Abstract
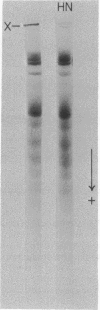
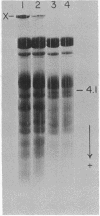
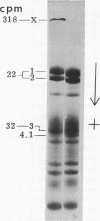
Transamidase (i.e., "transglutaminase") activity of human erythrocytes, lysed by a single freezing and thawing to 37 degrees, was measured by a method of incorporating [14C]putrescine into N,N'-dimethylcasein. In the absence of added calcium ions, virtually no enzyme activity could be detected. An increase in concentration of the cation to about 0.5 mM, however, turned on the enzyme to appreciable levels of activity. Simultaneously, Ca2+ produced formation of high molecular weight, nondisulfied bonded protein polymers either directly in the lysate or in fresh cells when the cation was added together with the A23187 ionophore. The polymers could be readily identified in the isolated cell ghosts by means of disc gel electrophoresis. If the Ca2+-promoted formation of polymers was allowed to take place in the presence of 14C-putrescine, then this tracer became incorporated into the polymeric material. The incorporation indicated that polymerization occurred through gamma-glutamyl-epsilon-lysine bridtes. It is suggested that the intrinsic transamidase mediates protein crosslinking of the erythrocyte membrane whenever there is an increase in intracellular Ca2+ concentration. The presence of suitable transglutaminase substrates, e.g. histamine, inhibited crosslinking when the cells were incubated with Ca2+ and ionophore.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carraway K. L., Triplett R. B., Anderson D. R. Calcium-promoted aggregation of erythrocyte membrane proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):571–581. doi: 10.1016/0005-2795(75)90163-4. [DOI] [PubMed] [Google Scholar]
- Dutton A., Singer S. J. Crosslinking and labeling of membrane proteins by transglutaminase-catalyzed reactions. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2568–2571. doi: 10.1073/pnas.72.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Lin Y., Means G. E., Feeney R. E. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969 Feb 10;244(3):789–793. [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Chenoweth D., Gray A. Titration of the acceptor cross-linking sites in fibrin. Ann N Y Acad Sci. 1972 Dec 8;202:155–171. doi: 10.1111/j.1749-6632.1972.tb16328.x. [DOI] [PubMed] [Google Scholar]
- Lorand L., Shishido R., Parameswaran K. N., Steck T. L. Modification of human erythrocyte ghosts with transglutaminase. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1158–1166. doi: 10.1016/0006-291x(75)90795-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Tishler P. V., Epstein C. J. A convenient method of preparing polyacrylamide gels for liquid scintillation spectrometry. Anal Biochem. 1968 Jan;22(1):89–98. doi: 10.1016/0003-2697(68)90262-5. [DOI] [PubMed] [Google Scholar]