Abstract
Here, we discuss potential explanations for the higher prevalence of hypertriglyceridemia in populations with an Amerindian background. Although environmental factors are the triggers, the search for the ethnic related factors that explains the increased susceptibility of the Amerindians is a promising area for research. The study of the genetics of hypertriglyceridemia in Hispanic populations faces several challenges. Ethnicity could be a major confounding variable to prove genetic associations. Despite that, the study of hypertriglyceridemia in Hispanics has resulted in significant contributions. Two GWAS reports have exclusively included Mexican mestizos. Fifty percent of the associations reported in Caucasians could be generalized to the Mexicans, but in many cases the Mexican lead SNP was different than that reported in Europeans. Both reports included new associations with apo B or triglycerides concentrations. The frequency of susceptibility alleles in Mexicans is higher than that found in Europeans for several of the genes with the greatest effect on triglycerides levels. An example is the SNP rs964184 in APOA5. The same trend was observed for ANGPTL3 and TIMD4 variants. In summary, we postulate that the study of the genetic determinants of hypertriglyceridemia in Amerindian populations which have major changes in their lifestyle, may prove to be a great resource to identify new genes and pathways associated with hypertriglyceridemia.
Keywords: Hispanics, Latin America, triglycerides
Introduction
The study of ethnic groups with an increased risk for having a disease has been a successful strategy to generate new knowledge [1]. The greater susceptibility of the Hispanics for having hypertriglyceridemia is a well-documented phenomenon [2], but, it has been subject of only a small set of studies. Hispanics is an admixed population [3]. A large percentage of the Hispanics living in the US or in Latin America have their origins in the Amerindian groups, the first residents of the continent. The Amerindians have suffered infections, wars and famine that have reshaped several times their environment, lifestyle and the size of the population [4]. As a consequence, it is likely that selection processes has occurred in this ethnic group. Hispanics has been poorly represented in genetic studies; for example, it is the only ethnic group not included in the lipid GWAS reports. The inclusion of individuals with other ethnic backgrounds may narrow the large loci in which associations have been detected. Furthermore, this approach increases the likelihood to detect rare variants with major effects. In this review, we discuss several approaches to identify potential explanations for the higher prevalence of hypertriglyceridemia in populations with an Amerindian background. By this mean, a better comprehension of the pathogenesis of hypertriglyceridemia may be achieved.
Epidemiology of hypertriglyceridemia: Focus in Hispanics
Hypertriglyceridemia is a common lipid disorder (Table 1). In the NHANES 1999-2004 survey (n=5610 adults) almost a third of the participants (33.1 ± 0.8%) had a value above 150 mg/dl [5]. As a result, more than 63.4 million Americans had hypertriglyceridemia. Triglycerides concentrations greater than 500 mg/dl are usually caused by genetic causes and are associated with an increased risk for having pancreatitis. This abnormality was estimated to occur in close to 3.4 million Americans [6]. The prevalence of hypertriglyceridemia was proportional to the age and it is greater in men (36.7 vs 29.6%, p<0.001). Smoking, scant physical activity, diabetes and a body mass index greater than 25 kg/m2 were conditions more commonly seen in hypertriglyceridemic individuals compared against the rest of the population.
Table 1.
Mean plasma lipid concentration in adults in Amerindian-derived populations and its comparison against other ethnic groups.
| LDL cholesterol | HDL cholesterol | Triglycerides | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NHANES1 | 1988- 994 |
1999- 2002 |
2007- 2010 |
1988- 1994 |
1999- 2002 |
2007- 2010 |
1988- 1994 |
1999- 2002 |
2007- 2010 |
|
Mexican
Americans |
|||||||||
| Total | 125 | 121 | 118* | 48.7 | 48.9 | 49.4 | 134 | 139 | 127 |
| Men | 127 | 125 | 120* | 45.2 | 45.0 | 45.4 | 136 | 142 | 136 |
| Women | 123 | 117 | 115* | 52.3 | 52.9 | 53.7 | 132 | 135 | 117* |
| Caucasians | |||||||||
| Total | 129 | 124 | 116* | 50.4 | 51.2 | 52.5* | 120 | 125 | 111* |
| Men | 132 | 126 | 115* | 44.8 | 45.5 | 46.7* | 130 | 134 | 119* |
| Women | 126 | 121 | 116* | 55.7 | 56.6 | 58.1* | 112 | 117 | 104* |
| Afro-Americans | |||||||||
| Total | 128 | 121 | 115* | 55.2 | 54.5 | 56.1 | 93 | 94 | 88 |
| Men | 129 | 121 | 116* | 52.4 | 51.0 | 52.6 | 100 | 99 | 94 |
| Women | 127 | 121 | 114* | 57.3 | 57.3 | 58.7 | 88 | 90 | 83 |
|
Mexican Health
Surveys |
19942 | 20003 | 20064 | 19942 | 20003 | 20064 | 19942 | 20003 | 20064 |
| Total | 106.4 | 130.1 | 131.5 | 34.2 | 38.4 | 38.9 | 133.0 | 181.7 | 139.6 |
| Men | 110.2 | 125.0 | 128.1 | 34.2 | 38.6 | 37.1 | 151.0 | 162.9 | 148.9 |
| Women | 106.0 | 120.8 | 136.1 | 38.0 | 38.1 | 40.1 | 124.6 | 199.5 | 131.5 |
Data are expressed as mg/dl.
p<0.05 between the 1988-2004 and the 2007-2010 surveys.
Modified from:
Carroll M, Kit B, Lacher D, Shero S, Mussolino M. Trends in serum lipids and lipoproteins in US adults, 1988-2010. JAMA. 2012;308:1545-1554
Aguilar-Salinas Carlos A. , Olaiz G,Valles V, Rios JM, Gómez Pérez FJ, Rull JA, Rojas R, Franco A, Sepúlveda J. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nation wide survey. J Lipid Research 2001;42:1298-307
Barquera S, Flores M, Olaiz-Fernández G, Monterrubio E,Villalpando S, González C, Rivera J, Sepúlveda J.Dyslipidemias and obesity in Mexico. Salud Publica Mex 2007;49 supl 3:S338-S347.
Aguilar-Salinas CA, Gómez Pérez FJ, Rull JA, Villalpando S, Barquera S, Rojas R. Prevalence of dyslipidemias in the 2006 Encuesta Nacional de Salud y Nutrición. 2009 Salud Pública Mex. 2010:52 (supl1) S44-S53
Hispanics is the ethnic group in the US with the highest prevalence of hypertriglyceridemia (40.4% for men and 34.9% for women). This abnormality is less common in Caucasians (39 % for men and 31.6% for women) and in African Americans (19.5% for men and 13.6% for women). Moderate (200-<500mg/dl) and severe (500-2000 mg/dl) forms of hypertriglyceridemia were also more frequent in Hispanics; 19% fall in any of these two categories [2,5,7]. Contrary to the observed in Caucasian males, the mean triglycerides did not decreased in the 1988-2010 period in Hispanic men. However, association between ethnicity and this lipid disorder should be carefully assessed [8] because several conditions associated with hypertriglyceridemia (i.e. diabetes, obesity) are also more common in Amerindian-derived populations.
Hypertriglyceridemia is a common abnormality in Latin American countries. Information derived from population-based surveys of eight countries of the region was collected by The “Latin American Consortium of Studies in Obesity” (LASO). The age adjusted prevalence of hypertriglyceridemia was 23.3 (95%CI 17.6-30.2%) [9]. In addition, the “CARMELA” study comprised 11,550 adults living in seven large urban centers across Latin America [10]. The mean triglycerides concentrations ranged from 114.3 mg/dl (in Buenos Aires, the participating center with the least Amerindian heritage) to 183.9 (in Mexico City). Wide variations in prevalence were reported. It varied from 20% in Buenos Aires to 50% in Mexico City. All other participating centers had prevalence close to 40%. Furthermore, the contribution of hypertriglyceridemia as a component of the metabolic syndrome is substantially greater in Hispanics. The CARMELA study included the estimation of the prevalence of the metabolic syndrome (using the National Cholesterol Education Program III criteria). Hypertriglyceridemia was present in 85.9% (95%CI 84.3-87.6%) of the cases. In contrast, this abnormality contributed to the diagnosis in only 31.2% of the Caucasian subjects with metabolic syndrome studied in the NHANES 1999-2004 survey.
The same trends above described were observed in the three nationwide population-based health surveys performed in Mexico from 1994 to 2006 [11,12,13]. Hypertriglyceridemia has been the second most frequent abnormality (behind hypoalphalipoproteinemia) found in Mexican adults. The percentage of affected adults varied from 31.6% (in 2006) to 49.1% (in 2000). Methodological differences between surveys are the main reason for the dispersion of the results. In the most recently published survey (2006), 4040 cases (1871 men and 2169 women) adults were included. Hypertriglyceridemia was more often seen in adults living in metropolitan areas or living in central Mexico. Its prevalence was not influenced by school education or socioeconomic status. The simultaneous elevation of cholesterol and triglycerides concentrations was observed in 18.2% (95%CI 16.3-20.2%) of the population. Hypertriglyceridemia/hypoalphalipoproteinemia was observed in 18.3% (95%CI 16.3-20.5%). Finally, isolated hypertriglyceridemia was found in 25.3% (95%CI 23.4-27.4%) of the study subjects.
Although the prevalence of dyslipidemia is difficult to compare among populations due to methodological differences, it is clear from the evidence described above that Hispanics have a higher prevalence of hypertriglyceridemia than the observed in other ethnic groups. However, this phenomenon has not been systematically studied.
In order to postulate potential explanations for the greater prevalence of hypertriglyceridemia reported in Hispanics, an integrative approach is needed. Hypertriglyceridemia should not be conceptualized as a single entity [14]. Abnormally high plasma triglycerides concentrations could result from primary or secondary etiologies. Primary etiologies are sufficiently characterized, although its etiology is unknown. The genetic component should be studied in well characterized populations, in which primary etiologies are studied apart. Environmental factors should be controlled as much as possible through a careful selection of the inclusion criteria. An unbiased descriptive approach (i.e. GWAS) is a possible option for the study of the polygenic forms.
Genetic forms of hypertriglyceridemia: Focus in Hispanics
-Familial combined hyperlipidemia: FCHL is a common condition characterized by the presence of different lipid phenotypes within the affected kindred; cases should have an increased apo B concentration [15]. It is frequently associated with the metabolic syndrome. The study of the genetics of familial combined hyperlipidemia has attracted the attention of several groups [16]. Despite that, the knowledge of the pathophysiology of this condition is incomplete. A detailed review of the genetics of FCHL is available elsewhere [17]. We want to highlight that although a significant proportion of the studies have been done in Caucasian populations (i.e. from Finland and the Netherlands, among others), some of the associations have been originally identified or have been replicated in Mexicans. These observations are described in detail in the following paragraphs.
FCHL has been consistently linked to the locus 1q21-23 in Caucasians, Asians and Mexicans [18]. Several candidates exist within this region to explain the association (TXNIP,RXRG,CRABP2,ATF6 and USF1). Results obtained in Finnish kindreds point out that USF1 variants are responsible for the association [19]. Since Mexicans and Finnish populations have remarkable differences in the prevalence of the risk alleles in this locus, the joint analysis of these populations was an adequate strategy to narrow the number of candidate genes. The same susceptibility haplotype associated with FCHL and TG was identified in Finnish and Mexican families, with the most significant association observed with the TG trait (p = 0.0009). It is especially significant to note that in the more outbred Mexican population with presumably different genomic linkage disequilibrium structure, the region of association was restricted from ≥ 46 kb observed in Finns to only 14 kb in Mexicans [20]. The USF1 gene product is a transcription factor that regulates close to 40 genes involved in lipid (i.e. APOA5, APOC3, APOA2, APOE, HSL and PNPLA2) and carbohydrate metabolism and in the regulation of immune response. USF1 forms a complex with USF2 to bind the e-boxes and initiate the transcription of the target genes [21]. Under insulin stimulation, USF1 is phosphorylated, blocking its ability to promote gene transcription. However, none of the FCHL associated USF1 SNPs (i.e. rs3737787) in the Finns or Mexicans result in an amino acid change. Thus, additional studies are needed to establish causal relationships. Both in Finnish and Mexicans were demonstrated that the rs3737787 genotype is associated with differences in the expression of the USF1 target genes in fat or lymphoblasts. Specifically in Mexicans, a co-expression analysis allowed to identify modules or groups of genes that are differentially expressed in fat samples stratified by the rs3737787 genotype [22]. Two modules were associated with the FCHL and hypertriglyceridemia. It was found that the rs3737787 genotype associated differences were the same when samples compared by the FCHL phenotype. One of the modules, called URFA (USF1-regulated, FCHL associated) contains 504 genes. In this sample, URFA module explains a significant portion of the FCHL variables (phenotype FCHL 10%, cholesterol 6%, triglycerides 17% and apolipoprotein B 9%). Expression analysis showed association between 18 genes and FCHL and 171 genes and the plasma triglycerides concentrations. Thirteen genes were associated to both genotypes. Among them are FADS3, which was previously identified in a GWAS as associated with the triglycerides concentrations. The FADS3 is involved in the synthesis of fatty acids omega-3 and - 6. Also ABCC6, AKT2, GCLM and HSD11B1 were part of the URFA module; all of them regulate lipid or carbohydrate metabolism and have been implicated in FCHL. Other associations between genetic variants and FCHL have resulted from interethnic studies including Mexicans. TCF7L2 is a transcription factor that plays an important role in the WNT signaling pathway. WNT proteins regulate the proliferation and cellular differentiation by the activation or repression of multiple genes; some of them are involved in the metabolism of lipids and carbohydrates. The variants rs7903146 and rs12255372 of the TCF7L2 have been associated with increased risk of type 2 diabetes in multiple populations. Due to the frequent coexistence of the FCHL and diabetes, Huertas-Vázquez et al. [23] explored whether the variants rs7903146 and rs12255372 are associated with some of the phenotypes associated FCHL in Mexican and Finnish families. These variants were associated with the triglycerides concentration in the Mexican population; the finding was replicated in Finns. Differences in the TCF7L2 expression were found in subcutaneous adipose tissue by dividing the population by the presence of the susceptibility allele. The same conclusion was raised when cases with or without FCHL were compared. Another example is the association with the 20q12-q13.1 locus explained by HNF4α variants. HNF4α is a MODY gene. However, it regulates the expression of APOB. HNF4α is a determinant of the concentration of cholesterol and triglycerides in FCHL patients both from Mexico and Finland [24]. The haplotypes associated with FCHL were the same in both populations. HNF4alfa is a transcriptional regulator of USF1.
The studies above described were followed by a GWAS in FCHL Mexican families (n=52, 567 subjects) [25]. As a replication sample, 1446 unrelated individuals with or without hypertriglyceridemia were studied. Apo B was selected as the main outcome variable. This report was the first GWAS performed exclusively in an admixed population containing Amerindian heritage. Ethnicity was carefully controlled to avoid spurious associations. It was estimated that the participants had an Amerindian proportion of 0.5 (SE=0.005). Apo B levels were not associated with ancestry. This approach allowed the replication of several previously reported loci associated with FCHL (2p.25, p21.31-14.2, 4p16.2-16.1 among others) or other lipid traits (for triglycerides, 4p15-16, 4q34, 13q22.33-31.1, 14q11.2-12 and 20q13.11). The HNF4α association was confirmed. Associations with markers within or close to the following genes were reported: FABP2, FOLH1, MADD, NR1H3, FADS, CETP, LCAT, APOE and PLTP. Two novel associations were informed. ApoB concentrations were associated with rs1424032 located on 16q21 and rs1349411 on 12p13.31. The 16q21 locus is a highly conserved non-coding region, and the 12p13.31 locus includes the APOBEC1 gene, which is an excellent candidate gene for serum apo B levels, as it is involved in the editing of APOB mRNA in the small intestine. Additional studies are ongoing to assess the physiologic consequences of these variants in FCHL patients.
In summary, interethnic studies have been useful for identify and/or replicate associations between genetic variants and FCHL. An association of the same haplotype in the same direction in two groups with different ethnic background is solid confirmatory evidence.
-Familial hypertriglyceridemia and other forms of severe hypertriglyceridemia: FHTG is a common form of hypertriglyceridemia characterized by isolated moderate to severe hypertriglyceridemia. Cholesterol concentrations are abnormal only if triglycerides values are greater than 1000 mg/dl; the ratio triglycerides/cholesterol is close to 5. This entity is common in Mexicans. Isolated hypertriglyceridemia was present in 13.3% (95%CI 11.6-15.2%) of the adults in the 2006 Mexican health survey (ENSANut2006) [16]. However, FHTG prevalence could not be estimated with precision due to the lack of information regarding the plasma lipid levels in the first degree relatives. Few candidate genes have been studied in this disorder without positive results [26].
-Dysbetalipoproteinemia: It is characterized by the plasma accumulation of remnants and IDLs. As a result, both cholesterol and triglycerides concentrations are abnormal (usually around 300 mg/dl). It is mainly caused by homozygous state for apoE2, an apolipoprotein E isoform with a decreased affinity for its binding receptors. This disorder in Hispanics is uncommon because the allele frequency of apoE2 is low in Amerindians [27]. Although it is controversial, apoE2 has been postulated as a marker of admixture. In Mestizo populations, the prevalence of the E2 variant is still lower (~3%) compared to that found in Caucasians (~7-13%).
-Polygenic forms of hypertriglyceridemia: Several meta-analysis of GWA studies have been published [28,29]. In the most recent version, samples of 188,577 Caucasian and 7,898 non Caucasian individuals from Africa and Asia were included [28]. Blood lipid levels were associated with variations of 157 loci; 62 new associations were reported. Triglycerides concentrations are associated with genetic variants of 37 genes; sixteen of them are not associated with any other lipid traits. This new report informed 8 new associations that explained 2.1% of the triglycerides variance in the Framingham offspring study. The genes with the strongest associations with triglycerides concentrations were: TRIB1, ANGPTL3, LPL, GCKR, FADS1-2-3 and MLXIPL. These results cannot be directly extended to every population due to interethnic differences in genetic architecture. Authors included an ancestry-specific analysis for the non-Caucasian participants. They identified five loci in which the associated SNPs were different to that reported in Caucasians. SORT1 and LDLR have ethnic specific variants in Africans; the same phenomenon was observed for APOA5 and CETP in South Asians. APOE had a different linkage disequilibrium pattern in Europeans. These observations reinforce the need to include multiethnic populations in the study of complex traits.
Amerindians have not included in the GWAS meta-analysis. Two GWA studies have included samples of Hispanics. However, in one of these reports, the Mexican samples were used only for replication. Kooner and coworkers [30] identified the contribution of MLXIPL in a two step GWAS. In the initial stage, 1005 European men and 1006 Asian men were included. Close to 900 SNPs were selected for the second stage, in which 4,568 individuals participated. Of them 1,560 were Mexican women and 968 Mexican men. Associations with triglycerides concentrations were found for 13 SNPs; four of them in the MLXIPL region. The other SNPs were in the vicinity of the APOA1/C3/A4/A5 cluster and in LPL. Mexicans had the highest prevalence of the risk allele of all four SNPs of MLXIPL. An additional relevant result was the replication of the remarkable effect that has the R230C variant of ABCA1 on HDL cholesterol in Mexicans. This genetic change is common in Amerindian populations, but it is absent in other ethnic groups. However, the number of SNPs genotyped and the sample size included in this report is insufficient to have an adequate representation of the genetic determinants of hypertriglyceridemia in Mexicans. Weissglass-Volkov and coworkers published a two-stage GWAS report in which only Mexicans cases and controls were included [31]. The objective of the study was to determine which variants are shared across populations. Also it was expected to find novel associations due to the existence of population-specific variants, differences in allele frequencies, patterns of linkage disequilibrium or gene-environment interactions. The initial stage included 2240 samples, 563,599 genotyped SNPs and 769,042 imputed SNPs. Cases were defined as Mestizo individuals with plasma triglycerides >200 mg/dl without use of lipid lowering therapies, diabetes, morbid obesity or any secondary cause of dyslipidemia. Controls should be otherwise healthy subjects with triglycerides <150 mg/dl. The second stage included 1235 genotyped SNPs and 2121 participants. A joint analysis of stages 1 and 2 data (n=4361) was performed. The main associations reported in Caucasians were replicated. The strongest signal was identified in APOA5, GCKR and LPL, followed by ANGPTL3, TIMD4-HAVCR1, MLXIPL and CILP2. The prevalence of the susceptibility allele of APOAV, ANGPTL3 and TIMD4 was significantly greater in Mexicans than in Caucasians (table 2). Close to 50% of the associations reported in Caucasians could be generalized to the Mexican populations. However, differences in the lead signals were observed in 82 of the 100 associations under study. Even more, some population specific variants of these loci were identified. For example the predominant Mexican SNP in the TIMD4-HAVCR1 was not associated with triglycerides levels in Caucasians. This signal is in strong linkage disequilibrium with a missense variant in HAVCR1, suggesting that HAVCR1 is the underlying gene responsible of the association. Furthermore, the different disequilibrium pattern found in Mexicans allowed the refinement of the length of the three loci (APOA5, MLXIPL and CILP2). As a result, a smaller region and a reduced number of SNPs were identified as responsible of the association. Specifically for APOA5, it was identified that the SNP rs964184 was the lead SNP both in Mexicans and Europeans. This SNP is in high linkage disequilibrium with 26 other SNPs in Caucasians, but not in Mexicans. This observation suggests that this SNP is the responsible variant of the association. The prevalence of this susceptibility allele is almost three times greater in Mexicans (0.3 vs 0.12).
Table 2.
Prevalence of risk alleles for hypertriglyceridemia in Mexicans and its comparison against Europeans
| Locus | Positi on |
Mexican Lead SNP |
MAF (MX/EUR) |
Effect (s.e) |
P-Value | European Lead SNP4 |
MAF (MX/CEU) |
|---|---|---|---|---|---|---|---|
| APOA5 | 11q2 3 |
rs964184 | G (0.30/0.12) |
1.77 (0.05) |
5.5E-35 | rs964184 | G (0.30/0.12) |
| GCKR | 2p23 | rs1260326 | T (0.26/0.42) |
1.41 (0.05) |
2.2E-13 | rs1260326 | T (0.26/0.42) |
| LPL | 8p21 | rs1267891 9 |
G (0.05/0.12) |
0.53 (0.1) | 2.7E-10 | rs1267891 9 |
G (0.05/0.12) |
| MLXIPL | 7q11 | rs2286276 | T (0.12/0.26) |
0.72 (0.07) |
2.2E-06 | rs1714573 8 |
T (0.06/0.12) |
| TIMD4 | 5q33 | rs2036402 | C (0.45/0.25) |
1.23 (0.04) |
3.4E-06 | rs1363232 | A (0.10/0.30) |
| CILP2 | 19p1 3 |
rs2228603 | T (0.03/0.10) |
0.34 (0.26) |
3.0E-05 | rs1040196 9 |
C (0.05/0.10) |
| ANGPTL3 | 1p31 | rs1088933 7 |
A (0.45/0.35) |
0.83 (0.04) |
3.3E-05 | rs2131925 | G (0.45/0.33) |
MAF= Minor allele frequency. MX=Mexican. EUR=European S.E.= Standard Error.
Modified from: Weissglas-Volkov D, Aguilar-Salinas CA, Nikkola E, et al. Genome-wide association study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J Med Genetics 2013;50:298-308
The Mexican lipid GWAS render the identification of new genes involved in the regulation of plasma triglycerides levels. This signal is located in chromosome 18q11 (p=2.43 × 10−08). The susceptibility allele is common in Mexican and rare is Caucasians. Differential expression analyses in fat samples of the genes covered by this locus suggested that the Niemann-Pick type C1 is the most likely candidate to explain the novel association. The susceptibility allele was associated with hypertriglyceridemia in a set of independent samples.
Environmental factors associated with hypertriglyceridemia: Focus in Hispanics
Environmental conditions play a major role as modulators of the severity of hypertriglyceridemia even in patients with primary dyslipidemias. In the last thirty years, the Mexican population (as a large percentage of the Latin American countries) was concentrated in major urban centers. The percentage of the population living in rural areas decreased from 40.5 to 26.8% from 1950 to 1990. The eating habits were modified, increasing the consumption of calories, fats and simple sugars. In rural areas the distribution of nutrients of the average diet is 64% carbohydrates, 12.1% protein and 22.7% fat [32]. After moving to an urban area, the consumption of fats increases (27.6 and 33% in areas of low and medium income respectively). The consumption of simple sugars increases or remains unchanged. As a result, negative trends have been reported for the consumption of meat (−18.7%), milk (−26.7%) and fruits/vegetables (−29%). In contrast, the consumption of soft drinks increased by 37.2% [33]. Excessive alcohol consumption remains to be a health problem, especially in men. In parallel, physical activity has decreased. Half of the teenagers expend more than 25 hours per week in front of a screen. Although these lifestyle changes have occurred in many areas of the world, it is remarkable the greater than expected impact occurred in Amerindian-derived populations. Hispanics living in the US and Mexicans are good examples of this phenomenon. In Mexico, the prevalence of obesity increased from 20.9% in 1994 to 32.4% in 2012 [34]. Therefore, less than 30% of adults have a normal weight. Overweight affects all age groups and is more common in women. The prevalence is high in all economic strata of the population, but its prevalence has grown especially among the lowest income group. Fat accumulation has a different pattern in Amerindians [35]. Fat is stored mainly in the intra-abdominal compartment. Subcutaneous fat has a small capacity to expand. In addition, height and lean mass is lower in this ethnic group. These physiological differences may be a potential explanation for the high prevalence of metabolic disorders seen in this group.
Perspectives and conclusions
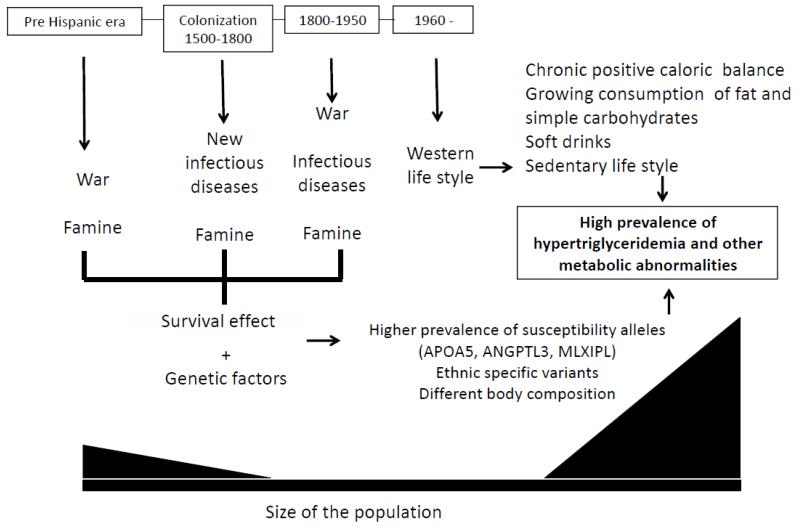
Amerindian derived populations living in the US and in other Latin American countries (i.e. Mexico) have suffered a remarkable change in their lifestyle during recent decades. The ancestors of these populations were survivors of multiple infections, famine and wars. Exposure to an affluent lifestyle is a new challenge to be faced. In a relatively short period of time, prevalence of metabolic disorders has dramatically changed. Now, Amerindian derived populations are among the ethnic groups with the highest prevalence of metabolic disorders. This socioeconomic phenomenon is an opportunity to generate new knowledge regarding the pathogenesis of dylipidemias, obesity and type 2 diabetes (Figure 1).
Figure 1.
Genetic and environmental factors involved in the increased susceptibility of Amerindian derived populations for having hypertriglyceridemia.
In this review, we have highlighted the epidemiology of hypertriglyceridemia in Ameriandian derived populations. Although it is clear that environmental factors are the triggers for the high prevalence of hypertriglyceridemia, the search for the ethnic related factors that explains the increased susceptibility of the Amerindians is a promising area for research.
The study of the genetics of hypertriglyceridemia in Hispanic populations faces several challenges. Ethnicity could be a major confounding variable to prove genetic associations. Ancestry informative markers and adequately planned statistical analyses have been applied to circumvent these limitations, although it may lead to a higher false negative rate and losing ancestry-specific variants. Thus, this is an area in which state of the art technology and innovative analyses strategies could provide powerful tools to identify new genetic factors and previously unrecognized pathways involved in the pathogenesis of hypertriglyceridemia.
Despite the limitations mentioned above, the study of hypertriglyceridemia in Hispanics has resulted in several significant contributions. Studies done in Mexican FCHL families redefined the USF1 region responsible for the association. In addition, using a system genetics approach, it was shown that the lead USF1 signal is associated with differential transcriptional patterns in subcutaneous fat. FADS3 was identified as a USF1 target gene; recent GWAS-derived evidence confirmed that FADS3 variants are associated with plasma triglycerides concentrations [28]. Furthermore, two GWAS reports have exclusively included Mexican mestizos. These studies showed that 50% of the associations reported in Caucasians could be generalized to the Mexican populations, but in many cases the Mexican lead SNP was different than that reported in Europeans. This information indicates that risk assessment models should be based in high resolution data from each ethnic group to capture the distant proxies and causal variants. Both reports included new associations with apo B or triglycerides concentrations. The different genomic linkage disequilibrium structure between ethnic groups, the diverging prevalence of the risk alleles and the existence of ethnic specific risk variants were the explanations for novel findings.
Additional studies are needed to identify reasons for the increased susceptibility for having hypertriglyceridemia of the Hispanic population. However, some clues could be found in the studies reviewed here. The frequency of susceptibility alleles in Mexicans is higher than that found in Europeans for several of the genes with the greatest effect on triglycerides levels. An example is the SNP rs964184 in APOA5. The prevalence of the susceptibility allele is almost three times greater in Mexicans (0.3 vs 0.12). The same trend was observed for ANGPTL3 and TIMD4 variants. However, contribution of these variants at the population level remains to be known. Furthermore, it is remarkable that susceptibility alleles of genes that may participate in thrifty phenotype are very common in Mexicans. This is the case for MLXIPL variants. MLXIPL forms heterodimers with ChREBP; these complexes regulate the expression of genes involved in glycolysis, gluconeogenesis and lipogenesis [36]. This pathway participates in the adaptation to famine. Fine mapping of genes involved in the adaptation to chronic caloric restriction (i.e. FGF21) in Hispanics is required to support a role of adaptation to famine in the increased susceptibility of this ethnic group to have hypertriglyceridemia and other metabolic disorders. Also, the search for ethnic specific variants is as an attractive goal. It has been demonstrated the existence of ethnic specific variants in Hispanics with hypoalphalipoproteinemia (R230C variant of ABCA1) [37] or type 2 diabetes (5 SNPs of SLC16A11) [38]. These variants have a relatively large population effect and could disclose a novel pathogenic mechanism (as the previously unknown role of SLC16A11 in type 2 diabetes). Finally, Hispanics are a potential source for doing whole exome sequencing in FHTG kindreds. The large number of cases and the size of their families may solve some of the logistic problems of such studies [39]. A family study design may allow the identification of rare genetic variants with large effect because the same mutation is found in several relatives and co-segregation with triglycerides concentration could be proved.
In summary, we postulate that the study of the genetic determinants of hypertriglyceridemia in Amerindians is a resource to identify new genetic variants and pathways associated with hypertriglyceridemia. Environmental factors have shaped the composition of this ethnic group, making likely the existence of a survival effect, ethnic specific variants or a genetic drift that made this population susceptible to metabolic disorders. Despite that, Amerindians have been underrepresented in GWA lipid studies. The broader recognition of this phenomenon may result in a greater number of international collaborations, interethnic studies and exchange of knowledge and infrastructure between institutions.
Acknowledgements
We express our gratitude to all investigators that have made significant contributions to the work reviewed here from the Department of Endocrinology and Metabolism (Ivette Cruz-Baustista, Roopa Mehta, Liliana Muñoz, Olimpia Arellano-Campos, Lizeth Gomez-Munguia, Juan Pablo del Rincon-Jarero, Maria Luisa Velasco-Perez, Luz Elizabeth Guillen-Pineda, Carmen Moreno, Magdalena Delgado among others), Endocrine Surgery (Miguel Herrera Hernandez) and the Unit of Molecular Biology and Genomic Medicine (Laura Riba, Maria Luisa Ordoñez-Sánchez, Maribel Rodríguez-Torres and Rosario Rodríguez-Guillen) of the Instituto Nacional de Ciencias Médicas y Nutrición and from the Department of Human Genetics (Daphna Weissglas-Volkov, Elina Nikkola, Christopher L. Plaisier, Adriana Huertas-Vazquez, Prasad MV Linga Reddy, Janet S. Sinsheimer, Rita M Cantor, Aldons J. Lusis) of the David Geffen School of Medicine at UCLA. Also, our gratitude for our study participants and their relatives.
Funding
This research was supported by NIH (grants HL-095056 and HL-2848) and CONACYT (grants 115250).
Abbreviations
- ABCA1
ATP binding cassette protein A1
- Apo B
Apolipoprotein B
- BMI
Body mass index
- ChREBP
Carbohydrate response element binding protein
- FADS3
Type 3 fatty acid desaturase
- FCHL
Familial combined hyperlipidemia
- FHTG
Familial hypertriglyceridemia
- GWAS
Genome wide association study
- HDL-C
High density lipoprotein cholesterol
- MLXIPL
Max-like protein X Interacting protein like
- MODY
Maturity onset diabetes of the young
- NHANES
National Health and Nutrition Examination Survey
- USF1
Upstream transcription factor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors do not have any conflicts of interest to disclose in relation to this manuscript.
References
- 1.Simino J, Rao DC, Freedman BI. Novel findings and future directions on the genetics of hypertension. Curr Opin Nephrol Hypertens. 2012;21:500–7. doi: 10.1097/MNH.0b013e328354e78f. [DOI] [PubMed] [Google Scholar]
- 2.Christian JB, Bourgeois BN, Snipes R, et al. Prevalence of Severe (500 to 2,000 mg/dl) Hypertriglyceridemia in United States Adults. Am J Cardiol. 2011;107:891–7. doi: 10.1016/j.amjcard.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Price A, Patterson N, Yu F, et al. A genomewide admixture map for Latino populations. American Journal of Human Genetics. 2007;80:1024–36. doi: 10.1086/518313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hünemeier T, Amorim CE, Azevedo S, et al. Evolutionary responses to a constructed niche: ancient Mesoamericans as a model of gene-culture coevolution. PLoS One. 2012;7:e38862. doi: 10.1371/journal.pone.0038862. doi: 10.1371/journal.pone.0038862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES, Li C, Zhao G, et al. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med. 2009;169:572–578. doi: 10.1001/archinternmed.2008.599. [DOI] [PubMed] [Google Scholar]
- 6.Toth P, Potter D, Ming E. Prevalence of lipid abnormalities in the United States: The National Health and Nutrition Examination Survey 2003–2006. J Clin Lipidology. 2012;6:325–330. doi: 10.1016/j.jacl.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Carroll M, Kit B, Lacher D, Shero S, Mussolino M. Trends in serum lipids and lipoproteins in US adults, 1988-2010. JAMA. 2012;308:1545–1554. doi: 10.1001/jama.2012.13260. [DOI] [PubMed] [Google Scholar]
- 8.Boullart AC, Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochimica et Biophysica Acta. 2012;1821:867–75. doi: 10.1016/j.bbalip.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Miranda JJ, Herrera VM, Chirinos JA, et al. Major cardiovascular risk factors in Latin America: A comparison with the United States. The Latin American consortium of studies in obesity (LASO) PLoS ONE. 2013;8:e54056. doi: 10.1371/journal.pone.0054056. doi:10.1371/journal.pone.0054056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinueza R, Boissonnet CP, Acevedo M. Dyslipidemia in seven Latin American cities: CARMELA study. Preventive Medicine. 2010;50:106–111. doi: 10.1016/j.ypmed.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Aguilar-Salinas Carlos A, Olaiz G, Valles V, Ríos JM, Gómez Pérez FJ, Rull JA, Rojas R, Franco A, Sepúlveda J. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nation wide survey. J Lipid Research. 2001;42:1298–307. [PubMed] [Google Scholar]
- 12.Barquera S, Flores M, Olaiz-Fernández G, Monterrubio E, Villalpando S, González C, Rivera J, Sepúlveda J. Dyslipidemias and obesity in Mexico. Salud Publica Mex. 2007;49(supl 3):S338–S347. [Google Scholar]
- 13.Aguilar-Salinas CA, Gómez Pérez FJ, Rull JA, Villalpando S, Barquera S, Rojas R. Prevalence of dyslipidemias in the 2006 Encuesta Nacional de Salud y Nutrición. 2009 Salud Pública Méx. 2010;52(supl1):S44–S53. doi: 10.1590/s0036-36342010000700008. [DOI] [PubMed] [Google Scholar]
- 14.Brunzell J. Hypertriglyceridemia. N Engl J Med. 2007;357:1009–17. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar Salinas CA, Zamora M, Gómez-Díaz RA, et al. Familial combined hyperlipidemia: controversial aspects of its diagnosis and pathogenesis. Seminars of Vascular Medicine. 2004;4:203–9. doi: 10.1055/s-2004-835379. [DOI] [PubMed] [Google Scholar]
- 16.Pajukanta P, Nuotio I, Terwilliger JD, et al. Linkage of familial combined hyperlipidaemia to chromosome 1q21-q23. Nat Genet. 1998;18:369–73. doi: 10.1038/ng0498-369. [DOI] [PubMed] [Google Scholar]
- 17.Brouwers M, van Greevenbroek M, Stehouwer C, et al. The genetics of familial combined hyperlipidaemia. Nat Rev Endocrinol. 2012;8:352–62. doi: 10.1038/nrendo.2012.15. [DOI] [PubMed] [Google Scholar]
- 18.Huertas-Vázquez A, del Rincón-Jarero JP, Canizales-Quinteros S, et al. Replication of Linkage of Familial Combined Hyperlipidemia to Chromosome 1q21-q23 in Mexican Families. Annals of Human Genetics. 2004;68(pte5):419–427. doi: 10.1046/j.1529-8817.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 19.Pajukanta P, Lilja HE, Sinsheimer JS, et al. Familial combined hyperlipidemia is associated with upstream transcription factor 1 (USF1) Nat Genet. 2004;36:371–6. doi: 10.1038/ng1320. [DOI] [PubMed] [Google Scholar]
- 20.Huertas-Vazquez A, Aguilar-Salinas C, Lusis AJ, et al. Familial combined hyperlipidemia in Mexicans: association with upstream transcription factor 1 and linkage on chromosome 16q24.1. Arterioscler Thromb Vasc Biol. 2005;25:1985–91. doi: 10.1161/01.ATV.0000175297.37214.a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Lusis AJ, Pajukanta P. Familial combined hyperlipidemia: upstream transcription factor 1 and beyond. Curr Opin Lipidol. 2006;17:101–9. doi: 10.1097/01.mol.0000217890.54875.13. [DOI] [PubMed] [Google Scholar]
- 22.Plaisier CL, Horvath S, Huertas-Vazquez A, et al. A systems genetics approach implicates USF1, FADS3, and other causal candidate genes for familial combined hyperlipidemia. PLoS Genet. 2009 Sep;5(9):e1000642. doi: 10.1371/journal.pgen.1000642. Epub 2009 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huertas-Vazquez A, Plaisier C, Weissglas-Volkov D, et al. TCF7L2 is associated with high serum triglycerides and differentially expressed in adipose tissue in families with familial combined hyperlipidemia. Diabetologia. 2008;51:62–9. doi: 10.1007/s00125-007-0850-6. [DOI] [PubMed] [Google Scholar]
- 24.Weissglas D, Huertas Vazquez A, Canizalez S, et al. Common hepatic nuclear factor 4 alpha variants are associated with high serum lipid levels and the metabolic syndrome. Diabetes. 2006;55:1970–7. doi: 10.2337/db06-0035. [DOI] [PubMed] [Google Scholar]
- 25.Weissglas-Volkov D, Plaisier C, Huertas-Vazquez A, et al. Identification of two common variants contributing to serum apolipoprotein B levels in Mexicans. Arteriosclerosis Thrombosis and Vascular Biology. 2010;30:353–9. doi: 10.1161/ATVBAHA.109.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MW, Craddock AC, Angelin B, et al. Analysis of the ileal bile acid transporter gene, SLC10A2, in subjects with familial hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2001;21:2039–45. doi: 10.1161/hq1201.100262. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar CA, Botet JP, Ordovas JM, et al. The apolipoprotein E4 allele is not associated with an atherogenic lipid profile in a Native-American population following its traditional lifestyle. Atherosclerosis. 1999;142:409–14. doi: 10.1016/s0021-9150(98)00251-2. [DOI] [PubMed] [Google Scholar]
- 28.Global Lipids Genetics Consortium. Willer CJ, Schmidt EM, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–83. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissglas-Volkov D, Aguilar-Salinas CA, Nikkola E, et al. Genome-wide association study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J Med Genetics. 2013;50:298–308. doi: 10.1136/jmedgenet-2012-101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kooner J, Chambers J, Aguilar-Salinas CA, et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nature Genetics. 2008;40:149–51. doi: 10.1038/ng.2007.61. [DOI] [PubMed] [Google Scholar]
- 31.Weissglas-Volkov D, Aguilar-Salinas CA, Nikkola E, et al. Genome-wide association study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J Med Genetics. 2013;50:298–308. doi: 10.1136/jmedgenet-2012-101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores M, Macias N, Rivera M, et al. Dietary patterns in Mexican adults are associated with risk of being overweight or obese. J Nutr. 2010;140:1869–73. doi: 10.3945/jn.110.121533. [DOI] [PubMed] [Google Scholar]
- 33.Barquera S, Campirano F, Bonvecchio A, et al. Caloric beverage consumption patterns in Mexican children. Nutr J. 2010;219:47. doi: 10.1186/1475-2891-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguilar-Salinas CA. Las enfermedades crónicas no transmisibles:el principal problema de salud en México. Salud Publica Mex. 2013;55(suppl 2):S347–S350. [PubMed] [Google Scholar]
- 35.Herrera VM, Casas JP, Miranda JJ, et al. Interethnic differences in the accuracy of anthropometric indicators of obesity in screening for high risk of coronary heart disease. Int J Obes. 2009;33:568–76. doi: 10.1038/ijo.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iizuka K. Recent progress on the role of ChREBP in glucose and lipid metabolism. Endocr J. 2013;60(5):543–55. doi: 10.1507/endocrj.ej13-0121. [DOI] [PubMed] [Google Scholar]
- 37.Aguilar-Salinas CA, Canizales-Quinteros S, Rojas-Martínez R, et al. The non-synonymous Arg230Cys variant (R230C) of the ATP-binding cassette transporter A1 is associated with low HDL cholesterol concentrations in Mexican adults: a population based nation wide study. Atherosclerosis. 2011;216:146–50. doi: 10.1016/j.atherosclerosis.2010.10.049. [DOI] [PubMed] [Google Scholar]
- 38.The SIGMA Type 2 Diabetes Consortium Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature. 2014;506:97–101. doi: 10.1038/nature12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenthal E, Ranchalis J, Crosslin DR, et al. Joint linkage and association analysis with exome sequence data implicates SLC25A40 in hypertriglyceridemia. American J Hum Genetics. 2013;93:1035–45. doi: 10.1016/j.ajhg.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]