Abstract
Microstructural white matter tract correlations have been shown to reflect known patterns of phylogenetic development and functional specialization in healthy subjects. The aim of this study was to establish intertract correlations in a group of controls and to examine potential deviations from normality in temporal lobe epilepsy (TLE). We investigated intertract correlations in 28 healthy controls, 21 left TLE (LTLE) and 23 right TLE (RTLE). Nine tracts were investigated, comprising the parahippocampal fasciculi, the uncinate fasciculi, the arcuate fasciculi, the frontoparietal tracts, and the fornix. An abnormal increase in tract correlations was observed in LTLE, while RTLE showed intertract correlations similar to controls. In the control group, tract correlations increased with increasing fractional anisotropy (FA), while in the TLE groups tract correlations increased with decreasing FA. Cluster analyses revealed agglomeration of bilateral pairs of homologous tracts in healthy subjects, with such pairs separated in our LTLE and RTLE groups. Discriminant analyses aimed at distinguishing LTLE from RTLE, revealing that tract correlations produce higher rates of accurate group classification than FA values. Our results confirm and extend previous work by showing that LTLE compared to RTLE patients display not only more extensive losses in microstructural orientation but also more aberrant intertract correlations. Aberrant correlations may be related to pathologic processes (i.e., seizure spread) or to adaptive processes aimed at preserving key cognitive functions. Our data suggest that tract correlations may have predictive value in distinguishing LTLE from RTLE, potentially moving diffusion imaging to a place of greater prominence in clinical practice. Hum Brain Mapp, 36:85–98, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: tracts, parahippocampal, cingulate, arcuate, uncinate, fornix, seizures, diffusion, anisotropy
INTRODUCTION
Epilepsy affects ∼70 million people worldwide [Ngugi et al., 2010] with its most common form consisting in seizures originating from the temporal lobe [Engel, 2001]. While the pathology in these patients is often localized and limited, functional and structural abnormalities outside the pathologic temporal lobe have been reported by our group and others [Bettus et al., 2009; Doucet et al., 2013; Gross et al., 2006; Kemmotsu et al., 2011; Rosenberger et al., 2009; Schoene‐Bake et al., 2009; Tracy et al., 2014a], contributing to the general impression that temporal lobe epilepsy (TLE) is a network disorder [Bernhardt et al., 2013].
Structural white matter (WM) networks in epilepsy have been investigated with diffusion tensor imaging (DTI). This method uses the anisotropic movement of water molecules in restricted compartments, such as axons, to investigate WM directionality. Virtual reconstruction of the underlying tracts can be obtained from diffusion data, offering the only possibility for in vivo assessments of specific WM fasciculi, along with their diffusion properties. One such property is fractional anisotropy (FA), which reflects the degree of monodirectionality of water diffusion and has been the principal choice of investigation in many studies. It should be noted that FA depends on several biologic factors, such as, axonal thickness, membrane thickness, myelination, edema, inflammation, Wallerian degeneration, and crossing fibers [Alexander et al., 2007; Jones et al., 2013; Winston, 2012]. Therefore, FA is a generic measure of orientation structure without specific or unique biologic significance.
DTI studies to date in epilepsy have generally found lower FA in TLE patients compared to healthy age‐matched controls. Tract‐specific decreases have been reported in the arcuate, uncinate, parahippocampal, inferior longitudinal, inferior fronto‐occipital, fornix, and the internal/external capsule tracts [Arfanakis et al., 2002; Concha et al., 2009; Diehl et al., 2008; Kim et al., 2011; Knake et al., 2009; Liacu et al., 2012; McDonald et al., 2008; Rodrigo et al., 2007]. Several studies comparing left TLE (LTLE) and right TLE (RTLE) patients also show that LTLE is associated with more extensive bilateral FA abnormalities, that is, in the cingulate, anterior thalamic radiations, and inferior frontal occipital fasciculi, as well as other tract, while RTLE is associated with more restricted abnormalities in the right ictal (pathologic) hemisphere [Ahmadi et al., 2009; Kemmotsu et al., 2011; Otte et al., 2012].
To date, all studies on TLE patients have considered WM abnormalities separately in each tract. Yet if epilepsy is a network disorder, each tract can be seen as existing in a context, and pathology as affecting not just the connection between brain regions, but the relationship among WM tracts themselves. Recent work in healthy subjects have shown that diffusion properties correlate between tracts, and these correlations reflect known phylogenetic development and interhemispheric (a)symmetries [Li et al., 2012; Mishra et al., 2013; Wahl et al., 2010]. Wahl et al. [2010] were the first to report these types of data based on tractography reconstruction of 12 tracts. The authors used hierarchical cluster analysis to merge tracts into groups based on their correlational similarity, reporting a dissociation between limbic (i.e., cingulate) and association tracts (i.e., arcuate, uncinate, inferior fronto‐occipital, and inferior longitudinal). They also observed that many tracts create symmetrical pairs based on the strong correlation with their contralateral homologue. More recently, Li et al. [2012] used voxel‐wise independent component analysis without tractography reconstruction and found similar bilateral correlations emerging from the data. These findings would seem to imply that structural pathways that implement similar or complementary cognitive functions will display similar changes in diffusion properties. Supporting this hypothesis, homologue tract correlations have been found to increase with brain maturation as one moves from birth to puberty [Mishra et al., 2013], suggesting that as cognitive or behavioral functionality gets solidified the association between tracts increases.
It is currently unknown whether the normative pattern of tract correlations observed in healthy controls is altered in TLE patients, and, if so, how these tract correlations relate to the known FA decreases in WM tracts of these patients. The primary aim of the current study was to establish the intertract correlations in a group of healthy controls, and then investigate deviations from normality in LTLE and RTLE patients. We considered LTLE and RTLE separately to identify the potentially distinct impact of ictal pathology on the dominant and nondominant hemispheres. Additionally, we examined the groups for difference in FA, and, based on the literature, we expect both TLE groups to differ from controls [Otte et al., 2012], and LTLE to have more extensive FA abnormalities [Ahmadi et al., 2009; Kemmotsu et al., 2011].
METHODS
Participants
A total of 44 epileptic patients were recruited from the Comprehensive Epilepsy Center at Thomas Jefferson University. Patients were excluded from the study for any of the following reasons: medical illness with central nervous system impact other than epilepsy; prior or current alcohol or illicit drug abuse; extratemporal epilepsy; bilateral mesial temporal sclerosis (MTS); any pathology outside the ictal temporal lobe; psychiatric diagnosis for any Axis I disorder listed in the Diagnostic and Statistical Manual of Mental Disorders – IV. Depressive Disorders were allowed in the patient sample, given the high comorbidity of depression and epilepsy [Tracy et al., 2007]. Patients with mental retardation (full‐scale IQ < 70) who were likely to have a different and unique course of brain development, and who were less able to cooperate with the MRI examination were also excluded.
All patients were considered good candidates for unilateral temporal lobectomy and were established to have unilateral temporal epileptogenic focus by board certified epileptologists based on at least 96 h of video‐EEG monitoring, MRI, PET, neuropsychological testing, and seizure semiology. When the data were not convergent between the various tests, intracranial electrodes were implanted to determine the seizure focus through electrocorticography. Full details of the algorithm for surgical decision making are described in Sperling et al. [1992]. After determining the epileptogenic hemisphere, the 44 TLE patients were split in two groups of 21 LTLE and 23 RTLE patients.
Another 28 healthy controls were recruited for the study, after passing a screening questionnaire to avoid individuals with a history of neuropathology, learning disorder, head trauma, drug abuse, alcohol abuse, seizures, or psychiatric disorders. This control group was matched to the TLE patients on age and handedness scores.
All subjects were left hemispheric dominant based on fMRI and/or handedness scores. The study was approved by the Thomas Jefferson University Institutional Review Board for Research with Human Subjects; consent was obtained from all patients and control subjects.
Imaging Protocol and Parameters
All scans were performed on a Philips Achieva 3T scanner (Amsterdam, the Netherlands) using an eight‐channel SENSE head coil. Data were acquired during a 6‐year period (2007–2013) and no single group was scanned on a schedule or time different than the other groups, thereby avoiding bias related to temporally dependent scanner calibration. A T1‐weighted anatomical MP‐RAGE volume was collected in sagittal orientation with in‐plane resolution of 256 × 256 and 1 mm slice thickness (1 mm3 voxels; TR = 650 ms; TE = 3.2 ms; FOV, 256 mm; and flip angle, 8°). The estimated total intracranial volume was obtained from this T1 image by running segmentation in Freesurfer v.5.3.0. Diffusion data were obtained with a single‐shot spin‐echo EPI pulse sequence, 66 axial slices, 2 mm thick, 0 mm gap, 256 mm FOV, 8,609 ms TR, 90 ms TE, 128 × 128 matrix, and 2 mm3 isotropic voxels. Thirty‐two volumes with noncollinear diffusion weighted orientations (850 s/mm2) were obtained, along with three nondiffusion (0 s/mm2) volumes. The sequence was repeated three times for each subject (total time = 15 min) and datasets were averaged during analysis to increase the signal‐to‐noise ratio.
Diffusion Imaging Analysis and Tractography Reconstruction
Images were imported into DTIstudio software [Jiang et al., 2006] and corrected for subject motion and eddy currents with a mutual information affine algorithm optimizing registration of each diffusion volume to the averaged nondiffusion volume. Following this correction, an inspection was performed on the data to check and exclude eventual slices with signal dropout [Li et al., 2013]. Tensor calculation was performed in DTIstudio using a standard linear regression. Normalization of the tensors in MNI space was performed in Diffeomap [Jiang et al., 2006] after obtaining a normalization matrix to register each subject's data on to the single‐subject template available with the software. The normalization matrix was the combination of an affine transformation matrix obtained during realignment of B0 images of the subject and the template, and the nonlinear transformation matrix obtained during alignment of FA maps [single‐channel LDDMM, threshold = 0.005, Cao et al., 2005]. Both normalization steps were performed on skull‐stripped volumes to improve accuracy and results were carefully inspected. The combined normalization matrix was applied to the tensor vectors, and whole brain tractography was performed on the normalized tensors using the FACT algorithm in DTIstudio [FA start = 0.21, FA stop = 0.19, and max tracking angle = 50°; Mori et al., 1999].
Specific tracts were selected from whole brain tractography using an atlas‐guided procedure. For each tract of interest, a set of predefined ROIs (template ROI set) was used to select fibers based on known start, end, and cross points. These 3D ROIs were created from anatomical parcellations of the Eve II template [for details on atlas‐guided tractography, see Zhang et al., 2010; template sets available at http://lbam.med.jhmi.edu/cmrm/Data_Yajing/longAssociation.htm]. For example, to select the arcuate fasciculus, the template set consisted of a 3D ROI of the frontal lobe, an additional “AND” ROI of the temporal lobe, and an “EXCLUSION” ROI of the parietal lobe and other subcortical structures. After isolating the tract with this method, manual inspection followed and streamlines that deviated from the known tract path were removed. FA values and the number of streamlines were obtained for each of the nine tracts. Note that tracts' FA is calculated as the average of each separate streamline, and, therefore, numerous streamlines within a voxel lead to a higher contribution of the voxel in the FA value of the tract.
A total of nine tracts were included in the study (four bilateral tracts and one mesial tract, Fig. 1). Specifically, these tracts were the uncinate fasciculi (uncL, uncR), the parahippocampal fasciculi (phipL, phipR), the arcuate fasciculi (arcL, arcR), the frontoparietal component of the superior longitudinal fasciculus (fpL, fpR), and the fornix (FX). These tracts were chosen for their known projections to the temporal lobe or for their involvement in language and memory, cognitive functions that are differently affected in LTLE and RTLE patients [Koylu et al., 2006; Tracy et al., 2014b; Trebuchon‐Da Fonseca et al., 2009].
Figure 1.
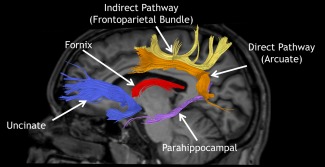
Example of tractography from one subject showing the tracts under investigation. The distinction between direct and indirect perisylvian language pathways is based on Catani et al. [2007] [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.].
Data Analysis
Group differences on relevant demographic and clinical variables were evaluated with chi‐square and analyses of variances, as appropriate. Within group FA values were tested for normality of distribution through the Shapiro–Wilk test.
Group differences in each of the nine tract's FA were investigated with separate ANCOVAs, after accounting for gender and intracranial volume. Results were thresholded at P = 0.0056 to correct for multiple comparisons with the Bonferroni method (original α = 0.05). Significant F‐tests were followed by planned comparisons aimed at comparing each TLE group with the healthy controls.
Pearson correlations were calculated between each tract and the other eight tracts for each group separately. An ANOVA on correlation values was run with experimental group (LTLE, RTLE, and Controls) and correlation type (intrahemispheric versus interhemispheric) as factors. Differences among groups were retested after recalculating tract correlations to account for gender, intracranial volume, epilepsy duration, and medial temporal sclerosis, through partial correlations.
Group differences in tract correlations were checked for reproducibility and robustness with a bootstrapping procedure. This step involved recalculation of tract correlations 10,000 times, each time with resampled FA values drawn randomly from the original sample and replaced before the next random pick. Each resample had the same size as the original sample. ANOVAs were run on these new 10,000 tract correlations and the ratio of significant findings was assessed with respect to the total number of samples.
The relationship between FA and tract correlations was investigated by splitting each group into two subgroups with high and low average FA. The median value of the average FA from all tracts was used as a threshold to split the groups in half. Subsequently, an ANOVA was run with the factors Group (LTLE, RTLE, and Controls) and “Average FA” (low, high).
Based on tract correlations, a bootstrapped hierarchical cluster analysis was performed and a dendrogram was created for each group separately. This procedure consists in an iterative agglomeration of the tracts based on their correlational similarity. A multiscale bootstrapped version of the clustering algorithm was used for this purpose (pvclust function in R), which yielded an approximately unbiased (AU) statistical probability of finding the cluster in the entire population based on the study sample [Suzuki and Shimodaira, 2006]. The same procedure was used by Wahl et al. [2010] to study tract correlations in a group of healthy controls. We used 100,000 bootstraps, average distance linkage, and Pearson correlations as distance method.
The potential utility of FA and tract correlations at distinguishing LTLE from RTLE patients was compared with discriminant analyses. The first discriminant function used a stepwise algorithm to automatically insert in the predicting algorithm the tracts that could distinguish LTLE from RTLE based on their FA value (insertion threshold P < 0.05, Wilk's lambda). The success rate of this analysis corresponds to the degree the subjects can be correctly assigned to their group based on FA. The second discriminant analysis used tract correlations as the only predictor of group pertinence. The success rate of this analysis corresponds to the degree at which tract correlations are correctly assigned to the group they belong to. Note, the predictive values of FA and the tract correlations could not be directly compared as the former involve single‐subject level variable, and the latter a group‐level variable.
RESULTS
Clinicodemographic Comparisons
Table 1 displays relevant clinic‐demographic data. The two TLE groups were compared on age, IQ, age at epilepsy onset, epilepsy duration, and proportion of patients with MTS. No significant differences were observed.
Table 1.
Clinical and demographic information for the three groups
| LTLE | RTLE | Controls | |
|---|---|---|---|
| N | 21 | 23 | 28 |
| Age | 42.5 ± 13.6 | 36.4 ± 14.5 | 39.3 ± 13.3 |
| Gender F/M | 15/7 | 10/13 | 21/7 |
| Intracranial volume (L) | 1.35 ± 0.23 | 1.48 ± 0.27 | 1.52 ± 0.18 |
| Edinburgh handedness | 87 ± 44 | 78 ± 57 | 76 ± 53 |
| W/o right arcuate | 13/8 | 19/4 | 25/3 |
| Full‐scale IQ | 91 ± 13.8 | 96 ± 10.4 | n/a |
| Age at epilepsy onset | 19 ± 16.1 | 22 ± 12.1 | n/a |
| Duration of epilepsy | 24 ± 19.1 | 15 ± 12.3 | n/a |
| Seizure types (no. of patients) | 13—CPS only | 12—CPS only | |
| 2—CPS + GTCS | 4—CPS + GTCS | ||
| 1—SPS only | 0—SPS only | ||
| 1—CPS w/2° GTCS | 3—CPS w/2° GTCS | ||
| 3—CPS + SPS | 3—CPS + SPS | ||
| 0—CPS w/2° GTCS + SPS | 1—CPS w/2° GTCS + SPS | ||
| MTS/non‐MTS (no. of patients) | 11/10 | 9/14 | n/a |
| Surgery | 14 (67%) | 14 (61%) | |
| 12—ATL | 14—ATL | ||
| 1—laser ablation | |||
| 1—gamma knife | |||
| Seizure‐free post‐surgery | 12 (86%) | 9 (64%) | |
| Range, 14–72 months | Range, 8–48 months | ||
| Post‐op | Post‐op | n/a | |
| Medication (no. of patients) | 4—Carbamazepine | 4—Carbamazepine | |
| 1—Depakote | 3—Depakote | n/a | |
| 2—Lacosamide | 5—Lacosamide | ||
| 7—Lamotrigine | 4—Lamotrigine | ||
| 10—Levetiracet. | 12—Levetiracet. | ||
| 1—Oxcarbazepine | 4—Oxcarbazepine | ||
| 2—Phenobarbital | 1—Phenobarbital | ||
| 3—Topiramate | 3—Topiramate | ||
| 2—Zonisamide | 3—Zonisamide | ||
| 5—Other | 6—Other |
Abbreviations: MTS, mesial temporal sclerosis; CPS, complex partial seizures; GTCS, generalized tonic‐clinic seizures; SPS, simple partial seizures; and w/2° GTCS, with secondary generalization.
A comparison of all three experimental groups showed no difference in age and handedness scores, but a different proportion of males/females between groups. More female subjects were present in the LTLE group compared with the control group (χ 2 = 8.51, P = 0.004), while RTLE did not change from controls. In addition, intracranial volume was found to be different between groups (F [2,69] = 3.44, P = 0.038), with LTLE patients having lower values compared to controls (95% CI = [−0.295, −0.037] liters, P = 0.012), while no difference existed between RTLE and controls (P > 0.1).
Twenty‐eight patients underwent surgery to control seizures (14 left). Of these, 11 LTLE and 9 RTLE patients are still seizure free to the present day [Engel class 1; Engel et al., 1993]. Histopathology of the resected tissue of 24 patients was assessed, of which 15 (63%) showed pathology in the hippocampus (reactive changes and/or neuronal loss), seven had inconclusive results due to the missing or damaged hippocampal specimen, and one had no pathology in the hippocampus. The neocortical histopathology showed reactive changes attributed to gliosis in 22 patients, cavernoma in 1 patient, and low grade glioma in 1 patient.
Tractography Results
All tracts, except the right arcuate, were successfully reconstructed in all participants. The right arcuate was not found in three healthy controls, four RTLE patients, and eight LTLE patients. In these patients, both the atlas‐based procedure and the manual placements of ROIs failed to extract the right arcuate. Further inspection showed the superior longitudinal fasciculus had no descending arcuate branch (see example in Supporting Information Figure 1). A chi‐square analysis showed that the proportion of subjects without the right arcuate was higher in LTLE compared to controls (χ 2 = 5.17, P = 0.023), and similar between RTLE and controls (χ 2 = 0.208, P > 0.1). Pearson correlations were run between the number of streamlines in the right arcuate and in the adjacent frontoparietal tract, both within the restricted group of 21 LTLE patients and within the entire sample of 72 subjects, in each case showing no statistical significance. Given the higher rates of “missing” right arcuate in LTLE patients, this group was further investigated with Pearson correlations between the number of streamlines in the right arcuate and clinical variables, such as, age at epilepsy onset and epilepsy duration. No significant correlation was found with age of onset (r = 0.04). An inverse correlation was found between epilepsy duration and the number of streamlines in the right arcuate (r = −0.587, P = 0.005). A t‐test showed that LTLE patients without the right arcuate had longer epilepsy duration (mean duration = 32.2 years) than those with a right arcuate (mean duration = 16.6 years; t[19] = 2.1, P = 0.05).
Tract‐Specific FA Abnormalities in TLE
Given the group differences in gender and intracranial volume, FA comparisons accounted for gender and intracranial volume as covariates. Table 2 displays FA values and ANCOVA comparisons for each tract, as well as on the average FA of all tracts. Compared to controls, the average FA of all tracts was lower both in LTLE and in RTLE patients, while LTLE and RTLE showed no difference with each other (P > 0.1). Individual tracts showed no difference between LTLE and RTLE (Table 2, right column), while each TLE group showed low FA compared to controls in specific tracts. In LTLE, low FA was found in uncR, fpR, phipR, and arcL. In RTLE, low FA was found in uncR, fpR, and phipR. Two other tracts, phipL and fpL, showed a trend toward significance in the main ANOVA results (0.006 < P < 0.007). Planned contrasts for these tracts showed abnormally low FA in LTLE, but not in RTLE patients. Figure 2 displays FA abnormalities in specific tracts as nodes with borders of different thicknesses and colors.
Table 2.
Fractional anisotropy values of the nine tracts, followed by statistical comparisons between groups after accounting for gender and intracranial volume
| Group | Main ANCOVA (F‐test) | LTLE vs. controls (95% CI) | RTLE vs. controls (95% CI) | LTLE vs. RTLE (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Controls (FA) | Left TLE (FA) | Right TLE (FA) | |||||
| Fornix | 0.37 ± 0.03 | 0.34 ± 0.04 | 0.35 ± 0.03 | F [2.67] = 3.1 P = 0.052 = 0.09 | n.s. | n.s. | n.s. |
| Parahip. left | 0.40 ± 0.03 | 0.36 ± 0.04 | 0.39 ± 0.02 | F [2.67] = 5.52a P = 0.006 = 0.14 | [−0.051, −0.012] P = 0.002 = 0.14 | n.s. | n.s. |
| Parahip. right | 0.38 ± 0.02 | 0.35 ± 0.03 | 0.36 ± 0.03 | F [2.67] = 7.39b P = 0.001 = 0.18 | [−0.043, −0.012] P = 0.001 = 0.16 | [−0.035, −0.007] P = 0.005 = 0.11 | n.s. |
| Arcuate left | 0.48 ± 0.02 | 0.44 ± 0.04 | 0.45 ± 0.03 | F [2.67] = 5.8b P = 0.005 = 0.15 | [−0.042, −0.009] P = 0.003 = 0.12 | n.s. | n.s. |
| Arcuate right | 0.45 ± 0.03 | 0.44 ± 0.03 | 0.43 ± 0.04 | F [2.67] = 1.6 P = 0.204 = 0.06 | n.s. | n.s. | n.s. |
| Frontopar. left | 0.43 ± 0.02 | 0.41 ± 0.03 | 0.42 ± 0.02 | F [2.67] = 5.4a P = 0.007 = 0.14 | [−0.034, −0.007] P = 0.003 = 0.12 | n.s. | n.s. |
| Frontopar. right | 0.45 ± 0.02 | 0.42 ± 0.03 | 0.42 ± 0.03 | F [2.67] = 7.6b P = 0.001 = 0.19 | [−0.040, −0.008] P = 0.003 = 0.12 | [−0.041, −0.012] P = 0.001 = 0.16 | n.s. |
| Uncinate left | 0.41 ± 0.02 | 0.39 ± 0.03 | 0.40 ± 0.03 | F [2.67] = 3.0 P = 0.058 = 0.08 | n.s. | n.s. | n.s. |
| Uncinate right | 0.42 ± 0.02 | 0.38 ± 0.04 | 0.39 ± 0.03 | F [2.67] = 11.2b P < 0.001 = 0.26 | [−0.047, −0.014] P < 0.001 = 0.17 | [−0.049, −0.019] P < 0.001 = 0.23 | n.s. |
| Average FA (all tracts) | 0.43 ± 0.02 | 0.40 ± 0.03 | 0.41 ± 0.02 | F [2.67] = 8.1b P = 0.001 = 0.19 | [−0.038, −0.010] P = 0.001 = 0.16 | [−0.034, −0.008] P = 0.001 = 0.14 | n.s. |
FA values for each tract (±standard deviation) and ANCOVA results for the “group” factor after accounting for gender and intracranial volume as covariates. Planned comparisons indicate 95% confidence interval, P‐value, and effect size ( = partial eta squared, n.s. = non significant at α = 0.0056).
ANCOVA shows statistical trend (0.006 < P < 0.007).
ANCOVA is significant at α < 0.0056.
Figure 2.
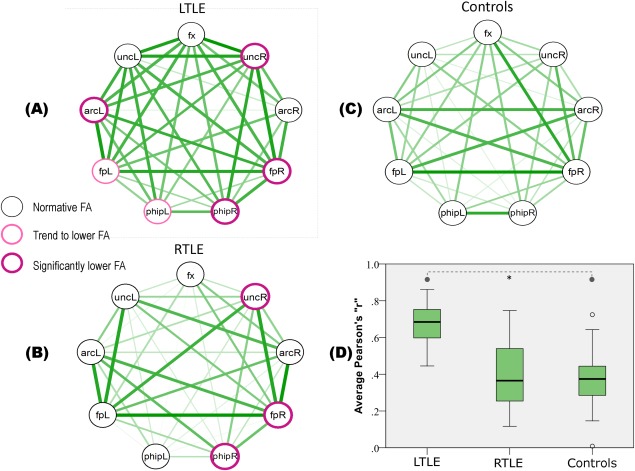
Nine tracts (nodes) and their correlations (edges) in LTLE (A), RTLE (B), and Controls (C). The border thickness and color of the nodes indicate departures from normality in the TLE groups. Edge color saturation and thickness reflect the correlation strength among tracts (Pearson r). (D) A plot of correlations in each group; the boxes span between 25th and 75th percentile, the thick black line within the box convey the median value, and the T‐bars extend 1.5 times the height of the box. Data points outside these ranges are depicted as circles. Tract abbreviations: fx, fornix; unc, uncinate; arc, arcuate; fp, frontoparietal; and phip, parahippocampal. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Tract Correlations
The Shapiro–Wilk tests on FA values showed no deviation from the normal distribution in any of the nine tracts in any group. Consequently, the Pearsons “r” was safely used as an appropriate measure of correlation. Figure 2 displays correlations among tracts as edges of variable thickness reflecting the strength of the correlation. In general, stronger correlations can be observed in the LTLE group. The Levene test showed variance inhomogeneity between group tract correlations, therefore, statistics were run on natural log transformed data. An ANOVA was run with experimental group (LTLE, RTLE, and Controls) and correlation type (intrahemispheric versus interhemispheric) as factors; correlations with arcR were excluded because this tract was absent at different rates in each group. The results revealed a significant difference between groups (F [2,57] = 21.8, P < 0.001), with tract correlations in LTLE being significantly higher than in RTLE (t[81] = 6.88, 95% CI = [+0.201, +0.364], P < 0.001) and higher than in controls (t[81] = 7.23, 95% CI = [+0.215, +0.379], P < 0.001), while no significant difference observed between RTLE and controls. The interaction between the group and inter/intrahemispheric factors was not significant, suggesting that the increased correlations observed in the LTLE patients occurred equivalently across the interhemispheric and intrahemispheric tracts. Partial tract correlations that accounted for gender and intracranial volume did not change the results; the LTLE group still showed significantly higher tract correlations compared to controls (t[81] = 3.54, 95% CI = [+0.115, +0.410], P < 0.001) and RTLE (t[81] = 4.5, 95% CI = [+0.187, +0.482], P < 0.001). A direct comparison of tract correlations in LTLE and RTLE after controlling for epilepsy duration and the presence of MTS showed higher tract correlations in LTLE compared to RTLE (t[54] = 6.1, 95% CI = [+0.307, +0.607], P < 0.001). Given the different histopathology found in two patients (one cavernoma, one low grade glioma; both in the LTLE group), we repeated the comparison of tract correlations with these two patients excluded. The results were the same, LTLE had significantly higher tract correlations than controls (t[81] = 7.78, 95% CI = [+0.218, +0.412], P < 0.001) and higher tract correlations than RTLE patients (t[81] = 7.42, 95% CI = [+0.104, +0.397], P < 0.001). A separate ANOVA on tract correlations for subjects with high and low average FA revealed an interaction Group × Average FA (F [2,162] = 6.55, P = 0.002). Figure 3 shows the opposing directions of change in tract correlation within groups, such that a change from low to high FA is associated with an increase in tract correlations in controls (independent t‐test[54] = 2.11, 95% CI = [+0.008, +0.328], P = 0.04), and with a decrease in tract correlations in LTLE (independent t‐test[54] = −2.23, 95% CI = [−0.220, −0.012], P = 0.03) and RTLE patients (independent t‐test[54] = −2.96, 95% CI = [−0.404, −0.078], P = 0.005).
Figure 3.
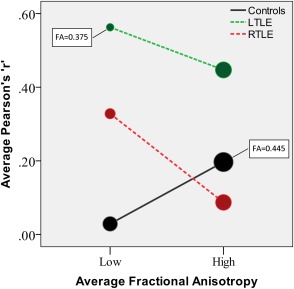
Tract correlations in subgroups with high and low average FA. The size of the circles is proportional to the average FA (minimum and maximum values are indicated in the plot). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Reproducibility of Group Differences in Tract Correlations
The bootstrapping of tract correlations (10,000 samples) produced 9,637 (96%) ANOVAs significant at α = 0.05 and 8,874 (89%) ANOVAs significant at α = 0.001. The frequency of different F values is shown with a histogram in Supporting Information Figure 2. Post hoc pairwise group comparisons (Tukeys's HSD) were performed for the significant ANOVAs (n = 9,637). Higher tract correlations in LTLE patients compared to healthy controls were found in 9,212 samples (96%; number obtained by counting 95% confidence intervals that did not include zero). Higher tract correlations in RTLE compared to controls were found in 1,589 samples (16%). The comparison of the two TLE groups revealed higher correlations in LTLE compared to RTLE in 8,886 samples (92%) and the inverse finding in 16 samples (0%). In general, these results closely resemble our original ANOVA findings, involving an increase in tract correlations in LTLE patients compared to the other two groups.
Tract Correlations in Seizure‐Free Patients Post‐Surgery
Despite the small number of patients who underwent surgery and were subsequently seizure free, we repeated the comparison of tract correlations within these groups (11 LTLE, 9 RTLE). A t‐test showed that, even in these smaller groups, tract correlations were significantly higher in LTLE compared to RTLE patients (t[41] = 5.4, P < 0.001; corrected for variance inhomogeneity).
Hierarchical Cluster Analysis
Figure 4 displays cluster dendrograms for the three groups. Note in the control group, three of the four bilateral tracts were associated with the homologous contralateral tract (94% probability for faL‐faR, 97% for phipL‐phipR, and 85% for arcL‐arcR). A disruption of the homologous pairs was observed in TLE patients. For example, the phipL‐phipR cluster was still present in LTLE patients (albeit with a lower probability of 72%) but not in RTLE patients, in whom the two tracts were part of completely different clusters. In the same manner, the cluster fpL‐fpR was still present in RTLE patients (82%), while fpL in LTLE was associated with the neighboring arcL (93%) before agglomerating with the contralateral homologous fpR (83%). The cluster arcL‐arcR was not preserved in either of the two TLE groups. In the LTLE group, arcR constituted an isolated cluster distant from all the other tracts, likely related to the higher number of missing arcuates, while in RTLE arcR was associated with uncL.
Figure 4.
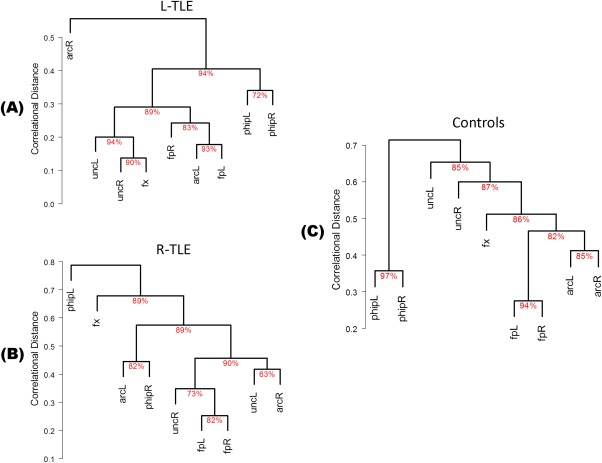
Dendrograms of bootstrapped hierarchical cluster analysis in LTLE (A), RTLE (B), and Controls (C). The y‐axis indicates the average linkage distance at which tracts merge into clusters. The approximately unbiased (AU) probability of the cluster formation is shown under each connection. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The y‐axis in Figure 4 shows the average distance linkage at which tracts merge into successive clusters. Note the abnormally low values in the y‐axis in the LTLE patients compared to the other two groups, reflecting the formal high tract correlations within the LTLE group. For example, 89% of the tracts in LTLE patients are already clustered at a linkage distance of 0.4, and the clustering process completed at 0.5. In the other two groups, only 45% of the tracts are clustered at a distance linkage of 0.4, and the clustering process is completed at values greater than 0.7.
Discriminant Analysis
To keep the full sample size in each group, the right arcuate was not used in discriminant analyses. Stepwise discriminant analyses using FA values as predictors introduced only one tract in the prediction algorithm, the left parahippocampal fasciculus (Wilk's lambda = 0.82, eigenvalue = 0.22, χ 2 = 8.3, P = 0.004). This model reached a 66% success rate in categorizing patients in the correct TLE group based on the FA value of phipL (leave‐one‐out validation), with the other seven tracts unable to significantly distinguish the groups. Specifically, 52% of LTLE patients and 78% of RTLE patients were categorized correctly based on FA of phipL.
The second discriminant analysis utilized the tract correlation values as predictor of group membership. This analysis reached a 80% success rate in categorizing correlations as pertaining to LTLE or RTLE (Wilk's lambda = 0.53, eigenvalue = 0.91, χ 2 = 34.6, P < 0.001). Specifically, 86% of LTLE correlations and 75% of RTLE correlations were correctly assigned to their group (leave‐one‐out validation).
DISCUSSION
The aim of this study was to investigate intertract correlations in a group of healthy controls and deviations from normality in TLE patients. Tract correlations have been shown to reflect known patterns of phylogenetic development and functional specialization [Li et al., 2012; Wahl et al., 2010]. As such, they have implication for understanding neurocognitive functioning and pathology progression in epilepsy. To our knowledge, this is the first study to test for potential patterns of abnormality in the tract correlations of epilepsy patients.
We found tract correlations in healthy controls to be analogous to those reported in the literature. In particular, we found that limbic tracts (i.e., the parahippocampal fasciculi) to dissociate from the other tracts, which were predominantly associative (Fig. 4, panel C). The same finding for the dorsal segment of this limbic bundle was reported by Wahl et al. [2010], in a group of 44 right‐handed normal controls. Thus, convergent evidence points to a distinction in correlational properties for limbic and associative tracts. However, we included another limbic tract in our study, the body of the fornix, which was not found to associate with the parahippocampal fasciculi. This tract failed to emerge from voxel‐wise correlations in the study of Li et al. [2012], who reported correlations for the more ventral fimbria, but not for the dorsal segment of the body of the fornix. Among the factors that may explain our findings and those of Li et al. [2012] are partial voluming and signal contamination from the CSF given the interventricular location, and the small size of the body of the fornix [Concha et al., 2005; Metzler‐Baddeley et al., 2012]. In addition, this tract contains WM fibers from both hemispheres, with a limited number possibly crossing to the contralateral hemisphere [Amaral et al., 1984; Demeter et al., 1985; Gloor et al., 1993], giving this tract potentially both limbic and commissural properties.
A second important finding in our healthy controls was the formation of bilateral pairs. Three of the four bilateral tracts were paired with their homologous counterpart (phipL‐phipR, fpL‐fpR, and arcL‐arcR). All three previous studies have reported a pairing effect [Li et al., 2012; Mishra et al., 2013; Wahl et al., 2010], though slightly different between studies. For example, in our study, we found direct pairing of the arcuate fasciculi, which were not paired in Wahl et al. [2010]. In contrast, the uncinate fasciculi were paired in Wahl et al.'s study but not in ours. These tracts were not investigated by Mishra et al. [2013] though pairing was the rule in all the tracts they investigated. Li et al. [2012] utilized a less constrained voxel‐based independent component analysis, which produced some components that appeared to contain the homologous elements of known tracts. All these previous studies and our current results suggest that correlation between homologous tracts may be an important feature of WM organization. The reason for strong contralateral pairing, that is, the strong association between the diffusion properties of homologous WM tracts, may be the consequence of several factors. First, homologous tracts likely connect homotopic areas of the cortex, and the functional correlation of homotopic areas is known to be very high [Damoiseaux et al., 2006]. Second, if both homologous tracts serve similar cognitive functions, or both are part of networks instantiated together to carry out a particular cognitive activity, the tracts would have the same “training” history, bearing similar experience‐dependent effects on their structure (i.e., plasticity). In this regard, recent literature has shown that FA changes can occur with even brief skill training (e.g., 2 h of juggling or playing videogames) [Hofstetter et al., 2013; Sagi et al., 2012; Scholz et al., 2009; Tang et al., 2010], or respond in coordination with relatively brief periods of active (FA increases) versus passive (FA decreases) cognitive activity [Mandl et al., 2008, 2013] Third, rates of FA increase during WM development are variable among different tract groups, but growth tends to be similar among homologous tracts, a process that would lead to an increase in homologous tract correlations [Mishra et al., 2013].
In moving the focus of our investigation to TLE patients, several WM abnormalities were noticed. Abnormally low FA was found in six tracts in LTLE patients, located in both hemispheres, and in three tracts in RTLE patients, located in the ipsilateral right pathologic hemisphere. Such findings are consistent with previous literature showing more extensive WM damage in LTLE than RTLE patients [Ahmadi et al., 2009; Kemmotsu et al., 2011]. While our results on FA abnormalities reiterate prior findings, we extend these findings by showing that FA abnormalities in LTLE patients are not just more extensive but are also associated with an increased coupling and association between WM tracts. The magnitude of tract correlations was significantly higher in LTLE patients than in controls, an effect that was not observed in RTLE. In fact, LTLE showed higher tract correlations also with respect to RTLE patients. This abnormal increase in tract correlations was not specific to intrahemispheric or interhemispheric pairs and was not explained by intracranial volume, gender, epilepsy duration, or the presence of MTS.
To examine the relationship between FA and tract correlations, we divided the groups using a median split on average FA values. Interestingly, healthy controls and TLE patients showed opposite directions of change from low to high FA. Healthy controls showed increased tract correlations in association with high FA, while the TLE groups decreased tract correlations with high FA, reflected in the significant interaction between these two factors. The effect observed in controls would fit the previous interpretation, whereby increased use (and activation) of cognitive networks that include the two WM homologues would potentially result in use‐dependent increases in FA [Sagi et al., 2012; Scholz et al., 2009] and, consequently, increased tract correlations. The exact reason for the reversal of this relationship between FA and tract correlations in TLE remains unknown. There may be some mechanism, either pathologic (e.g., seizure spread or generalization) or adaptive (flawed compensation and recruitment of tracts) that ties increased dependence between WM regions to diminished structural properties such as FA. In this case, the regular occurrence of seizure spread would cause multiple tracts to bear the burden of seizures to a similar degree, lowering FA, but also working to increase intertract correlations. Thus, our data suggest that the changes taking place in our TLE patients, regardless of whether these changes are pathologic or not, occur in a linked and dependent fashion. Interestingly, gray matter studies also suggest widespread network abnormalities in TLE patients, extending beyond the pathologic temporal lobe [Bernhardt et al., 2010; Bonilha et al., 2004; for a review, see Bernhardt et al., 2013]. One study in particular has shown increased interregional cortical thickness correlations in TLE patients compared to controls [Bernhardt et al., 2011], a finding that complements our data, supporting the idea that wide network changes occur in epilepsy, causing increased correlations both between cortical regions and between WM tracts.
As to why this process would be more evident in LTLE as opposed to RTLE is less clear. A possibility in explaining the increased microstructural correlations is related to neuroplastic adaptive responses. Evidence from task‐based and resting‐state fMRI show that LTLE patients exhibit large degrees of reorganization for language and cognitive tasks [Cousin et al., 2008; Rosenberger et al., 2009], and more extensive functional connectivity abnormalities involving language and memory areas, compared to RTLE patients [Doucet et al., 2013; Pereira et al., 2010]. In this context, as was noted earlier, a buildup of correlated structural WM properties may be seen as an adaptive (though flawed) neuroplastic response to the brain injury of seizures, potentially fostering recruitment of new gray matter regions into the burdened cognitive network to compensate and maintain functionality. The prominence of this process in LTLE, n.b., the language dominant hemisphere in our patients, suggests that there may be an increased drive for generating compensatory responses in WM tracts when language and memory are threatened. Future studies may be in a better position to investigate these hypotheses, perhaps by including variables reflecting the amount of seizure propagation or cognitive performance scores.
Besides the overall abnormality in the strength of tract correlations in LTLE, our tract clustering data showed departures from normal also in the pattern of tract associations, both in LTLE and RTLE groups (Fig. 4). Among the three homologous pairs formed in the control group (arcL‐arcR, fpL‐fpR, and phipL‐phipR), the two arcuates were the most vulnerable to change in TLE, with both the left and right patients losing this association. However, a direct link between the arcuates is not always found in healthy controls [Wahl et al., 2010] suggesting that this link may be sensitive to small changes in methods (Wahl et al. used Spearman values for clustering, we used Pearson values) or the presence of pathology in one of the hemispheres (our TLE groups).
The left and right parahippocampal fasciculi both showed low FA in LTLE patients, but their link was preserved (Figs. 2 and 4). A different pattern was observed in RTLE patients, where only the ipsilateral phipR was affected by low FA, and the link with the contralateral phipL was disrupted. These findings suggest that pathologic processes occurring in the limbic tracts in the setting of dominant hemispheric pathology may consistently affect bilateral structural orientation within the tracts but allow the normative link between them to be maintained. In contrast, pathologic processes occurring in the nondominant hemisphere disrupt structural orientation only in ipsilateral limbic tracts without preserving the link with the contralateral healthy tract. With regard to the previous literature, our study supports the notion that the damage to limbic tracts extends bilaterally when pathology affects the dominant hemisphere [Ahmadi et al., 2009], while the relationship between bilateral damage and the presence of MTS, as suggested by Liacu et al. [2012], is not supported given that both our LTLE and RTLE groups had comparable levels of MTS.
In contrast with the parahippocampal fasciculi, the link between homologous frontoparietal tracts was disrupted in LTLE patients but was preserved in RTLE patients. Yet the FA of these tracts showed the same abnormalities as the parahippocampal fasciculi, a bilateral decrease in LTLE and only an ipsilateral decrease in RTLE. Thus, the inconsistent disruption of homologous pairs for the parhippocampal and frontoparietal tracts is not readily explained. As noted, one possibility is that the dominant hemisphere, with regard to temporal lobe functions, harbors stronger forces for adaptive reorganization given the importance of maintaining receptive/expressive language. Thus, keeping a closer link between the limbic homologues may still be possible in the setting of dominant pathology, while perisylvian language pathways (Catani et al., 2005) may require stronger forces of reorganization, or damage to other brain areas, as catalyst for reorganization. As a result, perisylvian tracts may be more inclined to split from the homologous partner in LTLE patients.
Among the tracts that we investigated, the right arcuate fasciculus was missing in a number of subjects, with LTLE patients showing higher rates of missing arcuate (38%) than RTLE patients (17%) or controls (11%). While several studies have reported an absent arcuate in healthy control samples, the proportion of these subjects varies widely, ranging from 15% to 53% (a list of these studies is presented in Supporting Information Table 1). A careful investigation of this issue by Yeatman et al. [2011] showed that the use of more advanced probabilistic tractography methods does reveal the missing tract in cases where deterministic tractography fails, clearly attributing the failure to methodologic limitations. In particular, Yeatman et al. found that a specific segment of the arcuate, where it bends toward the temporal lobe, contains an abnormal mesiolateral diffusion orientation in subjects with a missing arcuate, possibly caused by prominent frontoparietal fibers crossing and bending the principal diffusion component in this unusual direction [Yeatman et al., 2011]. Following their finding, we checked whether subjects with missing arcR have a more prominent fpR bundle and found no relationship between the number of streamlines of the two tracts. Therefore, the interpretation advanced by Yeatman et al. [2011] could not be confirmed. However, other tracts in this area have a mesiolateral orientation (i.e., the tapetum, the medial longitudinal fasciculus) which could affect tractography. While we concur with Yeatman et al. [2011], who point out the methodologic artifact in reports of the missing arcuate, among our LTLE patients we did observe an interesting correlation between the number of streamlines in this tract and epilepsy duration, with fewer streamlines associated with longer duration. This does raise questions about the relationship between disease progression and structural orientation changes in the contralateral hemisphere of LTLE patients.
Previous studies have shown that FA values can be used to distinguish between LTLE and RTLE patients, with classification rates as high as 90% reported [i.e., Ahmadi et al., 2009]. There is great clinical value in developing analytic methods that would justify incorporating DTI data more fully into the surgical algorithms utilized for identifying epilepsy surgery candidates. Toward this end, we examined the relative power of our FA and tract correlation data to distinguish between RTLE and LTLE. Discriminant analyses revealed that FA values are less successful than tract correlations in this regard. Tract correlations were correctly classified 80% of the time in terms of LTLE or RTLE designations, while FA values correctly classified only 66% of cases, with nearly half of the LTLE patients (48%) miscategorized as RTLE. Although the method used to obtain tract correlations relies on group‐level information, and, therefore, is not directly applicable to clinical decision making, when the relationship between FA values and tract correlations is better understood, this information could potentially be incorporated into presurgical algorithms, particularly for cases where the localization of the seizure onset zone is equivocal by other methods.
As a caveat, we must note several limitations to our study. The sample size of our TLE groups may limit the reproducibility of our tract clustering findings. Li et al. [2012] made an interesting comparison of results obtained from a smaller (N = 44) and bigger (N = 53) number of subjects and found that the measure of reproducibility they used had decreased when using fewer subjects. Another issue is the use of a single diffusion measure (i.e., FA) to investigate tract correlations. Wahl et al. [2010] and Mishra et al. [2013] used also axial, radial, and mean diffusivities. However, as noted by Wahl et al. [2010], these measures showed significant departures from the normality distribution (forcing the authors to use nonparametric tests), while, in contrast, the “FA correlational matrix appeared to reflect known functional similarities and differences between tracts better than the other three DTI metrics” [Wahl et al., 2010]. In keeping with these conclusions, the follow‐up study by the same group used only FA to investigate voxel‐wise correlations [i.e., Li et al., 2012]. A third issue may arise from overlapping tracts, which may share the same voxels, potentially biasing the tracts toward similar FA values. Wahl et al. [2010] corrected overlapping voxels by adjusting their contribution to the average tract FA. In our study, we chose temporal lobe tracts that generally do not overlap, though the more superior arcuate and the frontoparietal tracts do overlap at the level of the superior longitudinal fasciculus. These adjacent tracts, however, did not show similar FA and did not pair with each other in the control group, but with their contralateral homologue, indicating that the potential influence of any shared voxels was not strong enough to cancel the similarity with the contralateral homologue. Fourth, we acknowledge that alternative methods of analyses, such as graph theory, could provide additional measurements of interest, though typical analyses with these methods rely on a higher number of tracts and regions [Bernhardt et al., 2011; Bonilha et al., 2013]. Last, it should be noted that the FACT algorithm we used is likely to reconstruct a higher number of streamlines in voxels with high FA, thus biasing the computation of average tract FA. This procedure, however, balances the more numerous voxels from the periphery of the tract with the larger number of streamlines in the tract core, where the streamlines converge to form known and certain bundles.
CONCLUSION
This study showed that in healthy controls the microstructural properties of WM, such as FA, can show some degree of heterogeneity across tracts, yet still display the expected strong association and pairing between tract homologues. We demonstrate that tract correlations are disrupted in TLE, with LTLE showing an increased magnitude of tract correlations, and both RTLE and LTLE patients showing disrupted correlations of homologous tracts compared to healthy, matched controls. These differences relative to controls cannot be accounted for by gender, brain volume, epilepsy duration, nor the presence of MTS. While higher tract correlations in controls were associated with higher FA, higher tract correlations were related to lower FA in both of our TLE groups, regardless of the side of pathology and seizures. Our results confirm and extend previous literature findings by showing that LTLE compared to RTLE patients display not only more regionally extensive losses in microstructural orientation but also more aberrant intertract correlations, reflecting an abnormal level of intertract dependence. Our data also show that both the FA values of specific WM tracts and the pattern of tract correlations may have predictive value in distinguishing between RTLE and LTLE, potentially moving DTI to a place of greater prominence in algorithms devoted to determining surgical candidacy in epilepsy. While we focused on the tracts most likely impacted by TLE, interdependence among other tracts is certainly worthy of investigation, with a particular eye on clarifying the inverse relationship with FA that we report here. We plan future studies investigating the degree to which the tract correlations observed in LTLE patients are related to pathologic processes often inherent to TLE (i.e., seizure spread) or to adaptive neuroplastic processes emergent from the dominant hemisphere, potentially aimed at preserving key cognitive functions.
Supporting information
Supplementary Information
Supplementary Information Table 1.
Supplementary Information Figure 1.
Supplementary Information Figure 2.
ACKNOWLEDGMENTS
This work was supported, in part, by the National Institute for Neurological Disorders and Stroke (NINDS) to Dr. Joseph I. Tracy. The remaining funding was provided by other sources of internal organizational support to Dr. Tracy. The authors report no conflicts of interest.
REFERENCES
- Ahmadi ME, Hagler DJ Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E (2009): Side matters: Diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol 30:1740–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM (1984): The commissural connections of the monkey hippocampal formation. J Comp Neurol 224:307–336. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME (2002): Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging 20:511–519. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A (2010): Cortical thickness analysis in temporal lobe epilepsy: Reproducibility and relation to outcome. Neurology 74:1776–1784. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N (2011): Graph‐theoretical analysis reveals disrupted small‐world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 21:2147–2157. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Hong S, Bernasconi A, Bernasconi N (2013): Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci 7:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort‐Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M (2009): Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, Li LM (2004): Voxel‐based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 61:1379–1384. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Helpern JA, Sainju R, Nesland T, Edwards JC, Glazier SS, Tabesh A (2013): Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology 81:1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Miller MI, Winslow RL, Younes L (2005): Large deformation diffeomorphic metric mapping of vector fields. IEEE Trans Med Imaging 24:1216–1230. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK (2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104:17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L, Gross DW, Beaulieu C (2005): Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol 26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- Concha L, Beaulieu C, Collins DL, Gross DW (2009): White‐matter diffusion abnormalities in temporal‐lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry 80:312–319. [DOI] [PubMed] [Google Scholar]
- Cousin E, Baciu M, Pichat C, Kahane P, Le Bas JF (2008): Functional MRI evidence for language plasticity in adult epileptic patients: Preliminary results. Neuropsychiatr Dis Treat 4:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter S, Rosene DL, Van Hoesen GW (1985): Interhemispheric pathways of the hippocampal formation, presubiculum, and entorhinal and posterior parahippocampal cortices in the rhesus monkey: The structure and organization of the hippocampal commissures. J Comp Neurol 233:30–47. [DOI] [PubMed] [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Luders HO (2008): Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia 49:1409–1418. [DOI] [PubMed] [Google Scholar]
- Doucet G, Osipowicz K, Sharan A, Sperling MR, Tracy JI (2013): Extratemporal functional connectivity impairments at rest are related to memory performance in mesial temporal epilepsy. Hum Brain Mapp 34:2202–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J Jr (2001): Mesial temporal lobe epilepsy: What have we learned? Neuroscientist 7:340–352. [DOI] [PubMed] [Google Scholar]
- Engel J Jr, Van Ness PC, Rasmussen TB, Ojemann LM (1993): Outcome with respect to epileptic seizures In: Engel J, Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; pp 609–621. [Google Scholar]
- Gloor P, Salanova V, Olivier A, Quesney LF (1993): The human dorsal hippocampal commissure: An anatomically identifiable and functional pathway. Brain 116:1249–1273. [DOI] [PubMed] [Google Scholar]
- Gross DW, Concha L, Beaulieu C (2006): Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 47:1360–1363. [DOI] [PubMed] [Google Scholar]
- Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y (2013): Short‐term learning induces white matter plasticity in the fornix. J Neurosci 33:12844–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006): DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106–116. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N, Girard HM, Bernhardt BC, Bonilha L, Lin JJ, Tecoma ES, Iragui VJ, Hagler DJ Jr, Halgren E, McDonald CR (2011): MRI analysis in temporal lobe epilepsy: Cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 52:2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chung CK, Koo BB, Lee JM, Kim JS, Lee SK (2011): Changes in language pathways in patients with temporal lobe epilepsy: Diffusion tensor imaging analysis of the uncinate and arcuate fasciculi. World Neurosurg 75:509–516. [DOI] [PubMed] [Google Scholar]
- Knake S, Salat DH, Halgren E, Halko MA, Greve DN, Grant PE (2009): Changes in white matter microstructure in patients with TLE and hippocampal sclerosis. Epileptic Disord 11:244–250. [DOI] [PubMed] [Google Scholar]
- Koylu B, Trinka E, Ischebeck A, Visani P, Trieb T, Kremser C, Bartha L, Schocke M, Benke T (2006): Neural correlates of verbal semantic memory in patients with temporal lobe epilepsy. Epilepsy Res 72:178–191. [DOI] [PubMed] [Google Scholar]
- Li Y, Shea SM, Lorenz CH, Jiang H, Chou MC, Mori S (2013): Image corruption detection in diffusion tensor imaging for post‐processing and real‐time monitoring. PLoS One 8:e49764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YO, Yang FG, Nguyen CT, Cooper SR, LaHue SC, Venugopal S, Mukherjee P (2012): Independent component analysis of DTI reveals multivariate microstructural correlations of white matter in the human brain. Hum Brain Mapp 33:1431–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacu D, Idy‐Peretti I, Ducreux D, Bouilleret V, de Marco G (2012): Diffusion tensor imaging tractography parameters of limbic system bundles in temporal lobe epilepsy patients. J Magn Reson Imaging 36:561–568. [DOI] [PubMed] [Google Scholar]
- Mandl RC, Schnack HG, Zwiers MP, van der Schaaf A, Kahn RS, Hulshoff Pol HE (2008): Functional diffusion tensor imaging: Measuring task‐related fractional anisotropy changes in the human brain along white matter tracts. PLoS One 3:e3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl RC, Schnack HG, Zwiers MP, Kahn RS, Hulshoff Pol HE (2013): Functional diffusion tensor imaging at 3 Tesla. Front Hum Neurosci 7:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E (2008): Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 71:1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler‐Baddeley C, O'Sullivan MJ, Bells S, Pasternak O, Jones DK (2012): How and how not to correct for CSF‐contamination in diffusion MRI. Neuroimage 59:1394–1403. [DOI] [PubMed] [Google Scholar]
- Mishra V, Cheng H, Gong G, He Y, Dong Q, Huang H (2013): Differences of inter‐tract correlations between neonates and children around puberty: A study based on microstructural measurements with DTI. Front Hum Neurosci 7:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC (1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269. [DOI] [PubMed] [Google Scholar]
- Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR (2010): Estimation of the burden of active and life‐time epilepsy: A meta‐analytic approach. Epilepsia 51:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte WM, van Eijsden P, Sander JW, Duncan JS, Dijkhuizen RM, Braun KP (2012): A meta‐analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia 53:659–667. [DOI] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F (2010): Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: Evidence from resting state fMRI. BMC Neurosci 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, Semah F, Mangin JF, Le Bihan D, Meder JF (2007): Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur Radiol 17:1663–1668. [DOI] [PubMed] [Google Scholar]
- Rosenberger LR, Zeck J, Berl MM, Moore EN, Ritzl EK, Shamim S, Weinstein SL, Conry JA, Pearl PL, Sato S, Vezina LG, Theodore WH, Gaillard WD (2009): Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology 72:1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur‐Moryosef S, Blumenfeld‐Katzir T, Assaf Y (2012): Learning in the fast lane: New insights into neuroplasticity. Neuron 73:1195–1203. [DOI] [PubMed] [Google Scholar]
- Schoene‐Bake JC, Faber J, Trautner P, Kaaden S, Tittgemeyer M, Elger CE, Weber B (2009): Widespread affections of large fiber tracts in postoperative temporal lobe epilepsy. Neuroimage 46:569–576. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen‐Berg H (2009): Training induces changes in white‐matter architecture. Nat Neurosci 12:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, O'Connor MJ, Saykin AJ, Phillips CA, Morrell MJ, Bridgman PA, French JA, Gonatas N (1992): A noninvasive protocol for anterior temporal lobectomy. Neurology 42:416–422. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Shimodaira H (2006): Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22:1540–1542. [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI (2010): Short‐term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA 107:15649–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JI, Lippincott C, Mahmood T, Waldron B, Kanauss K, Glosser D, Sperling MR (2007): Are depression and cognitive performance related in temporal lobe epilepsy? Epilepsia 48:2327–2335. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Osipowicz K, Spechler P, Sharan A, Skidmore C, Doucet G, Sperling MR (2014a): Functional connectivity evidence of cortico‐cortico inhibition in temporal lobe epilepsy. Hum Brain Mapp 35:353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JI, Pustina D, Doucet G, Osipowicz K (2014b): Seizure‐induced neuroplasticity and cognitive network reorganization in epilepsy In: Tracy JI, Hampstead B, Sathian K, editors. Plasticity of Cognition in Neurologic Disorders. New York: Oxford University Press. [Google Scholar]
- Trebuchon‐Da Fonseca A, Guedj E, Alario FX, Laguitton V, Mundler O, Chauvel P, Liegeois‐Chauvel C (2009): Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain 132(Pt 10):2772–2784. [DOI] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, Lahue SC, Cooper SR, Sherr EH, Mukherjee P (2010): Microstructural correlations of white matter tracts in the human brain. Neuroimage 51:531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston GP (2012): The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, Ben‐Shachar M (2011): Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci 23:3304–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, Akhter K, Rosa‐Neto P, Pike GB, Evans A, Toga AW, Woods R, Mazziotta JC, Miller MI, van Zijl PC, Mori S (2010): Atlas‐guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 52:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information Table 1.
Supplementary Information Figure 1.
Supplementary Information Figure 2.


