalmology. (2014);157:1282–1290. doi: 10.1016/j.ajo.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Kamei M, Fujikado T, Yonezawa E, Ozawa M, Cecilia-Gonzalez C, Ustariz-Gonzalez O. Artificial vision by direct optic nerve electrode (AV-DONE) implantation in a blind patient with retinitis pigmentosa. The International Journal of Artificial Organs: The Official Journal of the Japanese Society for Artificial Organs. (2009);12:206–209. doi: 10.1007/s10047-009-0467-2. [DOI] [PubMed] [Google Scholar]
- Saunders A.L, Williams C.E, Heriot W, Briggs R, Yeoh J, Nayagam D.A, McCombe M. Development of a surgical procedure for implantation of a prototype suprachoroidal retinal prosthesis. Clinical & Experimental Ophthalmology. (2014);42:665–672. doi: 10.1111/ceo.12287. doi: 10.1111/ceo.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz A, Rock T, Naycheva L, Willmann G, Wilhelm B, Peters T, Bartz-Schmidt K.U. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Investigative Ophthalmology & Visual Science. (2011);52:4485–4496. doi: 10.1167/iovs.10-6932. [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, Hottowy P, Sher A, Dabrowski W, Litke A.M, Chichilnisky E.J. High-resolution electrical stimulation of primate retina for epiretinal implant design. Journal of Neuroscience. (2008);28:4446–4456. doi: 10.1523/JNEUROSCI.5138-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerhalder J, Oueghlani E, Bagnoud M, Leonards U, Safran A.B, Pelizzone M. Simulation of artificial vision: I. Eccentric reading of isolated words, and perceptual learning. Vision Research. (2003);43:269–283. doi: 10.1016/s0042-6989(02)00481-9. [DOI] [PubMed] [Google Scholar]
- Sommerhalder J, Rappaz B, de Haller R, Fornos A.P, Safran A.B, Pelizzone M. Simulation of artificial vision: II. Eccentric reading of full-page text and the learning of this task. Vision Research. (2004);44:1693–1706. doi: 10.1016/j.visres.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Stingl K, Bartz-Schmidt K.U, Besch D, Braun A, Bruckmann A, Gekeler F, Greppmaier U. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proceedings Biological Sciences/The Royal Society, (2013) doi: 10.1098/rspb.2013.0077. 280, 20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A, Borgonovi E, Taylor R.S, Sahel J.A, Rizzo S, Stanga P.E, Kukreja A. The cost-effectiveness of the Argus II retinal prosthesis in retinitis pigmentosa patients. BMC Ophthalmology, (2014) doi: 10.1186/1471-2415-14-49. 14, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J.D, Cho A.K, Humayun M.S. Retinal prostheses: current clinical results and future needs. Ophthalmology. (2011);118:2227–2237. doi: 10.1016/j.ophtha.2011.08.042. [DOI] [PubMed] [Google Scholar]
- Wrobel W.G. & AG. Retina Implant. (2010). The value of retinal implants. Biomedizinische Technik. 55, 1. [Google Scholar]
- Zrenner E. Fighting blindness with microelectronics. Science Translational Medicine, (2013) doi: 10.1126/scitranslmed.3007399. 5, 210ps16. [DOI] [PubMed] [Google Scholar]
- Zrenner E, Bartz-Schmidt K.U, Benav H, Besch D, Bruckmann A, Gabel V.P, Gekeler F. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proceedings Biological Sciences/The Royal Society. (2011);278:1489–97. doi: 10.1098/rspb.2010.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]