Abstract
The properties of purified mammalian adenosine 3′:5′-cyclic monophosphate (cAMP)- and guanosine 3′:5′-cyclic monophosphate (cGMP)-dependent protein kinases were compared. Several physical characteristics of the two enzymes were similar, including size, shape, affinity for cyclic nucleotide binding, and Km for ATP. In addition, the amino acid composition of the two proteins indicated a close composition homology (70-90%). Both cyclic nucleotide-dependent protein kinases catalyzed phosphorylation of rat liver pyruvate kinase (EC 2.7.1.40) and fructose 1,6-diphosphatase (EC 3.1.3.11), rabbit skeletal muscle glycogen synthase (EC 2.4.1.11) and phosphorylase b kinase (EC 2.7.1.38), and calf thymus histone H2b. The phosphorylation of several synthetic peptides and of trypsin-sensitive and trypsin-insensitive sites in glycogen synthase suggested similar recognition sites on the protein substrates for the two kinases. The cAMP-dependent protein kinase was the better catalyst with each protein or peptides substrate. The results suggest that the two enzymes evolved from a common ancestral protein.
Keywords: protein phosphorylation, amino acid composition, substrate specificity, protein evolution
Full text
PDF
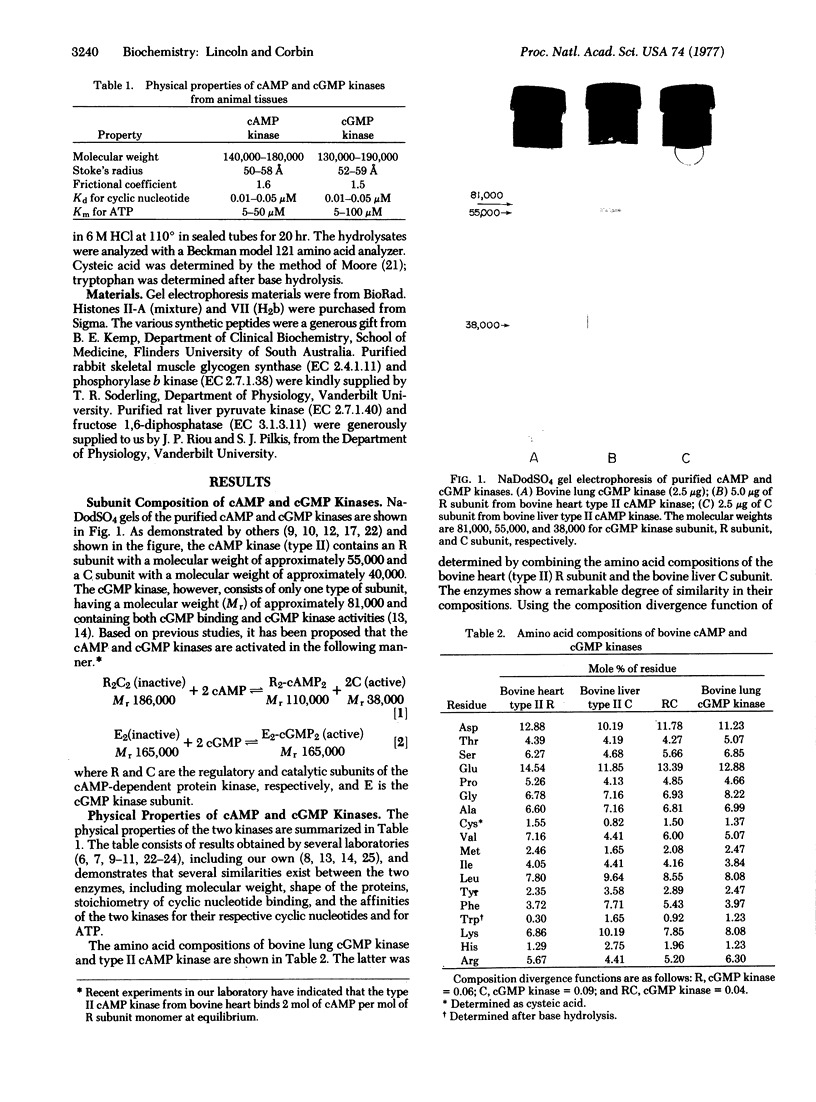
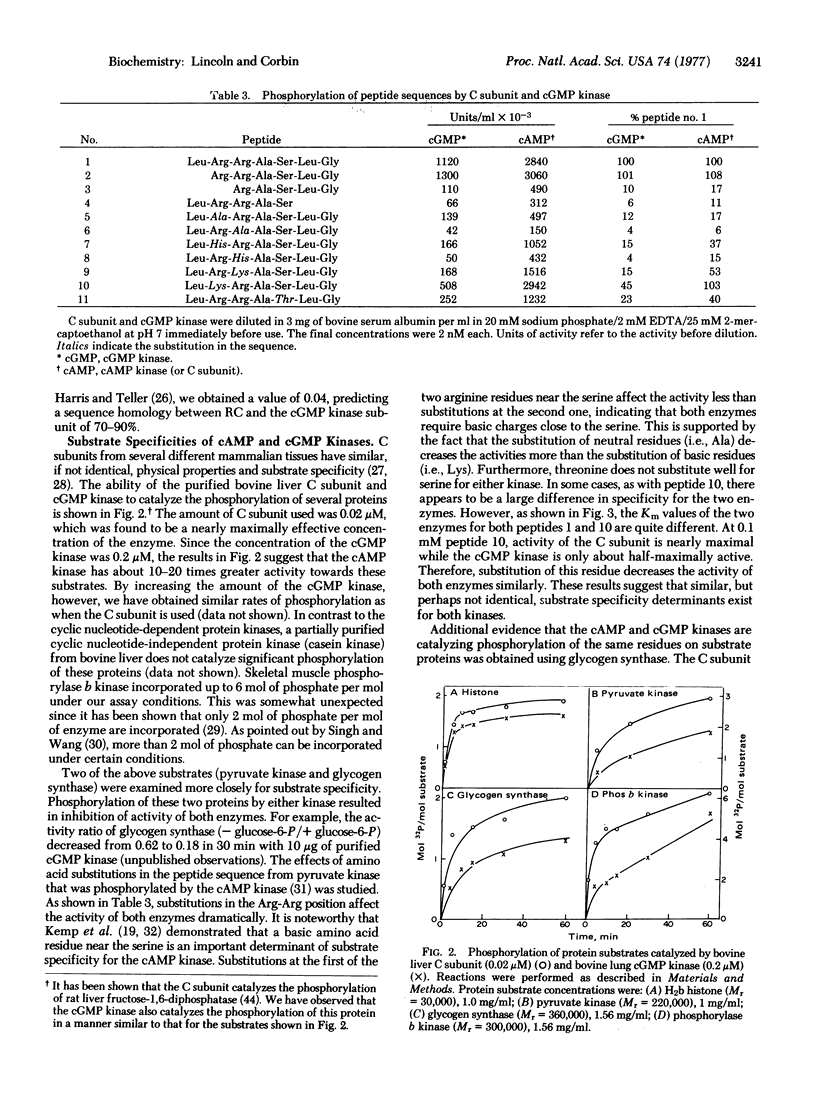


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera C. R., Namihira G., Hamilton L., Munk P., Eley M. H., Linn T. C., Reed L. J. -Keto acid dehydrogenase complexes. XVI. Studies on the subunit structure of the pyruvate dehydrogenase complexes from bovine kidney and heart. Arch Biochem Biophys. 1972 Feb;148(2):343–358. doi: 10.1016/0003-9861(72)90152-x. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Interaction of the subunits of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of muscle. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2444–2447. doi: 10.1073/pnas.68.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Davis P. F., Pettit F. H., Reed L. J. Peptides derived from pyruvate dehydrogenase as substrates for pyruvate dehydrogenase kinase and phosphatase. Biochem Biophys Res Commun. 1977 Apr 11;75(3):541–549. doi: 10.1016/0006-291x(77)91506-6. [DOI] [PubMed] [Google Scholar]
- Dills W. L., Jr, Beavo J. A., Bechtel P. J., Krebs E. G. Purification of rabbit skeletal muscle protein kinase regulatory subunit using cyclic adenosine-3':5'-monophosphate affinity chromatography. Biochem Biophys Res Commun. 1975 Jan 6;62(1):70–77. doi: 10.1016/s0006-291x(75)80406-2. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Hirsch A. H., Rosen O. M. Interconversion of cyclic nucleotide-activated and cyclic nucleotide-independent forms of a protein kinase from beef heart. Proc Natl Acad Sci U S A. 1971 Apr;68(4):731–735. doi: 10.1073/pnas.68.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. Role of the receptor in the mechanism of action of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):786–790. doi: 10.1073/pnas.68.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Holdy K. E., Walton G. M., Kanstein C. B. Purification and characterization of 3':5'-cyclic GMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3918–3922. doi: 10.1073/pnas.73.11.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Kanstein C. B. Guanosine 3',5'-monophosphate receptor protein: separation from adenosine 3',5'-monophosphate receptor protein. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1113–1122. doi: 10.1016/0006-291x(75)90684-1. [DOI] [PubMed] [Google Scholar]
- Harris C. E., Teller D. C. Estimation of primary sequence homology from amino acid composition of evolutionary related proteins. J Theor Biol. 1973 Feb;38(2):347–362. doi: 10.1016/0022-5193(73)90179-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Takeda M., Nishizuka Y., Hamana K., Iwai K. Studies on the sites in histones phosphorylated by adenosine 3':5'-monophosphate-dependent and guanosine 3':5'-monophosphate-dependent protein kinases. J Biol Chem. 1976 Oct 25;251(20):6287–6293. [PubMed] [Google Scholar]
- Hjelmquist G., Andersson J., Edlund B., Engstroöm L. Amino acid sequence of a (32P) phosphopeptide from pig liver pyruvate kinase phosphorylated by cyclic 3',5'-AMP-stimulated protein kinase and gamma-(32P)ATP. Biochem Biophys Res Commun. 1974 Nov 27;61(2):559–563. doi: 10.1016/0006-291x(74)90993-0. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L., Glinsmann W. H., Robinson J. C. Regulation of glycogen synthetase activity by two kinases. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1163–1169. doi: 10.1016/s0006-291x(75)80351-2. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Guanosine 3':5'-monophosphate-dependent protein kinase from silkworm, properties of a catalytic fragment obtained by limited proteolysis. J Biol Chem. 1976 Jul 25;251(14):4476–4478. [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. VI. Isolation and partial purification of a protein kinase activated by guanosine 3',5'-monophosphate. J Biol Chem. 1970 May 25;245(10):2493–2498. [PubMed] [Google Scholar]
- Kuo J. F., Kuo W. N., Shoji M., Davis C. W., Seery V. L., Donnelly T. E., Jr Purification and general properties of guanosine 3':5'-monophosphate-dependent protein kinase from guinea pig fetal lung. J Biol Chem. 1976 Mar 25;251(6):1759–1766. [PubMed] [Google Scholar]
- Kuo J. F., Wyatt G. R., Greengard P. Cyclic nucleotide-dependent protein kinases. IX. Partial purification and some properties of guanosine 3',5'-monophosphate-dependent and adenosine 3',5'-monophosphate-dependent protein kinases from various tissues and species of Arthropoda. J Biol Chem. 1971 Dec 10;246(23):7159–7167. [PubMed] [Google Scholar]
- Lincoln T. M., Dills W. L., Jr, Corbin J. D. Purification and subunit composition of guanosine 3':5'-monophosphate-dependent protein kinase from bovine lung. J Biol Chem. 1977 Jun 25;252(12):4269–4275. [PubMed] [Google Scholar]
- Lincoln T. M., Hall C. L., Park C. R., Corbin J. D. Guanosine 3':5'-cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2559–2563. doi: 10.1073/pnas.73.8.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Glycogen synthetase kinase 2 (GSK 2); the identification of a new protein kinase in skeletal muscle. FEBS Lett. 1974 Oct 1;47(1):162–166. doi: 10.1016/0014-5793(74)80450-3. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Katakami H., Yamamura H., Takai Y., Shimomura R. Functional specificity of guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases from silkworm. J Biol Chem. 1975 Feb 25;250(4):1297–1300. [PubMed] [Google Scholar]
- Schlender K. K., Reimann E. M. Isolation of a glycogen synthase I kinase that is independent of adenosine 3':5'-monophosphate. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2197–2201. doi: 10.1073/pnas.72.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T. J., Wang J. H. Effect of Mg2+ concentration on the cAMP-dependent protein kinase-catalyzed activation of rabbit skeletal muscle phosphorylase kinase. J Biol Chem. 1977 Jan 25;252(2):625–632. [PubMed] [Google Scholar]
- Soderling T. R. Regulation of glycogen synthetase. Effects of trypsin on the structure, activity, and phosphorylation of the skeletal muscle enzyme. J Biol Chem. 1976 Jul 25;251(14):4359–4364. [PubMed] [Google Scholar]
- Sugden P. H., Corbin J. D. Adenosine 3':5'-cyclic monophosphate-binding proteins in bovine and rat tissues. Biochem J. 1976 Nov;159(2):423–437. doi: 10.1042/bj1590423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Nakaya S., Inoue M., Kishimoto A., Nishiyama K., Yamamura H., Nishizuka Y. Comparison of mode of activation of guanosine 3':5'-monophosphate-dependent and adenosine 3':5'-monophosphate-dependent protein kinases from silkworm. J Biol Chem. 1976 Mar 10;251(5):1481–1487. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson M. J., Ahmed K. Localization of protein phosphokinase activities in the nucleolus distinct from extra-nucleolar regions in rat ventral prostate nuclei. Exp Cell Res. 1975 Jul;93(2):261–266. doi: 10.1016/0014-4827(75)90449-8. [DOI] [PubMed] [Google Scholar]
- Yamamura H., Nishiyama K., Shimomura R., Nishizuka Y. Comparison of catalytic units of muscle and liver adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1973 Feb 27;12(5):856–862. doi: 10.1021/bi00729a012. [DOI] [PubMed] [Google Scholar]



