Abstract
Objective
Inflammation plays an important role in atherosclerosis. Elevated C-reactive protein (CRP) levels are associated with a greater risk of cardiovascular disease. Our goal was to study CRP metabolism, and to determine its relationship with lipoprotein metabolism using stable isotope methodology.
Material/Methods
Eight subjects with combined hyperlipidemia underwent a 15-h primed-constant infusion with deuterated leucine. CRP was purified from the plasma density fraction greater than 1.21g/ml by affinity chromatography. Lipoprotein fractions were separated by sequential ultracentrifugation. Isotope enrichment was determined by gas chromatography/mass spectrometry.
Results
The subjects had mean LDL-C levels of 147.5mg/dl and mean CRP levels of 3.4mg/ l. The mean CRP production rate (PR) was 0.050±0.012mg/kg/day and the mean CRP fractional catabolic rate (FCR) was 0.343±0.056 pools/day (residence time 2.92days). CRP pool size (PS) was significantly related to production (r=0.93; p<0.001), but not FCR. CRP PS was also related to body mass index (r=0.79; p=0.02). There was a significant association between CRP FCR and TRL apoB-100 FCR (r=0.74, p=0.04), as well as between CRP PS and TRL apoB-48 FCR (r=-0.90, p=0.002), indicating linkage between CRP and TRL metabolism.
Conclusion
The main determinant of plasma CRP levels was CRP production rate. Moreover a significant linkage between CRP metabolism and both TRL apoB-100 and apoB-48 catabolism was noted.
Keywords: C-reactive protein, Lipoprotein, Metabolism
1. Introduction
C-reactive protein (CRP) is a member of the pentraxin family of proteins. It contains five identical, non-convalently associated protomers arranged symmetrically around a central pore. CRP is a major acute phase reactant produced by hepatocytes. The concentration rises rapidly (4–6h), and markedly (as much as 1000 folds) after acute tissue injury or inflammation [1]. It is widely accepted that inflammation plays a clinically significant role in the development and progression of atherosclerosis [2]. Clinical studies also support a link between chronic inflammation and coronary heart disease [3,4]. As a marker of inflammation, CRP has been included in some cardiovascular risk stratification and treatment guidelines [5]. In a 2010 meta-analysis, high sensitivity CRP (hs-CRP) was found to be an independent predictor of cardiovascular disease [6]. CRP has also been linked to insulin resistance, obesity, and metabolic syndrome [7,8]. Weight loss has been shown to decrease hs-CRP levels in obese and diabetic patients [9,10]. In addition, weight loss improves cardiovascular risk factor and reduces triglyceride (TG) levels [11]. Studies show that weight loss reduces VLDL–TG secretion rate [12] and intrahepatic triglyceride (IHTG) content [13–15]. Moreover several lipid lowering drugs such as statin, fibrates, and ezetimibe decrease both CRP and lipid levels. Our goal was to investigate the potential interrelations between CRP metabolism, and the metabolism of lipoprotein apolipoproteins B-100, B-48, and A-I using stable isotope methodology [16]. The detail of CRP isolation method was also shown in this study.
2. Methods
2.1. Study Subjects and Design
Eight subjects with combined hyperlipidemia, five men and three postmenopausal women without hormonal replacement therapy, were recruited for the study. Plasma lipid criteria for enrollment were LDL-C levels ≥160mg/dl, TG levels ≥150mg/dl, and low high density lipoprotein cholesterol (HDL-C) levels (≤40mg/dl in men, and ≤50mg/dl in women). Exclusion criteria were as follows: age<40years, smoking, thyroid dysfunction, liver or kidney disease, diabetes mellitus, stroke, myocardial infarction in the past 6months, and current use of medications known to affect lipid metabolism. Subjects were instructed to follow the therapeutic lifestyle changes diet as recommended by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) throughout the study. After 11weeks on the therapeutic lifestyle diet, subjects had 12h fasting blood samples drawn for the measurement of plasma lipids and CRP concentrations. Plasma was separated at 1000g for 30min at 4°C and stored at −70°C until used. At this time subjects were admitted to the General Clinical Research Center of Tufts Medical Center and underwent a stable isotope study. Subjects were fed hourly for 20h with small identical meals starting 5h before and continuing throughout the infusion. A primed-constant infusion of 10μmol/kg body weight/h deuterated leucine ([5,5,5-2H3] L-leucine; C/D/N Isotopes, Pointe-Claire, Quebec, Canada) was carried out for 15h. Blood samples were collected in EDTA tubes just before the infusion (0h) and at 30, 35, 45min and 1, 1.5, 2, 3, 4, 6, 9, 12, 14, 15h during the infusion. The details of the study design and apolipoprotein metabolism results were published previously [17]. All study participants provided written informed consent, and the study protocol was approved by the Institutional Review Board of Tufts University-School of Medicine and Tufts Medical Center.
2.2. Lipid and Apolipoprotein measurements
Plasma total cholesterol (TC) and TG were measured by automated enzymatic assays standardized through the Centers for Disease Control [18]. Plasma LDL-C and HDL-C concentrations were measured directly with kits from Equal Diagnostics (Exton, PA) and Roche Diagnostics (Indianapolis, IN), respectively. Sequential density ultracentrifugation in a Beckman ultracentrifuge (Beckman, Palo Alto, CA) was used to separate each lipoprotein fraction from 5ml of plasma from each infusion time point as follows; triglyceride rich lipoproteins (TRL) d <1.006g/ml, intermediate density lipoprotein (IDL) d= 1.006–1.019g/ml, low density lipoprotein (LDL) d=1.019–1.063, and high density lipoprotein (HDL) d=1.063–1.21g/ml. Apolipoprotein B (apoB) concentrations in plasma, and in the TRL and IDL fractions were measured by ELISA using polyclonal antibodies from BioDesign (Saco, ME). LDL apoB concentrations were calculated by subtracting TRL and IDL apoB from total plasma apoB levels. ApoB-48 concentrations were assessed by running TRL fractions on SDS-PAGE gels, stained with 0.1% Coomassie blue R-250, and the relative proportion of apoB-48 was assessed by densitometric scanning. Plasma CRP concentrations were measured using a high-sensitivity immunoturbidimetric assay (Kamiya Biomedical Company, Seattle, WA). The averages of 8 infusion time points (1, 2, 3, 4, 6, 9, 12, and 15h) were used in the calculation.
2.3. CRP isolation
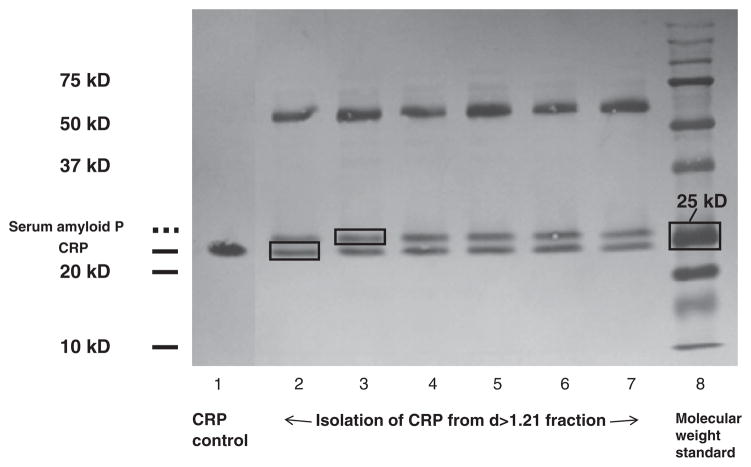
CRP was isolated from the d>1.21g/ml fraction of each time point by affinity chromatography and gel electrophoresis. Briefly, EDTA was removed from the samples by an overnight dialysis against 0.05M Tris-buffered saline, pH 8 at 25°C. The EDTA-free d>1.21g/ml protein fractions were then incubated with immobilized p-aminophenyl phosphoryl choline resin (Thermo Scientific, Rockford, IL) in a chromatography column for 1h at room temperature. The bound fraction, which contained CRP, was eluted with Tris buffer containing 2mM EDTA. The CRP monomer unit was separated by 12% monogradient SDS-PAGE gel electrophoresis for 17h at 50 V, transferred to polyvinylidene fluoride membranes, and visualized with 0.1% Coomassie blue R250. Purified human CRP (Meridian Life Science, Inc, ME) and a molecular weight standard (Biorad, Hercules, CA) were used to identify the isolated CRP monomer bands. CRP immunoblotting and protein identification by LC/MS/MS, using in-gel digestion and A Sequest search of the NCBI non-redundant protein database, confirmed the presence and purity of the isolated CRP proteins. Antibody to human CRP was obtained from Meridian Life Science, Inc, ME.
During the process of CRP monomer isolation on the SDS-PAGE gel; we found a protein band running just above the CRP band (Fig. 1). This protein has a size of approximately 25kDa according to the molecular weight control standards. We used the in-gel digestion LC/MS/MS method, and A Sequest search of the NCBI non-redundant protein database, to identify and confirm that this protein was serum amyloid P. This protein has a molecular weight similar to CRP and also binds to phosphoryl choline; therefore it elutes with CRP during chromatography column isolation. For this reason it is vital to further purify CRP using SDS-PAGE gel after the affinity chromatography column.
Fig. 1.
CRP isolation on 12% SDS page gel. The first lane is CRP control; Lanes 2–7 contain eluents from chromatography column; Lane 8 is molecular weight standard. CRP protein bands were located below serum amyloid P protein bands on the gels. The two proteins were both isolated from chromatography column and have similar sizes. SDS-Page gel electrophoresis is necessary in the process of isolating these two proteins after chromatography column.
2.4. Isotopic enrichment measurements and Kinetic analysis
Isolated CRP bands were excised from the membrane, hydrolyzed in 12N HCl at 110°C for 24h, and evaporated to dryness. Amino acids were converted to heptafluorobutyramide derivatives and analyzed by a gas chromatography/ mass spectrometry (Agilent Technologies 6890/5973N). Selected ion monitoring at m/z 349 (derivatized leucine – HF-) and m/z 352 (derivatized d3-leucine – HF-) was used to determine the areas under the chromatographic peaks of each ion. Mole percentage enrichment for each sample was calculated from the areas under the curve and converted to tracer–tracee ratio as previously described [19]. The Simulation Analysis and Modeling II (SAAM II) program was used for determination of CRP kinetic parameters. The model that was used for the analysis had input into a precursor pool and then input and catabolism from a single plasma pool.
2.5. Statistical analyses
Plasma lipid, apolipoproteins, and CRP concentrations were checked for their distributions. Data were expressed as mean±SD if they were normally distributed, and median (interquartile range) if they were non-normally distributed. CRP kinetic parameters of male and female subjects were compared using independent sample T test. The correlation between CRP kinetic parameters and apolipoprotein kinetic parameters was determined by Spearman’s Rho analyses. All analyses were performed using the SPSS statistical Package (SPSS, Chicago, IL).
3. Results
The eight subjects studied had a mean age of 55.4±8.4years. The mean body mass index (BMI) was 28.3±3.3kg/m2.
Table 1 shows non-fasting plasma lipids, apolipoproteins and CRP levels of the study participants. The mean LDL-C was 147.5mg/dl while the mean HDL-C was 34.9mg/dl. Median TG level was 254.5mg/dl and median CRP level was 2.7mg/l.
Table 1.
Non-fasting plasma lipid, Apolipoprotein B, and CRP concentrations of the study participants.
| Parameters | Results (n=8) |
|---|---|
| Total cholesterol (mg/dl) | 233.9±24.5 |
| Triglyceride (mg/dl) * | 254.5 (235.5, 362.4) |
| LDL-C (mg/dl) | 147.5±25.0 |
| HDL-C (mg/dl) | 34.9±5.93 |
| VLDL apoB (mg/dl) | 9.90±2.46 |
| TRL apoB-48 (mg/dl) | 0.65±0.27 |
| IDL apoB (mg/dl) | 3.30±1.00 |
| LDL apoB (mg/dl) | 96.6±12.2 |
| CRP (mg/l) * | 2.70(1.43, 5.73) |
Values are expressed as mean±SD or *median (interquartile range).
LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; VLDL, very low density lipoprotein; apoB, apolipoprotein B; TRL, triglyceride rich lipoprotein; IDL, intermediate density lipoprotein.
Individual CRP kinetic parameters of the study subjects are shown in Table 2.
Table 2.
Individual CRP kinetic parameters in the study subjects.
| Subject number | Non-fasting CRP concentration (mg/l) | CRP FCR (pool/day) | CRP PR
|
CRP PS (mg) | |
|---|---|---|---|---|---|
| mg/kg/day | mg/day | ||||
| 1 | 1.72 | 0.668 | 0.052 | 5.72 | 8.56 |
| 2 | 1.33 | 0.144 | 0.009 | 0.76 | 5.27 |
| 3 | 3.19 | 0.296 | 0.042 | 2.99 | 10.12 |
| 4 | 2.32 | 0.284 | 0.030 | 2.19 | 7.68 |
| 5 | 3.07 | 0.426 | 0.059 | 4.86 | 11.40 |
| 6 | 1.25 | 0.312 | 0.018 | 1.19 | 3.83 |
| 7 | 6.57 | 0.394 | 0.117 | 10.13 | 25.69 |
| 8 | 7.61 | 0.215 | 0.074 | 7.30 | 33.94 |
CRP, C-reactive protein; FCR, fractional catabolic rate; PR, production rate; PS, pool size.
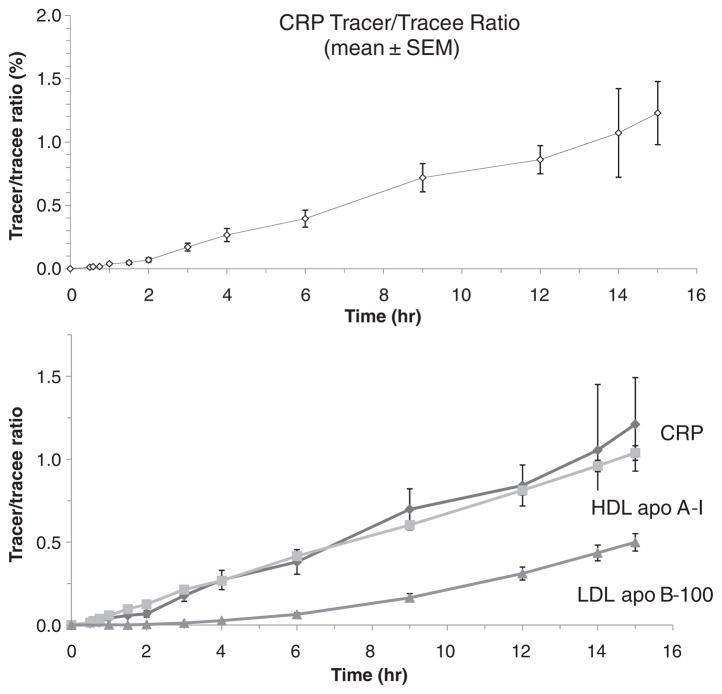
Our CRP and apolipoprotein kinetic data for all subjects are shown in Table 3. CRP had a mean residence time of 3.5days while TRL apoB-100 and TRL apoB-48 had a residence time of 0.27 and 0.36days respectively. Fig. 2 shows leucine tracer/ trace (t/t) ratios of CRP and the association of these CRP t/t ratios with HDL apoA-I and LDL apoB t/t ratios.
Table 3.
Comparison of CRP kinetics with lipoprotein kinetics.
| Protein | Pool size, mg | Fractional catabolic rate, pools/day | Production rate, mg/kg/day | Residence time, days |
|---|---|---|---|---|
| CRP | 13.31±3.78 | 0.343±0.056 | 0.050±0.012 | 3.5±0.6 |
| TRL apoB-100 | 376±28 | 4.13±0.55 | 17.3±1.6 | 0.27±0.03 |
| LDL apoB-100 | 3726±365 | 0.25±0.02 | 10.7±0.8 | 4.3±0.4 |
| TRL apoB-48 | 24.9±4.0 | 2.88±0.24 | 0.84±0.13 | 0.36±0.02 |
| HDL apoA-I | 4000±345 | 0.26±0.02 | 12.2±0.9 | 3.9±0.2 |
Data are expressed as mean±SEM.
LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; VLDL, very low density lipoprotein; apo, apolipoprotein; TRL, triglyceride rich lipoprotein; IDL, intermediate density lipoprotein.
Fig. 2.
Leucine tracer/trace ratio of CRP, HDL apoA-I and LDL apoB-100 during a 15-h primed-constant infusion with deuterated leucine.
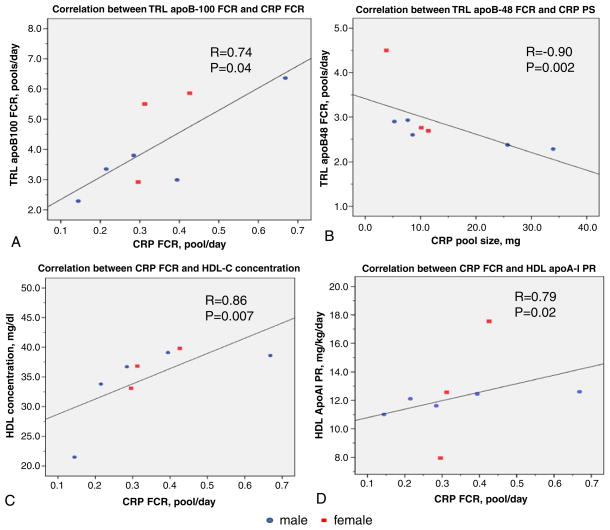
CRP PS was significantly related with CRP PR (r=0.93, p= 0.001), but not CRP FCR (r=0.14, p=0.74). In addition, CRP PS was significantly correlated with BMI (r=0.79, p=0.02). Moreover we documented a significant association between TRL apoB-100 FCR and CRP FCR (r=0.74, p=0.04), as well as a significant inverse correlation between TRL apoB-48 FCR and CRP PS (r=−0.90, p=0.002) (Fig. 3A, B). In addition, there was a significant association between CRP FCR and HDL-C concentrations (r=0.86, p=0.007), as well as between CRP FCR and HDL apoA-I PR. (r=0.79, p=0.02) (Fig. 3C, D).
Fig. 3.
Correlation between CRP, apolipoprotein B and HDL metabolism. (A) Correlation between TRL apoB-100 FCR and CRP FCR. (B) Correlation between TRL apoB-48 FCR and CRP pool size. (C) Correlation between CRP FCR and HDL-C concentration. (D) Correlation between CRP FCR and HDL apoA-I production rate. FCR, fractional catabolic rate; PR, production rate; PS, pool size; HDL, high density lipoprotein; apoA-I, apolipoprotein A-I; TRL, triglyceride rich lipoprotein; apoB, apolipoprotein B.
Multivariate linear regression analysis for correlation between CRP FCR and HDL-C, and CRP FCR and HDL apoA-I PR adjusted for TRL apoB-100 FCR was performed. Model 1: HDL-C and TRL apoB-100 FCR: The beta-coefficients for HDL-C and TRL apoB-100 FCR were 0.009; p=0.38, and 0.036, p=0.19 respectively. Model 2: HDL apoA-I PR and TRL apoB-100 FCR. The beta-coefficients for HDL apoA-I PR and TRL apoB-100 FCR were −0.196; p=0.61, and 0.091, p=0.059 respectively.
There were no significant associations between TRL apoB-48 FCR and CRP FCR (r=−0.21, p=0.61), and TRL apoB-100 FCR and CRP PS (r=−0.048, p=0.91). In addition, there was no significant relationship between CRP metabolism and LDL apoB-100 metabolism.
4. Discussion
CRP has been shown to be an independent risk factor for cardiovascular disease [6]. The protein is synthesized in the liver in response to infection, trauma, systemic inflammation, and also excess liver fat. CRP levels can be significantly reduced by both weight loss and statin therapy [9,20–22]. CRP binds to phosphoryl choline, and this characteristic allows for its isolation from lipoprotein free plasma (density>1.21g/ml) using phosphoryl choline affinity column chromatography. However another protein, serum amyloid P, has similar binding characteristics and is therefore isolated at the same time as CRP. The separation of these two proteins (see Fig. 1) which have similar molecular weight requires an additional gel electrophoresis step using 12% SDS PAGE.
Vigushin and colleagues have studied the metabolism of radiolabeled CRP in humans and reported a CRP FCR of 0.9 pools/day (residence time of 1.11days) in control subjects [23]. They documented that patients with various disease states (rheumatoid arthritis, systemic lupus erythematosis, infection, and cancer) had similar CRP FCR values as controls, indicating that the elevated CRP levels observed in these patients were due to increased production rates [23]. Mauger and colleagues have studied human CRP metabolism using a primed constant infusion of deuterated leucine and a very similar CRP isolation method to what we used, except that they isolated CRP from the 1.25g/ml infranatant fraction, and used 16% SDS PAGE for the additional purification step [24]. These investigators did not mention serum amyloid P in their paper as a potential contaminant. They reported that CRP PR was a significant determinant of plasma CRP levels [24]. Moreover they noted that CRP production was significantly correlated with waist circumference and interleukin-6 levels, and inversely correlated with HDL-C levels [24].
The focus of our research on CRP metabolism was somewhat different. Our goal was to ascertain whether CRP metabolism was linked to the metabolism of apolipoproteins A-I, B-48, and apoB-100 within plasma lipoproteins in the fed state. Like the previous investigators [24] we also noted that CRP PR was a significant determinant of plasma CRP concentrations. We also saw a significant correlation between CRP PS and BMI. Moreover the CRP kinetic parameters that we have reported are similar to those of Mauger and colleagues [24]. They reported mean CRP PR and FCR values of approximately 2.1mg/day and 0.40 pools/day (residence time 2.70 days), respectively. We reported mean CRP PR and FCR values of approximately 4.4mg/day and 0.34 pools/day (residence time 3.5days). It should be noted that Mauger and colleagues studied relatively young healthy subjects with mean CRP levels of 2.22mg/l, while our subjects were older and had mean CRP values of 4.1mg/l, which may account for the higher production rates that we observed. In our view endogenous labeling of proteins is much more likely to provide more accurate measures of plasma kinetics than studies using radiolabeled proteins.
The novel features of our study are that we documented the following significant relationships: 1) CRP PS was inversely linked to TRL apoB-48 FCR (r=−0.90, p=0.002), 2) CRP FCR was linked to TRL apoB-100 FCR (r=0.74, p=0.04), and 3) CRP FCR was linked to both HDL-C levels (r=0.86, p=0.007) and HDL apoA-I production rate (r=0.79, p=0.02). We saw no significant relationships between CRP metabolism and LDL apoB-100 metabolism. Moreover the links between CRP metabolism and HDL metabolism were no longer significant after correcting for the relation with TRL apoB-100 FCR while the correlation between CRP FCR and TRL apo-100 FCR still remained borderline significant in multivariate regression analysis.
CRP metabolism has been linked to liver fat deposition [25–27], and therefore altered uptake of TRL would be predicted to affect CRP metabolism. Moreover CRP levels are higher in obese subjects [28,29], who often have elevated TG levels and decreased levels of HDL-C [30]. It is also known that obese subjects often have overproduction of CRP, decreased fractional catabolism of TRL apoB-100 and apoB-48, as well as enhanced fractional catabolism of HDL apoA-I [31,32]. In our view as previously mentioned the amount of liver TG is a key driver of CRP production and its relationship to excess BMI. Moreover the amount of lipid in the liver is determined by 1) the amount and uptake of lipid via chylomicron remnants containing apoB-48, 2) the endogenous liver TG synthesis from fatty acids, 3) the secretion of TG by the liver onto VLDL apoB-100 containing lipoproteins, as well as 4) the liver uptake of lipoproteins containing TG, mainly VLDL apoB-100 particles. In the fasting state most of the serum TG is found on VLDL apoB-100 containing lipoproteins, and their levels in our studies are determined mainly by VLDL apoB-100 fractional catabolism (r=−0.726, p=0.04), and not production (r=0.62, p=0.10).
Consistent with prior studies CRP PS was significantly correlated with CRP production and not fractional catabolism. The next strongest association that we observed was an inverse correlation between CRP PS and TRL apoB-48 FCR. These data suggest to us that delayed clearance of chylomicron remnants probably is associated with elevated chylomicron levels and enhanced delivery of TG to the liver; resulting in excess liver fat and increased CRP production. Finally we noted a significant association of CRP FCR with TRL apoB-100 FCR. This association still remained after multivariate analysis, while the link between HDL apoA-I and CRP metabolism disappeared. Why this linkage occurred is unclear except that both CRP and TRL apoB-100 are catabolized by the liver, and their catabolism appears to be regulated by common mechanisms.
A previous study shows that subjects with higher physical activity and consuming healthy diets have significantly higher HDL-C, lower apoB and lower CRP levels than subjects who are physically inactive and consuming non-healthy diets [33]. Weight loss and statins lower CRP levels. However it is not clear how these interventions lower CRP levels in humans. It is known that statins enhance the clearance of apoB containing lipoproteins [34,35]. Future research needs to be carried out to further understand human CRP metabolism and its linkage to plasma lipoprotein metabolism.
A major limitation of our study was its small sample size. Its strength is that it is a carefully carried out human study using state of the art stable isotope technology with endogenous labeling of CRP and apolipoproteins.
In conclusion, our data indicate a linkage between CRP and TRL metabolism, and that optimizing TRL levels may be important for maintaining normal CRP levels and controlling coronary heart disease risk.
Acknowledgments
Funding
Dr. Thongtang was supported by a postdoctoral fellowship from Siriraj Hospital, Mahidol University, Bangkok, Thailand. Dr. Ooi was supported by a National Health and Medical Research Council of Australia Postdoctoral Research Fellowship. Dr. Schaefer was supported by grants R01 HL-60935, HL 74753, and PO50HL083813 from the National Institutes of Health and contract 53-3K-06 from the United Department of Agriculture Research Service.
Abbreviations
- CRP
C-reactive protein
- PR
production rate
- FCR
fractional catabolic rate
- PS
pool size
- TRL
triglyceride-rich lipoprotein
- apoB
apolipoprotein B
- apoA-I
apolipoprotein A-I
Footnotes
Author contributions
The study was designed by Dr. Schaefer. The study was supervised by Dr. Schaefer and Dr. Lamonfava. Laboratory analysis was performed by Dr. Thongtang and Dr. Diffenderfer. Dr. Ooi performed data modeling. Dr. Thongtang analyzed the data and wrote the manuscript. All authors provide intellectual input and edited the manuscript.
Conflict of interest
All authors report no potential conflict of interest.
References
- 1.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–20. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 3.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 5.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 6.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-reactive protein to abdominal adiposity. Am J Cardiol. 2010;106:56–61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Festa A, D’Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–9. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–9. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Fanghanel G, Sanchez-Reyes L, Felix-Garcia L, Violante-Ortiz R, Campos-Franco E, Alcocer LA. Impact of waist circumference reduction on cardiovascular risk in treated obese subjects. Cir Cir. 2011;79:175–81. [PubMed] [Google Scholar]
- 12.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–56. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 13.Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011;93:1048–52. doi: 10.3945/ajcn.110.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–8. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–6. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- 16.Barrett PH, Chan DC, Watts GF. Thematic review series: patient-oriented research. Design and analysis of lipoprotein tracer kinetics studies in humans. J Lipid Res. 2006;47:1607–19. doi: 10.1194/jlr.R600017-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Matthan NR, Lichtenstein AH, et al. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J Lipid Res. 2007;48:1746–53. doi: 10.1194/jlr.M700067-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 19.Cobelli C, Toffolo G, Bier DM, Nosadini R. Models to interpret kinetic data in stable isotope tracer studies. Am J Physiol. 1987;253:E551–64. doi: 10.1152/ajpendo.1987.253.5.E551. [DOI] [PubMed] [Google Scholar]
- 20.Quist-Paulsen P. Statins and inflammation: an update. Curr Opin Cardiol. 2010;25:399–405. doi: 10.1097/HCO.0b013e3283398e53. [DOI] [PubMed] [Google Scholar]
- 21.Asher J, Houston M. Statins and C-reactive protein levels. J Clin Hypertens (Greenwich) 2007;9:622–8. doi: 10.1111/j.1524-6175.2007.06639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biasucci LM, Biasillo G, Stefanelli A. Inflammatory markers, cholesterol and statins: pathophysiological role and clinical importance. Clin Chem Lab Med. 2010;48:1685–91. doi: 10.1515/CCLM.2010.277. [DOI] [PubMed] [Google Scholar]
- 23.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–7. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauger JF, Levesque J, Paradis ME, Bergeron N, Tchernof A, Couture P, et al. Intravascular kinetics of C-reactive protein and their relationships with features of the metabolic syndrome. J Clin Endocrinol Metab. 2008;93:3158–64. doi: 10.1210/jc.2007-2585. [DOI] [PubMed] [Google Scholar]
- 25.Maffeis C, Silvagni D, Bonadonna R, Grezzani A, Banzato C, Tato L. Fat cell size, insulin sensitivity, and inflammation in obese children. J Pediatr. 2007;151:647–52. doi: 10.1016/j.jpeds.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 26.Reinehr T, Stoffel-Wagner B, Roth CL, Andler W. High-sensitive C-reactive protein, tumor necrosis factor alpha, and cardiovascular risk factors before and after weight loss in obese children. Metabolism. 2005;54:1155–61. doi: 10.1016/j.metabol.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 28.Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122–8. doi: 10.1210/jc.2007-2089. [DOI] [PubMed] [Google Scholar]
- 29.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 30.Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32:855–67. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 31.Batista MC, Welty FK, Diffenderfer MR, Sarnak MJ, Schaefer EJ, Lamon-Fava S, et al. Apolipoprotein A-I, B-100, and B-48 metabolism in subjects with chronic kidney disease, obesity, and the metabolic syndrome. Metabolism. 2004;53:1255–61. doi: 10.1016/j.metabol.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Barrett PH, Watts GF. Kinetic studies of lipoprotein metabolism in the metabolic syndrome including effects of nutritional interventions. Curr Opin Lipidol. 2003;14:61–8. doi: 10.1097/00041433-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Lavoie ME, Faraj M, Strychar I, Doucet E, Brochu M, Lavoie JM, et al. Synergistic associations of physical activity and diet quality on cardiometabolic risk factors in overweight and obese postmenopausal women. Br J Nutr. 2012:1–10. doi: 10.1017/S0007114512001699. [DOI] [PubMed] [Google Scholar]
- 34.Ooi EM, Barrett PH, Chan DC, Nestel PJ, Watts GF. Dose-dependent effect of rosuvastatin on apolipoprotein B-100 kinetics in the metabolic syndrome. Atherosclerosis. 2008;197:139–46. doi: 10.1016/j.atherosclerosis.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Verges B, Florentin E, Baillot-Rudoni S, Monier S, Petit JM, Rageot D, et al. Effects of 20 mg rosuvastatin on VLDL1-, VLDL2-, IDL- and LDL-ApoB kinetics in type 2 diabetes. Diabetologia. 2008;51:1382–90. doi: 10.1007/s00125-008-1046-4. [DOI] [PubMed] [Google Scholar]