Abstract
Over the past decade, evidences of an integration of metabolic and inflammatory pathways, referred to as metaflammation in several aspects of metabolic syndrome, have been accumulating. Fatty acid-binding protein 4 (FABP4), also known as adipocyte FABP (A-FABP) or aP2, is mainly expressed in adipocytes and macrophages and plays an important role in the development of insulin resistance and atherosclerosis in relation to metaflammation. Despite lack of a typical secretory signal peptide, FABP4 has been shown to be released from adipocytes in a non-classical pathway associated with lipolysis, possibly acting as an adipokine. Elevation of circulating FABP4 levels is associated with obesity, insulin resistance, diabetes mellitus, hypertension, cardiac dysfunction, atherosclerosis, and cardiovascular events. Furthermore, ectopic expression and function of FABP4 in several types of cells and tissues have been recently demonstrated. Here, we discuss both the significant role of FABP4 in pathophysiological insights and its usefulness as a biomarker of metabolic and cardiovascular diseases.
Keywords: fatty acid-binding protein, metabolic syndrome, inflammation, cardiovascular disease
Introduction
It has recently been reported that metabolically driven low-grade and chronic inflammation is observed in various types of metabolic disorders, including obesity, insulin resistance, type 2 diabetes, and cardiovascular disease.1,2 The atypical inflammation emerging from metabolic tissues is referred to as metaflammation. Metaflammation is triggered by a surplus of nutrients and metabolites that engage a group of molecules with signaling pathways involved in canonical inflammation. Such signal pathways are mediated by c-Jun N-terminal kinase (JNK), inhibitor of nuclear kappa B kinase (IKK), double-stranded RNA-dependent protein kinase (PKR), and others,1,2 and are responsible for the inhibition of insulin signaling and a vicious cycle of further production of inflammatory mediators through transcriptional regulation by activating protein-1 (AP-1) and nuclear factor-kappa B (NF-κB).3–5
In this article, we focus on fatty acid-binding proteins (FABPs) derived from metabolically active cells, especially FABP4, and discuss both the significant role of FABP4 in pathophysiological insights and its usefulness as a biomarker in the context of metabolic and cardiovascular diseases.
Fatty Acid-Binding Proteins
FABPs are a family of 14–15-kDa proteins, known as intracellular lipid chaperones, that regulate lipid trafficking and responses in cells.6,7 FABPs can reversibly bind to hydrophobic ligands, such as saturated and unsaturated long-chain fatty acids (FAs), eicosanoids, and other lipids, with high affinity and broad selectivity.8,9 FABPs are found in all species, from Caenorhabditis elegans and Drosophila melanogaster to rodents and humans, demonstrating strong evolutionary conservation.10–12 Since the first report of FABPs in 1972,13 at least nine different isoforms have been identified in mammals to date. Different isoforms of the FABP family are uniquely expressed in tissues involved in active lipid metabolism. In general, the amount of FABP in cells is proportional to the rates of FA metabolism.14 The family consists of liver (L-FABP/FABP1), intestinal (I-FABP/FABP2), heart (H-FABP/FABP3), adipocyte (A-FABP/FABP4/aP2), epidermal (E-FABP/FABP5/mal1), ileal (Il-FABP/FABP6), brain (B-FABP/FABP7), myelin (M-FABP/FABP8), and testis (T-FABP/FABP9) isoforms.
FABPs exhibit about 15–70% sequence identity between different isoforms.11 However, FABPs share almost similar three-dimensional structures, showing two orthogonal five-stranded β-sheets by a 10-stranded anti-parallel β-barrel structure.6,11 The FA-binding pocket is located inside the β-barrel, and usually, one long-chain FA can be bound to the interior cavity of FABPs except for FABP1. There are differences between the different isoforms in binding affinity and ligand selectivity because of structural differences.11 In general, hydrophobic ligand has a tight binding affinity for FABPs, except for unsaturated FAs.
FABPs have been proposed to actively facilitate the transport of FAs to specific organelles in the cell for lipid oxidation in the mitochondrion or peroxisome; lipid-mediated transcriptional regulation in the nucleus; signaling, trafficking, and membrane synthesis in the endoplasmic reticulum (ER); and regulation of enzyme activity and storage as lipid droplets in the cytoplasm.6 FABPs are also involved in the conversion of FAs to eicosanoids and the stabilization of leukotriene.15,16
FABPs in Adipocytes: FABP4 and FABP5
FABP4, also known as adipocyte FABP (A-FABP), was first detected in adipose tissue and mature adipocytes.17,18 This protein has also been termed adipocyte P2 (aP2) since there is high sequence similarity (67%) with the myelin P2 protein (M-FABP/FABP8).18 FABP4 is highly expressed in adipocytes and consists of about 1% of all soluble proteins in adipose tissue.19 Expression of FABP4 is highly induced during adipocyte differentiation and transcriptionally controlled by peroxisome proliferator-activated receptor (PPAR) γ agonists, FAs, dexamethasone, and insulin.20–24
FABP4-deficient mice showed an increase in body weight but reduced insulin resistance in both high-fat diet-induced and genetic obesity mouse models, but the effect of FABP4 on insulin sensitivity was not observed in lean mice (Fig. 1).25,26 It has been postulated that FABP4 activates hormone-sensitive lipase (HSL) in adipocytes, regulating lipolysis.27,28 In fact, FABP4-deficient adipocytes exhibited reduced efficiency of lipolysis.29,30 Knockdown of Fabp4 gene by RNA interference in dietary obese mice increased body weight and fat mass without significant changes in glucose and lipid homeostasis.31 The phenotypic feature of Fabp4-knockdown mice is similar to the phenotype of FABP4 heterozygous knockout mice on a high-fat diet,25 indicating that remaining FABP4 protein sustains some elements of FABP4 function in control mice. The loss of FABP4 in adipocytes is compensated by FABP5, an FABP known as E-FABP or mal1.25
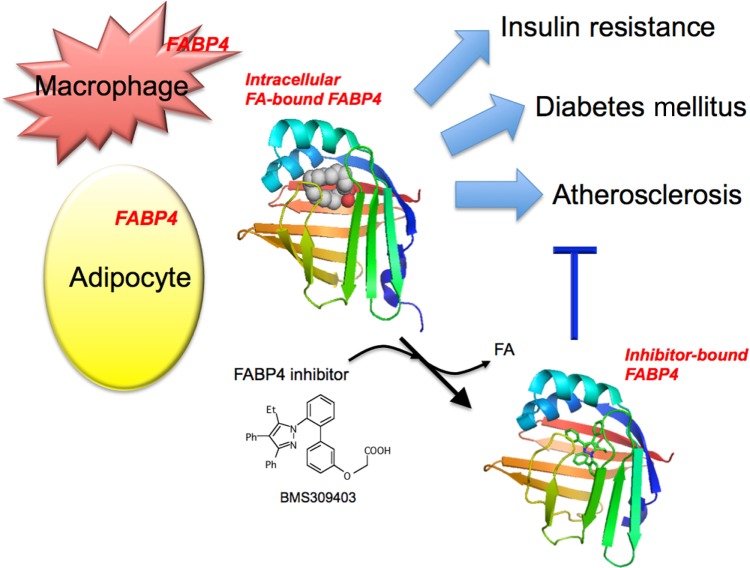
Figure 1.
Association of FABP4 in adipocytes and macrophages with metabolic and cardiovascular diseases. FABP4 acts at the interface of metabolic and inflammatory pathways in adipocytes and macrophages and plays important roles in the development of insulin resistance, diabetes mellitus, and atherosclerosis. Chemical inhibition of FABP4 could be a therapeutic strategy against metabolic and cardiovascular diseases. BMS309403, one of the specific FABP4 inhibitors, is an orally active small molecule and interacts with the fatty acid-binding pocket within the interior of FABP4 to inhibit binding of endogenous fatty acid (FA). Intracellular FA-bound FABP4 (PDB code: 2hnx). Inhibitor-bound FABP4 (PDB code: 2nnq).
FABP5 is most abundantly present in epidermal cells of the skin but is also expressed in several other cells and tissues including adipocytes.6 The amount of FABP5 is about 100-fold lesser than that of FABP4 in adipocytes.32 FABP4 and FABP5 have 52% amino acid similarity and bind to several FAs with similar affinity and selectivity.10
In transgenic mice in which FABP5 was overexpressed in adipose tissue, basal lipolysis and hormone-stimulated lipolysis were enhanced, and insulin sensitivity was reduced in mice on a high-fat diet.33,34 FABP5 deficiency mildly increased systemic insulin sensitivity in dietary and genetic obesity mouse models.33 FABP5-deficient adipocytes demonstrated an increased capacity for insulin-stimulated glucose transport. Except for increased FABP3 (H-FABP) in the liver,35 there was no compensatory increase in the expression of FABPs in tissues of FABP5-deficient mice.33
FABPs in Macrophages: FABP4 and FABP5
Both FABP4 and FABP5 are also expressed in macrophages and dendritic cells,36,37 though the amount of FABP4 in adipocytes is about 10,000-fold higher than that in macrophages.38 Under physiological conditions, the stoichiometry of FABP4 and FABP5 appears to be nearly equal in macrophages.36 Unlike in adipocytes, FABP5 did not appear to be significantly upregulated in FABP4-deficient macrophages.36 The mechanism underlying compensatory changes in FABPs is unknown.
Expression of FABP4 is induced during differentiation from monocytes to macrophages and by treatment with lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate, PPARγ agonists, oxidized low-density lipoprotein, and advanced glycation end products.36,39–43 Conversely, atorvastatin, a cholesterol-lowering drug, has been shown to reduce FABP4 expression in macrophages.44 Metformin, an antidiabetic drug, has also been reported to inhibit forkhead box protein O1 (FOXO1)-mediated transcription of FABP4 and reduce lipid accumulation in macrophages.45
In macrophages, FABP4 increases accumulation of cholesterol ester and foam cell formation via inhibition of the PPARγ-liver X receptor α (LXRα)-ATP-binding cassette A1 (ABCA1) pathway and induces inflammatory responses through activation of the IKK-NF-κB and JNK-AP-1 pathways.46,47 Ablation of FABP4 protected against atherosclerosis in apolipoprotein E (ApoE)-deficient mice with or without high-cholesterol western-style diets (Fig. 1).36,48 Bone marrow transplantation studies demonstrated that actions in macrophages are predominant for the protective effect of FABP4 deficiency on atherosclerosis rather than actions in adipocytes.36 FABP4 in dendritic cells has been shown to regulate the IKK-NF-κB pathway and T-cell priming,37 which might contribute to the development of atherosclerosis since both dendritic cells and T-cells are involved in the pathogenesis of atherosclerosis.49 As evidence of atherosclerosis in humans, expression of FABP4 in macrophages has been shown to be increased in unstable carotid plaques of human endarterectomy samples.50
Expression of FABP5 in macrophages is increased by treatment with toll-like receptor (TLR) agonists: LPS, a TLR4 agonist, and zymosan, a fungal product that activates TLR2.51 In addition to FABP4, FABP5 may also play a role in atherosclerosis via TLRs and/or other mechanisms. Ablation of FABP5 suppressed atherosclerosis in LDL receptor-deficient mice on a western-style hypercholesterolemic diet, and the anti-atherosclerotic effect of FABP5 deletion was associated with reduction of the expression of inflammatory genes, interleukin 6 and cyclooxygenase-2, and suppression of macrophage recruitment in atherosclerotic lesions because of decreased expression of CC chemokine receptor 2.52
Combined Deficiency of FABP4 and FABP5
As described above, both FABP4 and FABP5 in adipocytes and macrophages play important roles in the development of insulin resistance and atherosclerosis (Fig. 1).25,26,33,36,48,52 Mice with combined deficiency of FABP4 and FABP5 (Fabp4−/−Fabp5−/−) on dietary and genetic obesity models exhibited improvement in insulin resistance and protection against type 2 diabetes and fatty liver disease more than did FABP4-or FABP5-deficient mice.53,54 Macrophage infiltration and accumulation in adipose tissue has been suggested to be an important feature of obesity-triggered metaflammation.55,56 Although the effect of FABP4 or FABP5 on atherosclerosis was mainly due to their actions in macrophages,36,52 bone marrow transplantation and cell-based co-culture experiments with adipocytes and macrophages using wild-type and Fabp4−/−Fabp5−/− mice showed that FABP actions in adipocytes and those in macrophages have distinct roles in regulation of insulin sensitivity through metabolic and inflammatory responses.57 Furthermore, Fabp4−/−Fabp5−/− mice intercrossed into an ApoE-deficient atherosclerosis model showed dramatically suppressed development of atherosclerosis compared to that in FABP4-deficient or wild-type mice on the same background.58 Interestingly, Fabp4−/−Fabp5−/−Apoe−/− mice on a hypercholesterolemic diet also had a significantly higher survival rate than that of Apoe−/− mice, probably due to better plaque stability and metabolic health.58
Lipidomic analyses using samples from different tissues, including adipose tissue, skeletal muscle, liver, and blood in Fabp4−/−Fabp5−/− and wild-type mice, showed significantly increased de novo lipogenesis by induction of stearoyl-CoA desaturase-1 (SCD-1) and FA synthase in adipose tissue.59 As a result, palmitoleate (C16:1n7), an unsaturated free FA, was identified as an adipose tissue-derived lipid hormone, referred to as lipokine, that decreases fatty liver and increases glucose uptake in skeletal muscle.59 Deletion of FABP4 in macrophages also increased de novo lipogenesis pathways through LXRα-mediated SCD-1 activation, resulting in production of palmitoleate and resistance to ER stress.60 Conversely, unsaturated FAs, including palmitoleate, modulated histone deacetylation, resulting in decreased basal and LPS-induced expression of FABP4 in macrophages.61
A role of a lipokine in human pathophysiology has been suggested by recent clinical studies. In a study in which 100 Caucasian subjects were recruited, palmitoleate level was positively correlated with insulin sensitivity assessed by euglycemic-hyperinsulinemic clamp studies, independent of age, gender, and adiposity.62 Another study in which 3630 subjects in the US were enrolled showed that high level of cis isomer palmitoleate, which is primarily produced by the liver in humans, was associated with adiposity and consumption of alcohol and carbohydrate.63 Interestingly, it has recently been reported that the level of trans isomer of palmitoleate, an exogenous source of C16:1n7, is associated with lower insulin resistance, lower incidence of diabetes, and higher HDL-cholesterol level, suggesting a potential strategy for intervention by metabolic benefits of dairy product consumption in human diseases.64
Potential Functional Domains of FABP4
A nuclear localization signal (NLS) and a nuclear export signal (NES) have been reported in potential functional domains of FABP4.6,65,66 The primary sequence of FABP4 does not reveal a typical NLS or NES. However, the signals could be found in the three-dimensional structure of FABP4 and were mapped to three basic residues (K21, R30, and K31) for NLS in the helix–loop–helix region and three nonadjacent leucine residues (L66, L86, and L91) for NES.65 The NLS in FABP4 is activated by closure of the portal loop and perturbation of a swinging doorway comprised F57.66 Non-activating ligands, such as oleate and stearate, protrude from the portal and prevent its closure, leading to the masking of the NLS, while activating ligands, such as linoleic acid, troglitazone, and anilinonaphthalene sulfonate, expose the NLS.66
As a small intracellular protein, FABP4 may access the nucleus and target FAs to transcription factors, such as members of the PPAR family, in the nuclear lumen. It has been reported that FABP4 expression itself is controlled by PPARγ.67 A recent study has indicated that continuous shuttling between the nucleus and cytoplasm is involved in transcriptional activation of PPARγ by FABP4.65 However, it has also been shown that FABP4 has inhibitory action of PPARγ in macrophages.46
Another domain of FABP4 includes an HSL binding site.6,28 A direct protein–protein interaction between FABP4 and HSL activity in adipocytes has been reported to regulate lipolysis.27,29,30 Adipocytes in FABP4-deficient mice have reduced lipolysis in vitro and in vivo. Interestingly, during experimentally induced lipolysis, FABP4-deficient mice showed reduction in insulin secretion.29
FABP4 has been reported to decrease Janus kinase 2 (JAK2) signaling via protein–protein interaction, indicating a novel role of FABP4 as a FA sensor affecting metabolism in the cell.68 Phosphatase and tensin homolog on chromosome 10 (PTEN), which negatively regulates the phosphoinositide 3-kinase pathway, has also been reported to interact with FABP4, possibly regulating adipocyte differentiation and lipid metabolism.69 Interestingly, PTEN-deficient keratinocytes increased expression of FABP4, suggesting that PTEN transcriptionally regulates FABP4 expression.70
Secretion of FABP4 as an Adipokine
FABP4 lacks an N-terminal secretory signal sequence,6 which is necessary for the classical secretory pathway, ie, ER–Golgi-dependent pathway. However, proteins can also be actively secreted from eukaryotic cells via ER–Golgi-independent pathways.71 Previous studies demonstrated that FABP4 secretion was not modulated by treatment with brefeldin A and monensin, inhibitors of protein secretion by blocking vesicular traffic at the ER and the Golgi apparatus, indicating a non-classical secretion mechanism for FABP4.72,73
In adipocytes, there are two distinct signal pathways of activation of lipolysis by phosphorylation of HSL: β-adrenergic receptor-mediated adenyl cyclase (AC)-protein kinase A (PKA) and natriuretic peptide receptor-A (NPR-A)-mediated guanylyl cyclase (GC)-protein kinase G (PKG) pathways.74,75 We recently showed that FABP4 is secreted from adipocytes in association with lipolysis through both AC-PKA and GC-PKG pathways (Fig. 2).76 Since it has been reported that lipolysis is mediated in part through the interaction of FABP4 with HSL in adipocytes,27,29,30 there is a dual regulatory mechanism of FABP4 secretion in adipocytes. FABP4 may also be a carrier protein for transport of FAs generated by lipolysis from lipid droplets to extracellular and/or intracellular utilization. In humans, plasma FABP4 level significantly declined after an oral glucose tolerance test or after eating a high-fat meal, while the insulin level increased.76 Suppression of FABP4 secretion by insulin-induced anti-lipolytic signaling may be involved in this decline in FABP4 level.
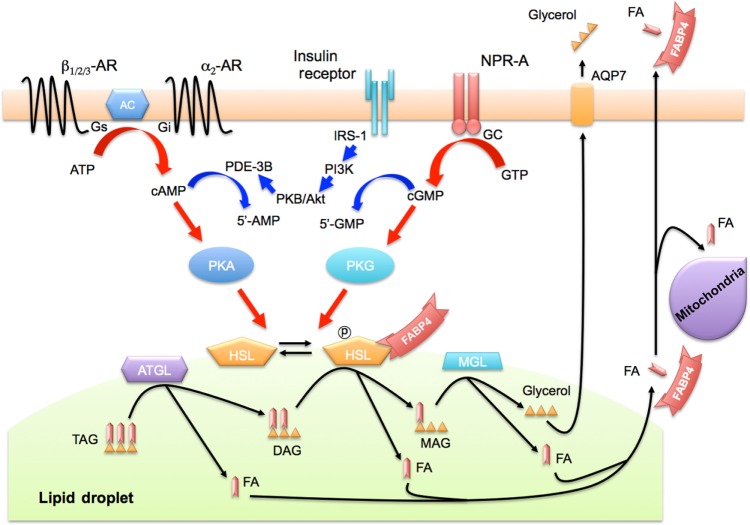
Figure 2.
Secretion of FABP4 in association with lipolysis. FABP4 lacks an N-terminal secretory signal sequence, which is necessary for the classical ER–Golgi-dependent secretory pathway, but FABP4 can be secreted via the ER–Golgi-independent pathway. FABP4 is secreted from adipocytes in association with two distinct signal pathways of activation of lipolysis by phosphorylation of HSL, including β-adrenergic receptor-mediated AC-PKA and NPR-A-mediated GC-PKG pathways. FABP4 also interacts with HSL for regulation of its activity. FABP4 may be a carrier protein for transport of FAs generated by lipolysis from lipid droplets to extracellular and/or intracellular utilization.
Abbreviations: AC, adenyl cyclase; AR, adrenergic receptor; ATGL, adipose triglyceride lipase; AQP7, aquaporin 7; DAG, diacylglyceride; ER, endoplasmic reticulum; FA, fatty acid; GC, guanylyl cyclase; HSL, hormone-sensitive lipase; MAG, monoacylgyceride; MGL, monoacylglycerol lipase; NPR-A, natriuretic peptide receptor-A; TAG, triacylglyceride; IRS-1, insulin receptor substrate 1; PDE-3B, phosphodiesterase 3B; PI3K, phosphatidylinositol-3-kinase; PKA, protein kinase A; PKB, protein kinase B; PKG, protein kinase G.
FABP4 secretion from human adipocytes has also been shown to be increased by ionomycin, an ionophore that raises intracellular Ca2+ levels, indicating contribution of a calcium-dependent secretory mechanism.77 In addition, FABP4 is secreted partially by microvesicles derived from adipocytes (an established mechanism for unconventional secretion from adipocytes71), and both microvesicle-free-mediated and microvesicle-secreted FABP4 are downregulated by insulin and upregulated by ionomycin.73,76,78 However, the release of FABP4 via adipocyte-derived microvesicles is a small fraction and conveys a minor activity.76,78 Taken together, the findings indicate that FABP4 is actively released by unconventional mechanisms and by adipocyte-derived microvesicles from adipocytes through AC-PKA- and GC-PKG-dependent signal pathways and/or an intracellular calcium-dependent mechanism.
Proteomic analysis showed the presence of FABP4 in cell supernatants derived from differentiated THP-1 macrophages,79 suggesting that FABP4 is also secreted from macrophages. In bone marrow transplantation using wild-type and FABP4/5-deficient mice, bone marrow-derived cells from wild-type mice could not sustain a detectable level of plasma FABP4 in FABP4/5-deficient mice, indicating that the predominant contributors of circulating FABP4 are adipocytes rather than macrophages.72 The biological roles of extracellular FABP4 derived from macrophages remain unknown, but there might be local effects of macrophage-derived FABP4 on metaflammation.
Evidences supporting extracellular roles of FABP4 as an adipokine are accumulating with great importance. The secreted form of FABP4 may be biologically active, leading to a paradigm shift in the understanding of FABPs as lipid chaperones and the local networking of metabolic and inflammatory responses (Fig. 3). Direct effects of exogenous FABP4 have been demonstrated in multiple types of cells; FABP4 enhanced hepatic glucose production in vivo and in vitro,72 decreased cardiomyocyte contraction in vitro,78 inhibited expression/activation of endothelial nitric oxide synthase (eNOS) in vascular endothelial cells,80 and increased proliferation/migration of vascular smooth muscle cells81 and glucose-stimulated insulin secretion in pancreatic β cells.82 Future investigation should provide further insights into extracellular roles of FABP4 and how they contribute to progression of diseases.
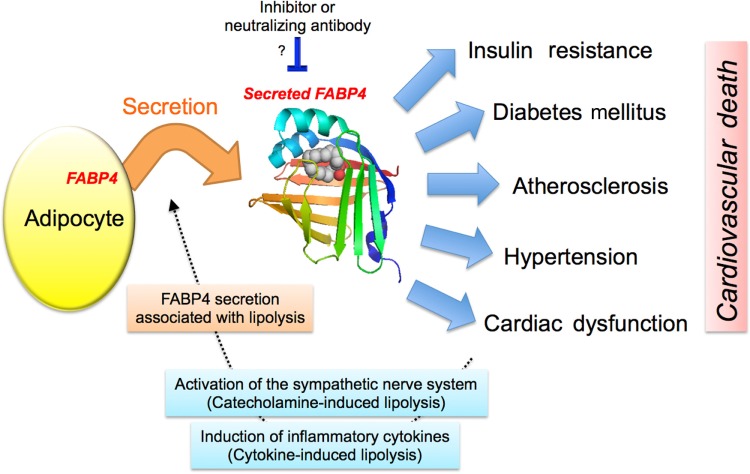
Figure 3.
Possible action of circulating FABP4. FABP4 is secreted from adipocytes in association with lipolysis and may contribute to the development of insulin resistance, diabetes mellitus, atherosclerosis, hypertension, and cardiac dysfunction, leading to poor prognosis by cardiovascular death. Metabolic and cardiovascular disease-mediated activation of the sympathetic nerve system and/or induction of inflammatory cytokines may increase lipolysis in adiocytes, resulting in a vicious spiral of additional production of secreted FABP4. Inhibition or neutralization of secreted FABP4 may represent an effective therapeutic strategy against metabolic and cardiovascular diseases.
Circulating Concentration of FABP4 as a Potent Biomarker
Recent studies have shown the presence of FABPs in human circulation. Since FABPs lack a secretory signal sequence,6 the detection of FABPs in circulation is thought to be a biochemical marker of tissue injury in related cells: FABP1 (L-FABP) for liver injury,83,84 FABP2 (I-FABP) for intestinal damage,83,85 FABP3 (H-FABP) for ongoing myocardial damage in acute myocardial infarction or heart failure,86,87 and FABP7 (B-FABP) for brain injury.83,88
On the other hand, it has been shown that FABP4 is secreted from adipocytes and may act as an adipokine as described above. FABP4 concentration was the highest among levels of FABP1–5 under a physiological condition in a general population without pharmacological treatments.89 FABP4 level is significantly higher in females than in males, possibly because of the larger amount of body fat in females than in males since there is an independent and strong correlation between FABP4 level and adiposity.89,90 Consistent with the results of previous studies showing that levels of several FABPs were increased in subjects with renal dysfunction,84,91,92 serum FABP4 level was shown to be negatively correlated with estimated glomerular filtration rate in recent studies,89,93 suggesting that FABPs are eliminated from the circulation mainly by renal clearance. We previously showed that FABP4 level in hemodialysis patients with end-stage kidney disease was about 20 times higher than that in controls with normal renal function and was decreased by 57.2% after hemodialysis.94
It has been reported that increased circulating FABP4 levels are associated with obesity, insulin resistance, type 2 diabetes, hypertension, cardiac dysfunction, and atherosclerosis.90,95–99 Circulating FABP4 level was significantly increased in obese subjects compared to the level in lean controls, and serum FABP4 level was positively correlated with waist circumference, blood pressure, and insulin resistance.90 High concentration of FABP4 at baseline was an independent predictor for the development of metabolic syndrome during a five-year follow-up period in a Chinese population.95 A 10-year prospective study also showed that high level of FABP4 at baseline independently predicted the development of type 2 diabetes.96 We previously demonstrated significant elevation of circulating FABP4 level in patients with essential hypertension and insulin resistance, correlation of FABP4 concentration with indices of insulin resistance, and association of elevation of FABP4 level with family history of hypertension, suggesting a role of FABP4 in genetic predispositions for the development of essential hypertension.97 Associations of FABP4 levels with the development of left ventricular (LV) hypertrophy and with systolic and diastolic cardiac dysfunction have also been reported.100–104 Our recent study demonstrated that FABP4 level was associated with LV diastolic dysfunction even in an apparently healthy population with no medication.98 It has also been reported that serum FABP4 level is associated with atherosclerosis assessed by carotid intima-media thickness.99 These findings support the notion that circulating FABP4 is not only a potent biomarker but also plays an important role, as an adipokine, in the development of metabolic syndrome and cardiovascular diseases (Fig. 3).
A genetic variant at the FABP4 locus associated with decreased FABP4 expression in adipose tissue has been suggested to reduce the risk of cardiovascular diseases in a population study.105 We and others previously showed that serum FABP4 level predicts long-term cardiovascular events.94,106,107 Furthermore, a large-scale prospective study showed that concentration of FABP4 predicts the risk of heart failure during a median follow-up of 10.7 years.108 Accumulating evidences of a causative role of FABP4 in cardiovascular events, including atherosclerosis and cardiac dysfunction, indicate that FABP4 is potentially a novel target for prevention of cardiovascular diseases (Fig. 3). Other than cardiovascular pathologic processes, FABP4 level has been shown to be a novel prognostic factor in patients with breast cancer independent of obesity.109
It has been reported that several drugs modify circulating FABP4 levels. Atorvastatin, a HMG-CoA reductase inhibitor, and several angiotensin II receptor blockers reduced FABP4 concentrations.110–112 On the other hand, pioglitazone, an insulin-sensitizing thiazolidinedione (a PPARγ agonist), increased FABP4 levels,113 presumably because of direct activation of PPARγ since the Fabp4 gene promoter includes the PPAR response element.114
Of note, similar to FABP4, circulating FABP5 has been reported to be detected at levels of about 1/10th or less of FABP4 concentrations, and FABP5 levels were associated with components of metabolic syndrome, although the correlation was not as strong as that of FABP4.89,115,116 Contribution of FABP5 to metabolic syndrome and cardiovascular diseases remains to be elucidated.
Ectopic FABP4 Expression
There are accumulating evidences indicating FABP4 is expressed in several types of cells, in addition to adipocytes and macrophages, under both physiological and pathological conditions (Fig. 4). For example, expression of FABP4 was observed in endothelial cells of capillaries and small veins in the heart and kidney.117 As a physiological function, FABP4 in capillary endothelial cells of the heart and skeletal muscle interstitial capillaries is involved in transendothelial FA transport into FA-consuming organs.118 Treatment of endothelial cells with vascular endothelial growth factor-A (VEGF-A) via VEGF-receptor-2 (VEGFR2) or basic fibroblast growth factor (bFGF) induced FABP4 expression.117 FABP4 in endothelial cells has also been reported to promote angiogenesis.119–121
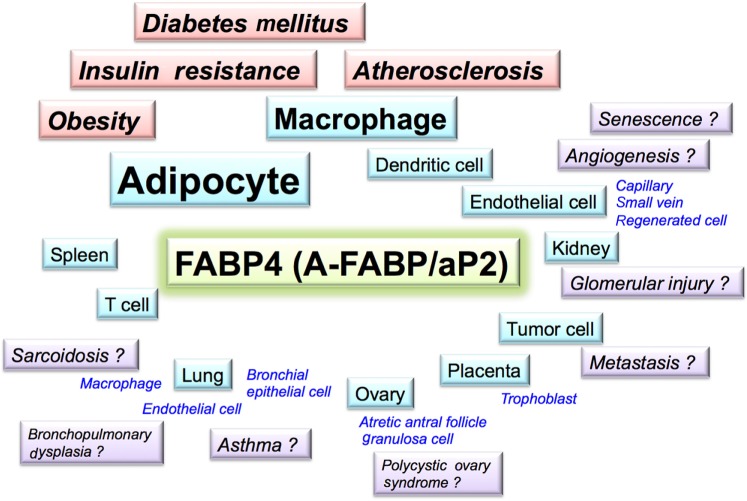
Figure 4.
Potential association of FABP4 in several pathological conditions. FABP4 (A-FABP/aP2) is expressed not only in adipocytes and macrophages but also in several types of tissues and cells under physiological and pathophysiological conditions and may contribute to several aspects of metabolic and cardiovascular diseases as well as renal, respiratory, gynecological, and oncological diseases.
Interestingly, roles of FABP4 in vascular injury have been reported. FABP4 was markedly induced in regenerated endothelial cells after endothelial balloon denudation in vivo.122 Intermittent hypoxia also increased expression of FABP4 in human aortic endothelial cells.123 Conversely, anigiopoietin-1 inhibited FOXO1-mediated FABP4 expression in endothelial cells.124 FABP4 was expressed in the aortic endothelium of 12-week old, but not 8-week old, ApoE-deficient mice showing endothelial dysfunction.125 Chronic treatment with BMS309403, a small molecule FABP4 inhibitor, significantly improved endothelial dysfunction in ApoE-deficient mice.125 Notably, it has recently been shown the possible involvement of FABP4 and FABP5 in senescence of endothelial cells.126,127 These observations support the notion that ectopic expression of FABP4 under pathological condition, but not physiological FABP4 expression, in the endothelium contributes to the pathogenesis of atherosclerosis and vascular injury.
In normal kidneys, FABP4 is expressed in endothelial cells of the tubulointerstitial peritubular capillary and vein in both the cortex and medulla, but not in glomerular or arterial endothelial cells, under normal physiological condition.117 We recently demonstrated that ectopic expression of FABP4 in the glomerulus is associated with progression of proteinuria and renal dysfunction.128 Among FABPs, FABP1 (L-FABP) is expressed in proximal tubular epithelial cells in the kidney, and urinary FABP1 has been reported to reflect damage of proximal tubular epithelial cells.129,130 Our recent study131 suggests that urinary FABP4 is a novel biomarker reflecting glomerular damage and that determination of both FABP1 and FABP4 in urine enables better characterization of renal injury.
Evidences for the involvement of FABP4 in respiratory diseases have also been accumulating. FABP4 was detected in endothelial cells of peribronchial blood vessels and a subset of macrophages in lungs and bronchoalveolar samples from patients with bronchopulmonary dysplasia (BPD).132 Several studies using lung lavage cells suggested that expression of FABP4 is involved in pathogenesis of sarcoidosis.133 Furthermore, the Th2 cytokines IL-4 and IL-13, which are involved in the development of asthma, increased FABP4 expression in bronchial epithelial cells, and the Th1 cytokine interferon γ suppressed expression of FABP4.38 Interestingly, FABP4-deficient mice showed protection against asthma and airway inflammation through FABP action in stromal cells but not bone marrow-derived cells.38
It has been reported that FABPs, including FABP4, are expressed in human placental trophoblasts and that FABP4 is a key regulator of trophoblastic lipid transport and accumulation during placental development.134–136 FABP4 was detected in apoptotic granulosa cells in atretic antral follicles of the mouse ovary,137 suggesting a possible relevance to polycystic ovary syndrome (PCOS), which is known to frequently coexist with insulin resistance. Expression of Fabp4 mRNA in isolated granulosa cells was higher in patients with PCOS than in controls.138 Association of FABP4 gene polymorphisms with the development of PCOS has also been reported.139
Roles of FABP4 in modifications of tumor cell growth and apoptosis have been suggested by several studies. In ovarian cancer metastasis, FABP4 was detected in human ovarian cancer cells at the adipocyte–tumor cell interface, and FABP4 deficiency in mice substantially impaired metastatic tumor growth.140 FABP4 expression was also detected in lipoblasts of lipoblastoma and liposarcoma.141 Moreover, expression of FABP4 has been linked to glioblastoma142 and urothelial carcinomas.143 Additionally, treatment of mouse spleen and cultured T lymphocytes with dexamethasone induced FABP4 expression and its nuclear localization in association with apoptosis process.144
FABP4 as a Therapeutic Target
The expression, regulation, and metabolic function of intracellular and extracellular human FABP4 have been reported to be similar to those of mouse FABP4.10,25,36,72 In fact, individuals with T-87C, a genetic variant of the human FABP4 gene within the promoter region, were protected from obesity-induced type 2 diabetes and exhibited diminished FABP4 expression in adipose tissue, lower triglyceride levels, and reduced cardiovascular disease risk.105 These findings provide a critical proof-of-principle that the biological functions of FABP4 are similar in mice and humans.
Since FABP4 acts at the interface of metabolic and inflammatory pathways and plays a significant role in the development of obesity, insulin resistance, type 2 diabetes, and atherosclerosis as described above, it is expected that agents capable of modifying FABP4 function would become a new class of therapeutic agents with multi-indications. To date, several series of FABP4 inhibitors have been synthesized.6,145–150 We previously demonstrated the effect of BMS309403, a specific FABP4 inhibitor, on insulin resistance, diabetes mellitus, fatty liver disease, and atherosclerosis in experimental models,151 indicating that chemical inhibition of FABP4 could be a therapeutic strategy against several aspects of metabolic syndrome. BMS309403 is an orally active small molecule and interacts with the FA-binding pocket within the interior of FABP4 to inhibit binding of endogenous FAs (Fig. 1).6,147,151 In addition, a recent study showed that neutralization of secreted FABP4 by neutralizing antibodies reduced hepatic glucose production and corrected the diabetic phenotype of obese mice.72 Neutralizing or eliminating secreted FABP4 may also represent an effective therapeutic strategy against metabolic and cardiovascular diseases (Fig. 3). Further studies are obviously needed to investigate whether chemical or other types of inhibition and neutralization of FABP4 can be safely used in humans and to show the efficacy of agents for metabolic and cardiovascular diseases.
Concluding Remarks
FABP4 is involved in the regulation of glucose and lipid metabolism in relation to inflammatory and metabolic processes in target cells, especially adipocytes and macrophages. Under normal physiologic conditions, FABP4-deficient mice have no compromised phenotype.25,53 However, FABP4-deficient mice on dietary or genetic obesity were protected from systemic pathologic stresses such as metaflammation, suggesting that the FABP4 gene would be one of the thrifty genes.152 FABPs have been evolutionarily preserved from invertebrates (lower eukaryotes) to vertebrates including humans,12 indicating a close link between inflammatory and metabolic responses by the conserved function of FABP. The presence of FABP4 in cells may have been beneficial for storing energy in adipose tissue or for acting as a strong immune response in macrophages against pathogens. In addition, secreted FABP4 in association with lipolysis during fasting may regulate hepatic glucose production as the thrifty phenotype to survive in famine. Under contemporary lifestyle with excessive caloric intake and decreased energy expenditure, the presence, induction, or enhanced secretion of FABP4 may be rather disadvantageous for regulating inflammatory or metabolic homeostasis. In recent metabolic disorders, as an evolutionary bottleneck in humans, inhibition or neutralization of FABP4 could be a promising novel class of therapeutics against several metabolic and cardiovascular diseases, and possibly other diseases such as asthma and cancer metastasis as well.
Acknowledgments
The authors are grateful to group members of their department for their scientific contribution. They also regret the inadvertent omission of many important references because of space limitations.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: MF. Contributed to the writing of the manuscript: MF, SS, KS, TM. Agree with manuscript results and conclusions: MF, SS, KS, TM. Jointly developed the structure and arguments for the paper: MF, SS, KS, TM. Made critical revisions and approved final version: MF, SS, KS, TM. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: In relation to this review article, Masato Furuhashi has been supported by grants from JSPS KAKENHI, Uehara Memorial Foundation, SENSHIN Medical Research Foundation, Japan Diabetes Foundation, Takeda Medical Research Foundation, Ono Medical Research Foundation, Takeda Science Foundation, Akiyama Life Science Foundation, Yamaguchi Endocrine Research Foundation, and Kondou Kinen Medical Foundation. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE)
REFERENCES
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 4.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293(5535):1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Furuhashi M, Li P, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140(3):338–48. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7(6):489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuhashi M, Ishimura S, Ota H, Miura T. Lipid chaperones and metabolic inflammation. Int J Inflam. 2011;2011:642612. doi: 10.4061/2011/642612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coe NR, Bernlohr DA. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim Biophys Acta. 1998;1391(3):287–306. doi: 10.1016/s0005-2760(97)00205-1. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid-binding proteins. Cell Mol Life Sci. 2002;59(7):1096–116. doi: 10.1007/s00018-002-8490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haunerland NH, Spener F. Fatty acid-binding proteins – insights from genetic manipulations. Prog Lipid Res. 2004;43(4):328–49. doi: 10.1016/j.plipres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47(1):39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- 12.Esteves A, Ehrlich R. Invertebrate intracellular fatty acid binding proteins. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142(3–4):262–74. doi: 10.1016/j.cbpc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Ockner RK, Manning JA, Poppenhausen RB, Ho WK. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972;177(4043):56–8. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- 14.Veerkamp JH, van Moerkerk HT. Fatty acid-binding protein and its relation to fatty acid oxidation. Mol Cell Biochem. 1993;123(1–2):101–6. doi: 10.1007/BF01076480. [DOI] [PubMed] [Google Scholar]
- 15.Ek BA, Cistola DP, Hamilton JA, Kaduce TL, Spector AA. Fatty acid binding proteins reduce 15-lipoxygenase-induced oxygenation of linoleic acid and arachidonic acid. Biochim Biophys Acta. 1997;1346(1):75–85. doi: 10.1016/s0005-2760(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 16.Zimmer JS, Dyckes DF, Bernlohr DA, Murphy RC. Fatty acid binding proteins stabilize leukotriene A4: competition with arachidonic acid but not other lipoxygenase products. J Lipid Res. 2004;45(11):2138–44. doi: 10.1194/jlr.M400240-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Spiegelman BM, Frank M, Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983;258(16):10083–9. [PubMed] [Google Scholar]
- 18.Hunt CR, Ro JH, Dobson DE, Min HY, Spiegelman BM. Adipocyte P2 gene: developmental expression and homology of 5’-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986;83(11):3786–90. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxa CA, Sha RS, Buelt MK, et al. Human adipocyte lipid-binding protein: purification of the protein and cloning of its complementary DNA. Biochemistry. 1989;28(22):8683–90. doi: 10.1021/bi00448a003. [DOI] [PubMed] [Google Scholar]
- 20.Amri EZ, Bertrand B, Ailhaud G, Grimaldi P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res. 1991;32(9):1449–56. [PubMed] [Google Scholar]
- 21.Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992;267(9):5937–41. [PubMed] [Google Scholar]
- 22.Kletzien RF, Foellmi LA, Harris PK, Wyse BM, Clarke SD. Adipocyte fatty acid-binding protein: regulation of gene expression in vivo and in vitro by an insulin-sensitizing agent. Mol Pharmacol. 1992;42(4):558–62. [PubMed] [Google Scholar]
- 23.Cook JS, Lucas JJ, Sibley E, et al. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A. 1988;85(9):2949–53. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melki SA, Abumrad NA. Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J Lipid Res. 1993;34(9):1527–34. [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–9. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 26.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141(9):3388–96. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 27.Shen WJ, Sridhar K, Bernlohr DA, Kraemer FB. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proc Natl Acad Sci U S A. 1999;96(10):5528–32. doi: 10.1073/pnas.96.10.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AJ, Sanders MA, Juhlmann BE, Hertzel AV, Bernlohr DA. Mapping of the hormone-sensitive lipase binding site on the adipocyte fatty acid-binding protein (AFABP). Identification of the charge quartet on the AFABP/aP2 helix-turn-helix domain. J Biol Chem. 2008;283(48):33536–43. doi: 10.1074/jbc.M806732200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheja L, Makowski L, Uysal KT, et al. Altered insulin secretion associated with reduced lipolytic efficiency in aP2-/-mice. Diabetes. 1999;48(10):1987–94. doi: 10.2337/diabetes.48.10.1987. [DOI] [PubMed] [Google Scholar]
- 30.Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res. 1999;40(5):967–72. [PubMed] [Google Scholar]
- 31.Yang R, Castriota G, Chen Y, et al. RNAi-mediated germline knockdown of FABP4 increases body weight but does not improve the deranged nutrient metabolism of diet-induced obese mice. Int J Obes. 2011;35(2):217–25. doi: 10.1038/ijo.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson MA, LiCata VJ, Ribarik Coe N, Bernlohr DA. Biochemical and biophysical analysis of the intracellular lipid binding proteins of adipocytes. Mol Cell Biochem. 1999;192(1–2):33–40. [PubMed] [Google Scholar]
- 33.Maeda K, Uysal KT, Makowski L, et al. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes. 2003;52(2):300–7. doi: 10.2337/diabetes.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hertzel AV, Bennaars-Eiden A, Bernlohr DA. Increased lipolysis in transgenic animals overexpressing the epithelial fatty acid binding protein in adipose cells. J Lipid Res. 2002;43(12):2105–11. doi: 10.1194/jlr.m200227-jlr200. [DOI] [PubMed] [Google Scholar]
- 35.Owada Y, Suzuki I, Noda T, Kondo H. Analysis on the phenotype of E-FABP-gene knockout mice. Mol Cell Biochem. 2002;239(1–2):83–6. [PubMed] [Google Scholar]
- 36.Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7(6):699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolph MS, Young TR, Shum BO, et al. Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J Immunol. 2006;177(11):7794–801. doi: 10.4049/jimmunol.177.11.7794. [DOI] [PubMed] [Google Scholar]
- 38.Shum BO, Mackay CR, Gorgun CZ, et al. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116(8):2183–92. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu Y, Luo N, Lopes-Virella MF, Garvey WT. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165(2):259–69. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 40.Kazemi MR, McDonald CM, Shigenaga JK, Grunfeld C, Feingold KR. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol. 2005;25(6):1220–4. doi: 10.1161/01.ATV.0000159163.52632.1b. [DOI] [PubMed] [Google Scholar]
- 41.Pelton PD, Zhou L, Demarest KT, Burris TP. PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem Biophys Res Commun. 1999;261(2):456–8. doi: 10.1006/bbrc.1999.1071. [DOI] [PubMed] [Google Scholar]
- 42.Fu Y, Luo N, Lopes-Virella MF. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res. 2000;41(12):2017–23. [PubMed] [Google Scholar]
- 43.Wang XQ, Yang K, He YS, Lu L, Shen WF. Receptor mediated elevation in FABP4 levels by advanced glycation end products induces cholesterol and triacylglycerol accumulation in THP-1 macrophages. Lipids. 2011;46(6):479–86. doi: 10.1007/s11745-011-3542-4. [DOI] [PubMed] [Google Scholar]
- 44.Llaverias G, Noé V, Peñuelas S, et al. Atorvastatin reduces CD68, FABP4, and HBP expression in oxLDL-treated human macrophages. Biochem Biophys Res Commun. 2004;318(1):265–74. doi: 10.1016/j.bbrc.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Song J, Ren P, Zhang L, Wang XL, Chen L, Shen YH. Metformin reduces lipid accumulation in macrophages by inhibiting FOXO1-mediated transcription of fatty acid-binding protein 4. Biochem Biophys Res Commun. 2010;393(1):89–94. doi: 10.1016/j.bbrc.2010.01.086. [DOI] [PubMed] [Google Scholar]
- 46.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280(13):12888–95. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui X, Li H, Zhou Z, et al. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein–1. J Biol Chem. 2010;285(14):10273–80. doi: 10.1074/jbc.M109.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boord JB, Maeda K, Makowski L, et al. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22(10):1686–91. doi: 10.1161/01.atv.0000033090.81345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 50.Agardh HE, Folkersen L, Ekstrand J, et al. Expression of fatty acid-binding protein 4/aP2 is correlated with plaque instability in carotid atherosclerosis. J Intern Med. 2011;269(2):200–10. doi: 10.1111/j.1365-2796.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 51.Feingold KR, Kazemi MR, Magra AL, et al. ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis. 2010;209(1):81–8. doi: 10.1016/j.atherosclerosis.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 52.Babaev VR, Runner RP, Fan D, et al. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-gamma-regulated genes. Arterioscler Thromb Vasc Biol. 2011;31(6):1283–90. doi: 10.1161/ATVBAHA.111.225839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maeda K, Cao H, Kono K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1(2):107–19. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Cao H, Maeda K, Gorgun CZ, et al. Regulation of metabolic responses by adipocyte/macrophage fatty acid-binding proteins in leptin-deficient mice. Diabetes. 2006;55(7):1915–22. doi: 10.2337/db05-1496. [DOI] [PubMed] [Google Scholar]
- 55.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furuhashi M, Fucho R, Gorgun CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest. 2008;118(7):2640–50. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boord JB, Maeda K, Makowski L, et al. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110(11):1492–8. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–44. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erbay E, Babaev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15(12):1383–91. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleman SL, Park YK, Lee JY. Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. Eur J Nutr. 2011;50(5):323–30. doi: 10.1007/s00394-010-0140-9. [DOI] [PubMed] [Google Scholar]
- 62.Stefan N, Kantartzis K, Celebi N, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care. 2010;33(2):405–7. doi: 10.2337/dc09-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mozaffarian D, Cao H, King IB, et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr. 2010;92(6):1350–8. doi: 10.3945/ajcn.110.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mozaffarian D, Cao H, King IB, et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med. 2010;153(12):790–9. doi: 10.1059/0003-4819-153-12-201012210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayers SD, Nedrow KL, Gillilan RE, Noy N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry. 2007;46(23):6744–52. doi: 10.1021/bi700047a. [DOI] [PubMed] [Google Scholar]
- 66.Gillilan RE, Ayers SD, Noy N. Structural basis for activation of fatty acid-binding protein 4. J Mol Biol. 2007;372(5):1246–60. doi: 10.1016/j.jmb.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan NS, Shaw NS, Vinckenbosch N, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22(14):5114–27. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson BR, Mazurkiewicz-Munoz AM, Suttles J, Carter-Su C, Bernlohr DA. Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. J Biol Chem. 2009;284(20):13473–80. doi: 10.1074/jbc.M900075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorbenko O, Panayotou G, Zhyvoloup A, Volkova D, Gout I, Filonenko V. Identification of novel PTEN-binding partners: PTEN interaction with fatty acid binding protein FABP4. Mol Cell Biochem. 2010;337(1–2):299–305. doi: 10.1007/s11010-009-0312-1. [DOI] [PubMed] [Google Scholar]
- 70.Tsuda M, Inoue-Narita T, Suzuki A, Itami S, Blumenberg M, Manabe M. Induction of gene encoding FABP4 in Pten-null keratinocytes. FEBS Lett. 2009;583(8):1319–22. doi: 10.1016/j.febslet.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 71.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10(2):148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 72.Cao H, Sekiya M, Ertunc ME, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013;17(5):768–78. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kralisch S, Ebert T, Lossner U, Jessnitzer B, Stumvoll M, Fasshauer M. Adipocyte fatty acid-binding protein is released from adipocytes by a non-conventional mechanism. Int J Obes (Lond) 2014;38(9):1251–4. doi: 10.1038/ijo.2013.232. [DOI] [PubMed] [Google Scholar]
- 74.Stich V, Berlan M. Physiological regulation of NEFA availability: lipolysis pathway. Proc Nutr Soc. 2004;63(2):369–74. doi: 10.1079/PNS2004350. [DOI] [PubMed] [Google Scholar]
- 75.Moro C, Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am J Physiol Heart Circ Physiol. 2013;304(3):H358–68. doi: 10.1152/ajpheart.00704.2012. [DOI] [PubMed] [Google Scholar]
- 76.Mita T, Furuhashi M, Hiramitsu S, et al. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring) 2014 Dec 17; doi: 10.1002/oby.20954. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Schlottmann I, Ehrhart-Bornstein M, Wabitsch M, Bornstein SR, Lamounier-Zepter V. Calcium-dependent release of adipocyte fatty acid binding protein from human adipocytes. Int J Obes (Lond) 2014;38(9):1221–7. doi: 10.1038/ijo.2013.241. [DOI] [PubMed] [Google Scholar]
- 78.Lamounier-Zepter V, Look C, Alvarez J, et al. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009;105(4):326–34. doi: 10.1161/CIRCRESAHA.109.200501. [DOI] [PubMed] [Google Scholar]
- 79.Fach EM, Garulacan LA, Gao J, et al. Mol Cell Proteomics; In vitro biomarker discovery for atherosclerosis by proteomics. ; 2004. pp. 1200–10. [DOI] [PubMed] [Google Scholar]
- 80.Aragones G, Saavedra P, Heras M, Cabre A, Girona J, Masana L. Fatty acid-binding protein 4 impairs the insulin-dependent nitric oxide pathway in vascular endothelial cells. Cardiovasc Diabetol. 2012;11(1):72. doi: 10.1186/1475-2840-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Girona J, Rosales R, Plana N, Saavedra P, Masana L, Vallve JC. FABP4 induces vascular smooth muscle cell proliferation and migration through a MAPK-dependent pathway. PLoS One. 2013;8(11):e81914. doi: 10.1371/journal.pone.0081914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu LE, Samocha-Bonet D, Whitworth PT, et al. Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol Metab. 2014;3(4):465–73. doi: 10.1016/j.molmet.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352(1–2):15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Pelsers MM, Morovat A, Alexander GJ, Hermens WT, Trull AK, Glatz JF. Liver fatty acid-binding protein as a sensitive serum marker of acute hepatocellular damage in liver transplant recipients. Clin Chem. 2002;48(11):2055–7. [PubMed] [Google Scholar]
- 85.Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36(7):529–35. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 86.Tanaka T, Hirota Y, Sohmiya K, Nishimura S, Kawamura K. Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clin Biochem. 1991;24(2):195–201. doi: 10.1016/0009-9120(91)90571-u. [DOI] [PubMed] [Google Scholar]
- 87.Setsuta K, Seino Y, Ogawa T, Arao M, Miyatake Y, Takano T. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. Am J Med. 2002;113(9):717–22. doi: 10.1016/s0002-9343(02)01394-3. [DOI] [PubMed] [Google Scholar]
- 88.Pelsers MM, Hanhoff T, Van der Voort D, et al. Brain- and heart-type fatty acid-binding proteins in the brain: tissue distribution and clinical utility. Clin Chem. 2004;50(9):1568–75. doi: 10.1373/clinchem.2003.030361. [DOI] [PubMed] [Google Scholar]
- 89.Ishimura S, Furuhashi M, Watanabe Y, et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One. 2013;8(11):e81318. doi: 10.1371/journal.pone.0081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu A, Wang Y, Xu JY, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52(3):405–13. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 91.Furuhashi M, Ura N, Hasegawa K, et al. Serum ratio of heart-type fatty acid-binding protein to myoglobin. A novel marker of cardiac damage and volume overload in hemodialysis patients. Nephron Clin Pract. 2003;93(2):C69–74. doi: 10.1159/000068520. [DOI] [PubMed] [Google Scholar]
- 92.Furuhashi M, Ura N, Hasegawa K, Tsuchihashi K, Nakata T, Shimamoto K. Utility of serum ratio of heart-type fatty acid-binding protein to myoglobin for cardiac damage regardless of renal dysfunction. Circ J. 2004;68(7):656–9. doi: 10.1253/circj.68.656. [DOI] [PubMed] [Google Scholar]
- 93.Yeung DC, Xu A, Tso AW, et al. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care. 2009;32(1):132–4. doi: 10.2337/dc08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Furuhashi M, Ishimura S, Ota H, et al. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One. 2011;6(11):e27356. doi: 10.1371/journal.pone.0027356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu A, Tso AW, Cheung BM, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115(12):1537–43. doi: 10.1161/CIRCULATIONAHA.106.647503. [DOI] [PubMed] [Google Scholar]
- 96.Tso AW, Xu A, Sham PC, et al. Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30(10):2667–72. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 97.Ota H, Furuhashi M, Ishimura S, et al. Elevation of fatty acid-binding protein 4 is predisposed by family history of hypertension and contributes to blood pressure elevation. Am J Hypertens. 2012;25(10):1124–30. doi: 10.1038/ajh.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fuseya T, Furuhashi M, Yuda S, et al. Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc Diabetol. 2014;13(1):126. doi: 10.1186/s12933-014-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeung DC, Xu A, Cheung CW, et al. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(8):1796–802. doi: 10.1161/ATVBAHA.107.146274. [DOI] [PubMed] [Google Scholar]
- 100.Balci MM, Arslan U, Firat H, et al. Serum levels of adipocyte fatty acid-binding protein are independently associated with left ventricular mass and myocardial performance index in obstructive sleep apnea syndrome. J Investig Med. 2012;60(7):1020–6. doi: 10.2310/JIM.0b013e31826868f2. [DOI] [PubMed] [Google Scholar]
- 101.Engeli S, Utz W, Haufe S, et al. Fatty acid binding protein 4 predicts left ventricular mass and longitudinal function in overweight and obese women. Heart. 2013;99(13):944–8. doi: 10.1136/heartjnl-2013-303735. [DOI] [PubMed] [Google Scholar]
- 102.Huang CL, Wu YW, Wu CC, et al. Association between serum adipocyte fatty-acid binding protein concentrations, left ventricular function and myocardial perfusion abnormalities in patients with coronary artery disease. Cardiovasc Diabetol. 2013;12:105. doi: 10.1186/1475-2840-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu M, Zhou M, Bao Y, et al. Circulating adipocyte fatty acid-binding protein levels are independently associated with heart failure. Clin Sci. 2013;124(2):115–22. doi: 10.1042/CS20120004. [DOI] [PubMed] [Google Scholar]
- 104.Baessler A, Lamounier-Zepter V, Fenk S, et al. Adipocyte fatty acid-binding protein levels are associated with left ventricular diastolic dysfunction in morbidly obese subjects. Nutr Diabetes. 2014;4:e106. doi: 10.1038/nutd.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tuncman G, Erbay E, Hom X, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci U S A. 2006;103(18):6970–5. doi: 10.1073/pnas.0602178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.von Eynatten M, Breitling LP, Roos M, Baumann M, Rothenbacher D, Brenner H. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler Thromb Vasc Biol. 2012;32(9):2327–35. doi: 10.1161/ATVBAHA.112.248609. [DOI] [PubMed] [Google Scholar]
- 107.Chow WS, Tso AW, Xu A, et al. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc. 2013;2(1):e004176. doi: 10.1161/JAHA.112.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Djoussé L, Bartz TM, Ix JH, et al. Fatty acid-binding protein 4 and incident heart failure: the cardiovascular health study. Eur J Heart Fail. 2013;15(4):394–9. doi: 10.1093/eurjhf/hfs196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res Treat. 2010;119(2):367–7. doi: 10.1007/s10549-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 110.Karpisek M, Stejskal D, Kotolova H, et al. Treatment with atorvastatin reduces serum adipocyte-fatty acid binding protein value in patients with hyperlipidaemia. Eur J Clin Invest. 2007;37(8):637–42. doi: 10.1111/j.1365-2362.2007.01835.x. [DOI] [PubMed] [Google Scholar]
- 111.Miyoshi T, Doi M, Hirohata S, et al. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart Vessels. 2011;26(4):408–13. doi: 10.1007/s00380-010-0060-x. [DOI] [PubMed] [Google Scholar]
- 112.Furuhashi M, Mita T, Moniwa N, et al. Angiotensin II receptor blockers decrease serum concentration of fatty acid-binding protein 4 in patients with hypertension. Hypertens Res. 2015 doi: 10.1038/hr.2015.2. In press. [DOI] [PubMed] [Google Scholar]
- 113.Cabré A, Lázaro I, Girona J, et al. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis. 2007;195(1):e150–8. doi: 10.1016/j.atherosclerosis.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 114.Schachtrup C, Emmler T, Bleck B, Sandqvist A, Spener F. Functional analysis of peroxisome-proliferator-responsive element motifs in genes of fatty acid-binding proteins. Biochem J. 2004;382(pt 1):239–45. doi: 10.1042/BJ20031340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yeung DC, Wang Y, Xu A, et al. Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur Heart J. 2008;29(17):2156–63. doi: 10.1093/eurheartj/ehn295. [DOI] [PubMed] [Google Scholar]
- 116.Bagheri R, Qasim AN, Mehta NN, et al. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. Am J Cardiol. 2010;106(8):1118–23. doi: 10.1016/j.amjcard.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elmasri H, Karaaslan C, Teper Y, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23(11):3865–73. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Iso T, Maeda K, Hanaoka H, et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol. 2013;33(11):2549–57. doi: 10.1161/ATVBAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elmasri H, Ghelfi E, Yu CW, et al. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis. 2012;15(3):457–68. doi: 10.1007/s10456-012-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saint-Geniez M, Ghelfi E, Liang X, et al. Fatty acid binding protein 4 deficiency protects against oxygen-induced retinopathy in mice. PLoS One. 2014;9(5):e96253. doi: 10.1371/journal.pone.0096253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harjes U, Bridges E, McIntyre A, Fielding BA, Harris AL. Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J Biol Chem. 2014;289(33):23168–76. doi: 10.1074/jbc.M114.576512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee MY, Tse HF, Siu CW, Zhu SG, Man RY, Vanhoutte PM. Genomic changes in regenerated porcine coronary arterial endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(11):2443–9. doi: 10.1161/ATVBAHA.107.141705. [DOI] [PubMed] [Google Scholar]
- 123.Han Q, Yeung SC, Ip MS, Mak JC. Effects of intermittent hypoxia on A-/E-FABP expression in human aortic endothelial cells. Int J Cardiol. 2010;145(2):396–8. doi: 10.1016/j.ijcard.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 124.Daly C, Wong V, Burova E, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18(9):1060–71. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee MY, Li H, Xiao Y, Zhou Z, Xu A, Vanhoutte PM. Chronic administration of BMS309403 improves endothelial function in apolipoprotein E-deficient mice and in cultured human endothelial cells. Br J Pharmacol. 2011;162(7):1564–76. doi: 10.1111/j.1476-5381.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ha MK, Soo Cho J, Baik OR, Lee KH, Koo HS, Chung KY. Caenorhabditis elegans as a screening tool for the endothelial cell-derived putative aging-related proteins detected by proteomic analysis. Proteomics. 2006;6(11):3339–51. doi: 10.1002/pmic.200500395. [DOI] [PubMed] [Google Scholar]
- 127.Lee MY, Wang Y, Vanhoutte PM. Senescence of cultured porcine coronary arterial endothelial cells is associated with accelerated oxidative stress and activation of NFkB. J Vasc Res. 2010;47(4):287–98. doi: 10.1159/000265563. [DOI] [PubMed] [Google Scholar]
- 128.Tanaka M, Furuhashi M, Okazaki Y, et al. Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron Clin Pract. 2015 Jan 9; doi: 10.1159/000368412. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 129.Kamijo A, Kimura K, Sugaya T, et al. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med. 2004;143(1):23–30. doi: 10.1016/j.lab.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 130.Noiri E, Doi K, Negishi K, et al. Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296(4):F669–79. doi: 10.1152/ajprenal.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okazaki Y, Furuhashi M, Tanaka M, et al. Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS One. 2014;9(12):e115429. doi: 10.1371/journal.pone.0115429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghelfi E, Karaaslan C, Berkelhamer S, Akar S, Kozakewich H, Cataltepe S. Fatty acid-binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2011;45(3):550–6. doi: 10.1165/rcmb.2010-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maver A, Medica I, Peterlin B. Search for sarcoidosis candidate genes by integration of data from genomic, transcriptomic and proteomic studies. Med Sci Monit. 2009;15(12):SR22–8. [PubMed] [Google Scholar]
- 134.Biron-Shental T, Schaiff WT, Ratajczak CK, Bildirici I, Nelson DM, Sadovsky Y. Hypoxia regulates the expression of fatty acid-binding proteins in primary term human trophoblasts. Am J Obstet Gynecol. 2007;197(5):e511–6. doi: 10.1016/j.ajog.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scifres CM, Chen B, Nelson DM, Sadovsky Y. Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J Clin Endocrinol Metab. 2011;96(7):E1083–91. doi: 10.1210/jc.2010-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Makkar A, Mishima T, Chang G, Scifres C, Sadovsky Y. Fatty acid binding protein-4 is expressed in the mouse placental labyrinth, yet is dispensable for placental triglyceride accumulation and fetal growth. Placenta. 2014;35(10):802–7. doi: 10.1016/j.placenta.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nourani MR, Owada Y, Kitanaka N, et al. Occurrence of immunoreactivity for adipocyte-type fatty acid binding protein in degenerating granulosa cells in atretic antral follicles of mouse ovary. J Mol Histol. 2005;36(8–9):491–7. doi: 10.1007/s10735-006-9024-y. [DOI] [PubMed] [Google Scholar]
- 138.Hu W, Qiao J. Expression and regulation of adipocyte fatty acid binding protein in granulosa cells and its relation with clinical characteristics of polycystic ovary syndrome. Endocrine. 2011;40(2):196–202. doi: 10.1007/s12020-011-9495-9. [DOI] [PubMed] [Google Scholar]
- 139.Wang J, Tang J, Wang B, et al. FABP4: a novel candidate gene for polycystic ovary syndrome. Endocrine. 2009;36(3):392–6. doi: 10.1007/s12020-009-9228-5. [DOI] [PubMed] [Google Scholar]
- 140.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bennett JH, Shousha S, Puddle B, Athanasou NA. Immunohistochemical identification of tumours of adipocytic differentiation using an antibody to aP2 protein. J Clin Pathol. 1995;48(10):950–4. doi: 10.1136/jcp.48.10.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cataltepe O, Arikan MC, Ghelfi E, et al. Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol Appl Neurobiol. 2012;38(5):400–10. doi: 10.1111/j.1365-2990.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 143.Ohlsson G, Moreira JM, Gromov P, Sauter G, Celis JE. Loss of expression of the adipocyte-type fatty acid-binding protein (A-FABP) is associated with progression of human urothelial carcinomas. Mol Cell Proteomics. 2005;4(4):570–81. doi: 10.1074/mcp.M500017-MCP200. [DOI] [PubMed] [Google Scholar]
- 144.Abdelwahab SA, Owada Y, Kitanaka N, et al. Enhanced expression of adipocyte-type fatty acid binding protein in murine lymphocytes in response to dexamethasone treatment. Mol Cell Biochem. 2007;299(1–2):99–107. doi: 10.1007/s11010-005-9050-1. [DOI] [PubMed] [Google Scholar]
- 145.Lehmann F, Haile S, Axen E, et al. Discovery of inhibitors of human adipocyte fatty acid-binding protein, a potential type 2 diabetes target. Bioorg Med Chem Lett. 2004;14(17):4445–8. doi: 10.1016/j.bmcl.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 146.Ringom R, Axen E, Uppenberg J, Lundback T, Rondahl L, Barf T. Substituted benzylamino-6-(trifluoromethyl)pyrimidin-4(1H)-ones: a novel class of selective human A-FABP inhibitors. Bioorg Med Chem Lett. 2004;14(17):4449–52. doi: 10.1016/j.bmcl.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 147.Sulsky R, Magnin DR, Huang Y, et al. Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP) Bioorg Med Chem Lett. 2007;17(12):3511–5. doi: 10.1016/j.bmcl.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 148.Hertzel AV, Hellberg K, Reynolds JM, et al. Identification and characterization of a small molecule inhibitor of fatty acid binding proteins. J Med Chem. 2009;52(19):6024–31. doi: 10.1021/jm900720m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barf T, Lehmann F, Hammer K, et al. N-Benzyl-indolo carboxylic acids: design and synthesis of potent and selective adipocyte fatty-acid binding protein (A-FABP) inhibitors. Bioorg Med Chem Lett. 2009;19(6):1745–8. doi: 10.1016/j.bmcl.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 150.Lan H, Cheng CC, Kowalski TJ, et al. Small-molecule inhibitors of FABP4/5 ameliorate dyslipidemia but not insulin resistance in mice with diet-induced obesity. J Lipid Res. 2011;52(4):646–56. doi: 10.1194/jlr.M012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Furuhashi M, Tuncman G, Görgün CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959–65. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42(9):1033–49. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]