A woman received intravenous (IV) acetaminophen every six hours (16 doses) for pain control. Tests showed high levels of markers for liver damage, but levels fell after the drug was stopped—raising the possibility of hepatotoxicity linked to IV acetaminophen.
Keywords: intravenous acetaminophen, hepatotoxicity
Abstract
We present a case of a 36-year-old female who came into the emergency department with right-side abdominal pain. She went to the operating room for a diagnostic laparoscopy and appendectomy. She received intravenous (IV) acetaminophen every six hours both preoperatively and postoperatively for pain control. The patient’s aspartate aminotransferase and alanine aminotransferase levels were elevated and peaked at 4,833 and 6,600 IU/L, respectively, from baselines of 14 and 15, respectively, while she was receiving 16 doses of IV acetaminophen. The patient was transferred to a regional liver transplant center for evaluation for a transplant. She was treated with IV N-acetylcysteine and discharged with a normal liver-function test without a transplant. This case report supports the possibility of hepatotoxicity associated with IV acetaminophen.
INTRODUCTION
Oral acetaminophen went on sale in the United States in 1955 under the brand name Tylenol.1 Fifty-five years later, on November 2, 2010, the U.S. Food and Drug Administration (FDA) granted marketing approval to Cadence Pharmaceuticals, Inc., for an IV acetaminophen formulation under the brand name of Ofirmev.2 IV acetaminophen is indicated for mild-to-moderate pain and in conjunction with opioids to manage moderate and severe pain. IV acetaminophen is also approved as an antipyretic.3 Current prescribing information for IV acetaminophen for adults and adolescents over 50 kg sets a maximum of 4 g of acetaminophen per day.3
IV acetaminophen may have a better safety profile than oral acetaminophen. In first-pass pharmacokinetic models, IV acetaminophen has been shown to expose the liver to 50% less initial acetaminophen compared with oral acetaminophen.4,5 Oral acetaminophen is absorbed in the proximal small intestine, delivered to the portal vein, and undergoes first-pass metabolism. IV acetaminophen is distributed to the systemic circulation first, before being delivered to the liver. Since first-pass metabolism is reduced, some authors have suggested that the risk of hepatic damage is decreased but not eliminated.5
Here we present the first known case in which a patient developed acute liver failure while receiving 16 doses of IV acetaminophen for postoperative pain relief, an amount within the therapeutic dosing range. This was determined to be the first such case via a literature search on PubMed using the search terms “intravenous,” “acetaminophen,” “liver,” and “hepatotoxicity.”
CASE PRESENTATION
A 36-year-old white female presented to the emergency department (ED) after four days of post-prandial right upper-quadrant pain, nausea, and chills. She was 5 feet, 5 inches (165.1 cm) tall and weighed 115 pounds (52.3 kg), with a body mass index of 20 kg/m2. She had no significant medical history or allergies and reported no use of medications (prescription and over the counter), dietary supplements, or illicit drugs prior to presentation to the ED. The patient denied any recent travel. Her medical record noted that she drank ethanol socially, although it was not specified whether she had consumed any recently, and no tests of ethanol levels or gamma-glutamyl transferase were drawn on admission. Her vital signs at presentation were as follows: temperature, 98.4° F; pulse, 91 beats per minute; respiratory rate, 16 breaths per minute; and blood pressure, 109/63 mm Hg. Liver function tests (LFTs) were as follows: alanine aminotransferase (ALT), 15 international units per liter (IU/L) (normal: 4–36 IU/L); aspartate aminotransferase (AST), 14 IU/L (normal: 13–39 IU/L); alkaline phosphatase, 44 IU/L (normal: 25–100 IU/L); total bilirubin, 0.7 mg/dL (normal: 0.2–1.1 mg/dL); and direct bilirubin, 0.2 mg/dL (normal: 0–0.5 mg/dL). Serum creatinine and phosphorus on admission were within normal limits, at 0.7 mg/dL and 3.3 mg/dL, respectively. A surgical consult was called to evaluate her abdominal pain and suspected acute appendicitis. In the ED, the patient received a one-time dose of 975 mg of oral acetaminophen (three 325-mg tablets) to control the pain. She later experienced worsening pain with guarding. A computed tomography (CT) scan of the abdomen showed she had a retrocecal appendix but no clear signs of appendicitis.
On hospital day 2, the patient was given her first dose of IV acetaminophen at 6 a.m. for abdominal pain because she could not tolerate oral medications as a result of nausea and vomiting. All IV acetaminophen doses were the standard adult amount of 1,000 mg infused over 15 minutes every six hours around the clock at 6 a.m., noon, 6 p.m., and midnight. All doses were administered and documented in the medication administration record (MAR) by nurses.
On hospital day 3, prior to surgery, the patient was found to have mildly elevat ed LFTs with total bilirubin of 0.7 mg/dL, direct bilirubin of 0.2 mg/dL, ALT of 197 IU/L, and AST of 159 IU/L. A laparoscopic appendectomy was performed; the postoperative diagnosis was acute appendicitis. However, the pathology report found the appendix was not inflamed. Ultimately, the cause of her initial symptoms was unclear.
Postoperatively, the patient experienced persistent nausea, vomiting, abdominal pain, and increased somnolence. It was unclear why she had these symptoms, although the causes could have been the anesthesia she had received for surgery or the underlying problem that led to the patient’s admission to the hospital. While the IV acetaminophen may also have contributed to these symptoms, she continued to complain of nausea and vomiting after IV acetaminophen was discontinued. She also developed mild hypotension (systolic blood pressure in the 80s) and acute anemia of an unknown origin, with a drop in hemoglobin from 9.5 g/dL to 8.2 g/dL. Cefazolin, metronidazole, and ondansetron were given postoperatively, and IV acetaminophen was continued every six hours for postoperative pain.
On hospital day 4, she received two units of packed red blood cells, and her hemoglobin rose to 9.7 g/dL. Her blood pressure remained stable, with no further clinical evidence of blood loss. CT scans of the abdomen and pelvis showed intraperitoneal blood consistent with postoperative bleeding.
On hospital day 6, the last dose of IV acetaminophen was given at 6 a.m. The patient received a total of 16 doses of IV acetaminophen in five days.
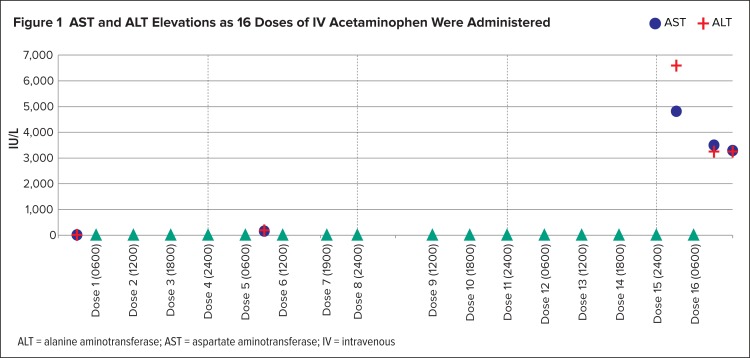
The patient remained nil per os (NPO, receiving nothing by mouth) throughout her entire hospital stay because of nausea and vomiting. On hospital day 6, AST was 4,833 IU/L and ALT was greater than 6,600 IU/L. AST and ALT became elevated as the IV acetaminophen doses were administered (Figure 1).
Figure 1.
AST and ALT Elevations as 16 Doses of IV Acetaminophen Were Administered
ALT = alanine aminotransferase; AST = aspartate aminotransferase; IV = intravenous
Total bilirubin was mildly elevated at 2.0 mg/dL, and alkaline phosphatase was normal. The international normalized ratio (INR) was 3.81. Hepatitis A and B panels were negative at the time. Due to the elevated LFTs, the IV acetaminophen was discontinued. The acetaminophen level was checked at 3 p.m., nine hours after the last dose, and was found to be 34 mcg/mL (normal: 5–26 mcg/mL).
The decision was made to start treatment with IV N-acetylcysteine (IV NAC), and the patient was transferred to the medical intensive care unit. The first dose of IV NAC consisted of 7,800 mg in 200 mL of 5% dextrose in water (D5W), which was administered at 6:15 p.m. at a rate of 200 mL per hour. A repeat acetaminophen level was drawn approximately 12.5 hours after the last dose at 6:18 p.m., which showed a level of 26 mcg/mL. Arterial blood gas, drawn at 6:27 p.m., showed that the patient had respiratory alkalosis with pH of 7.46 and PCO2 of 33 mg Hg. The patient received a second dose of IV NAC 2,600 mg in 500 mL of D5W at 7:30 p.m. at a rate of 125 mL (650 mg) per hour. While the second dose of IV NAC was infusing, the ALT and AST drawn at 10:15 p.m. showed decreases to 3,250 and 1,962, respectively. However, the INR remained elevated at 4.01. The third acetaminophen level, also measured at 10:15 p.m., showed a level of 12 mcg/mL. The patient was transferred to a regional liver transplant hospital for evaluation of a possible liver transplant on day 7. The patient was continued on IV NAC at the second institution, and was eventually discharged without a liver transplant and with normal LFTs. We were unable to determine the number of doses of IV NAC the patient received at the other institution and the duration of time before the LFTs normalized.
Throughout the patient’s stay at our institution, her renal function was stable, with a serum creatinine that ranged from 0.5 to 0.7, and a creatinine clearance (calculated via the Cockcroft-Gault equation) that ranged from 91.7 mL/min to 128.4 mL/min. The patient’s serum phosphorus declined after the initial measurement of 3.3 mg/dL to 1.5 mg/dL on hospital day 3 and 0.6 mg/dL on hospital day 7, the last day of her stay at our hospital.
Ultimately, the cause of her nausea and abdominal pain remains unknown; they may have been caused by an ovarian cyst. The patient’s case was included in the hospital’s adverse drug reaction report, which was presented to the P&T committee. Additionally, the case was submitted to the FDA’s MedWatch system.
DISCUSSION
Our patient experienced acute liver failure, as evidenced by the elevation of bilirubin and liver enzymes from normal baseline values. The differential diagnosis for the patient’s acute liver failure included acute viral hepatitis, ischemic hepatitis (shock liver), and acetaminophen toxicity. There was no evidence of a medication error with IV acetaminophen, as all doses were accounted for in the MAR and administered at intervals of six hours (one dose was administered one hour late). Based on hospital protocol, each Ofirmev order only allows a maximum of eight doses. In our patient, the second eight-dose order started at noon the next day, leaving a 12-hour dosing gap between the eighth and ninth doses. Hepatitis A and B panels were negative, ruling out viral hepatitis. While the patient did experience episodes of postoperative hypotension, her blood pressure values were not significantly lower than her baseline blood pressure. According to Seeto et al., systemic hypotension or hypoxia from a low-flow state alone is insufficient to cause typical ischemic hepatitis, as several patients in their study had no detectable blood pressure, following trauma, for prolonged periods, requiring rapid responses from clinicians. These patients had entirely normal LFTs throughout the hospital course.6
Risk assessment of hepatic failure in acute toxicity is traditionally done with the Rumack-Matthew nomogram. During chronic use of acetaminophen at therapeutic doses, asymptomatic elevations of ALT and AST may occur, but they usually are mildly elevated (less than three times the upper limit of normal), resolve spontaneously, and typically do not lead to hepatic dysfunction.7,8 In our patient, the acetaminophen level was drawn nine hours after the last dose. It was at this time that the patient was transferred to the medical intensive care unit and IV acetaminophen was suspected of causing the hepatotoxicity. The acetaminophen level was 34 mcg/mL, but the cause is unknown: This was not a case of a single acute overdose, which is the scenario the Rumack-Matthew nomogram is meant to address.9
In retrospect, it probably would have been beneficial to draw an acetaminophen level earlier in the course of therapy than was done. However, the clinicians managing the case did not suspect there might be a potential risk for hepatic toxicity to develop from the therapeutic use of IV acetaminophen. In addition, the manufacturer’s package insert does not recommend monitoring acetaminophen blood concentrations during the therapeutic use of IV acetaminophen.3
Unlike with acute acetaminophen toxicity, the Rumack-Matthew nomogram cannot be used in cases of chronic toxicity to determine the risk of hepatic failure. However, several risk factors have been shown to place patients at higher risk for chronic acetaminophen toxicity from oral ingestion. These factors include chronic alcoholism; chronic ingestion of isoniazid; ingestion of cytochrome P450 (CYP) 2E1 enzyme inducers, such as barbiturates or phenytoin; fever; malnutrition; acquired immunodeficiency syndrome; anorexia or prolonged fasting; and acetaminophen taken in repeated supratherapeutic doses of greater than 4 g or 100 mg/kg.10 Of these risk factors, our patient might have been affected by malnutrition and anorexia.
The Naranjo algorithm,11 an adverse drug reaction (ADR) causality assessment questionnaire, was used to determine the likelihood that the IV acetaminophen caused the increase in hepatic enzymes. The Naranjo score obtained for our patient was 5 out of a maximum of 12, suggesting that the reaction could have been associated with IV acetaminophen.
Although the manufacturer has reported that peak blood concentration (Cmax) is reached 30 minutes faster with IV acetaminophen than with oral absorption, the timing of the onset of hepatotoxicity with IV acetaminophen is not well known. Acetaminophen is metabolized by phase I and phase II in the liver. Approximately 10% of acetaminophen is metabolized by the CYP2E1 enzyme by phase I oxidation. After acetaminophen is oxidized, it is converted to a reactive intermediate called N-acetyl-para-benzoquinone (NAPQI), which is then conjugated and detoxified by glutathione to nontoxic cysteine and mercapturate metabolites. When NAPQI is not safely metabolized, it covalently binds and arylates proteins in the cells and ultimately causes cell necrosis and death. Approximately 85% of the therapeutic dose of acetaminophen undergoes phase II conjugation to sulfation and glucuronidation metabolites, which are eliminated renally.10,12 In theory, less IV acetaminophen is metabolized to NAPQI compared with that taken orally, so there is less chance of hepatotoxicity at a given acetaminophen concentration.
The administration of repeated doses of IV acetaminophen (16 doses over five days) could also have played a role in the hepatotoxicity in our patient. In the clinical studies noted in the Ofirmev prescribing information,3 17% of the 1,020 patients received more than 10 doses, indicating that in clinical trials, the majority of patients received fewer doses than our patient. Our patient’s hepatotoxicity seemed to occur after repeated therapeutic doses over several days and therefore did not represent acute toxicity. Though rare, previous case reports have shown that hepatotoxicity is possible with ingestion of acetaminophen at therapeutic doses for more than four days, despite the fact that the patients did not have risk factors for toxicity.13
Malnutrition may also have contributed to our patient’s acetaminophen toxicity. Malnutrition has been theorized to increase the risk of acetaminophen toxicity because CYP2E1 activity and glutathione stores are decreased in malnourished patients.8 However, the degree of malnutrition’s effect on the risk of acetaminophen toxicity is unknown. Our patient had a history of decreased oral intake prior to admission (because of her nausea) and continued to receive nothing by mouth throughout the course of the hospital stay, increasing her likelihood of malnutrition. Although albumin may not be a reliable indicator of malnutrition, it is noteworthy that the patient’s serum albumin levels decreased throughout her hospital stay, from 4.2 g/dL on admission to 2.6 g/dL on day 6.
Drug interactions are a distinct possibility in the setting of acetaminophen toxicity because drugs that are metabolized by CYP2E1, either by induction or regulation, could alter the levels of acetaminophen. Such drug interactions could lead to the possibility of an increase in NAPQI and more hepatocellular damage.12 Additionally, hepatotoxic medications may also cause additive or synergistic effects that would increase the risk of hepatotoxicity. There were no major CYP2E1 interactions on the list of medications that the patient received at our institution. However, the patient did receive medications that could possibly increase levels of LFTs, such as cefazolin, ciprofloxacin, enoxaparin, hydromorphone, ketorolac, morphine, ondansetron, and pipercillin/tazobactam.
Overall, it appears that IV acetaminophen may have played a role in the hepatotoxicity experienced by our patient, despite her receiving therapeutic doses. The patient’s alleged minimal ethanol use, lack of interacting medication use, and baseline laboratory values all indicate that the patient did not have a high risk of developing acetaminophen toxicity. The Naranjo score was 5, meaning the IV acetaminophen was only a possible cause of hepatotoxicity. However, the time course of the patient receiving IV acetaminophen and the LFT increases show that the patient did not have this condition on arrival. Additionally, other possible diagnoses were ruled out, as shown by the tests performed and her laboratory values. The patient had been unable to eat prior to admission (because of her nausea), was kept NPO during much of her stay, and might have been malnourished, which could have been a risk factor for acetaminophen toxicity. Finally, she received high but therapeutic doses of acetaminophen for more than four days, which has been reported to cause hepatotoxicity in case reports.13
IMPLICATIONS FOR PRACTICE
The authors encourage practitioners to follow the Ofirmev prescribing information in terms of indications, maximum daily dose, and monitoring. Although the prescribing information does not recommend the routine monitoring of LFTs while patients are receiving Ofirmev, it may be prudent to obtain baseline and follow-up LFTs for patients at risk for toxicity. Additionally, restricting the number of doses or the duration a patient can receive IV acetaminophen may decrease the risk of toxicity and decrease costs.
CONCLUSION
To our knowledge, this is the first case report of a patient who developed elevated liver enzymes and acetaminophen levels while receiving therapeutic doses of IV acetaminophen (16 doses over five days). Although our patient received the manufacturer’s recommended dosing of IV acetaminophen, she accumulated an acetaminophen level of 34 mcg/mL and developed elevated liver enzymes. The reasons for this are unclear.
When our patient developed elevated LFT results in the setting of a positive acetaminophen level, she was treated with IV N-acetylcysteine. This case also supports common recommendations to treat suspected acetaminophen toxicity with N-acetylcysteine, even in patients with mildly elevated acetaminophen levels.
REFERENCES
- 1.McNeil Pharmaceuticals. Our story. About Tylenol. Jul 1, 2014. Available at: http://www.tylenol.com/news/about-us. Accessed August 20, 2014.
- 2.U.S. Food and Drug Administration. Drug details. Ofirmev. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed January 14, 2015.
- 3.Ofirmev package insert. San Diego, California: Cadence Pharmaceuticals, Inc; 2013. [Google Scholar]
- 4.New drug: ofirmev (injectable acetaminophen) Pharmacist’s Letter/Prescriber’s Letter. 2011;27(2):270212. [Google Scholar]
- 5.Jahr JS, Lee VK. Intravenous acetaminophen. Anesthesiol Clin. 2010;28(4):619–645. doi: 10.1016/j.anclin.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med. 2000;109(2):109–113. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 7.Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499–516. doi: 10.1016/j.ccc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Dart RC, Erdman AR, Olson KR, et al. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol. 2006;44(1):1–18. doi: 10.1080/15563650500394571. [DOI] [PubMed] [Google Scholar]
- 9.Acetadote package insert. Nashville, Tennessee: Cumberland Pharmaceuticals Inc; 2013. [Google Scholar]
- 10.Ferner RE, Dear JW, Bateman DN. Management of paracetamol poisoning. BMJ. 2011;342:d2218. doi: 10.1136/bmj.d2218. [DOI] [PubMed] [Google Scholar]
- 11.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 12.Nelson L, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. New York: McGraw-Hill Medical; 2011. Acetaminophen. [Google Scholar]
- 13.Bolesta S, Haber SL. Hepatotoxicity associated with chronic acetaminophen administration in patients without risk factors. Ann Pharmacother. 2002;36(2):331–333. doi: 10.1345/aph.1A035. [DOI] [PubMed] [Google Scholar]