Abstract
The activation of α2-adrenoceptors with bilateral injections of moxonidine (α2-adrenoceptor and imidazoline receptor agonist) into the lateral parabrachial nucleus (LPBN) increases 1.8% NaCl intake induced by treatment with furosemide (FURO) + captopril (CAP) subcutaneously. In the present study, we analyzed licking microstructure during water and 1.8% NaCl intake to investigate the changes in orosensory and postingestive signals produced by moxonidine injected into the LPBN. Male Sprague-Dawley rats were treated with FURO + CAP combined with bilateral injections of vehicle or moxonidine (0.5 nmol/0.2 ll) into the LPBN. Bilateral injections of moxonidine into the LPBN increased FURO + CAP-induced 1.8% NaCl intake, without changing water intake. Microstructural analysis of licking behavior found that this increase in NaCl intake was a function of increased number of licking bursts from 15 to 75 min of the test (maximum of 49 ± 9 bursts/bin, vs. vehicle: 2 ± 2 bursts/bin). Analysis of the first 15 min of the test, when most of the licking behavior occurred, found no effect of moxonidine on the number of licks/burst for sodium intake (24 ± 5 licks/burst, vs. vehicle: 27 ± 8 licks/burst). This finding suggests that activation of α2-adrenoceptors in the LPBN affects postingestive signals that are important to inhibit and limit sodium intake by FURO + CAP-treated rats.
Keywords: parabrachial nucleus, α2-adrenoreceptors, postingestive, sodium intake
INTRODUCTION
Fluid intake is controlled by orosensory and postingestive Q signals. The orosensory properties of the consumed fluid are detected at the beginning of ingestion, when the fluid comes in contact with the mouth, especially the tongue. The type of solution, previous experience and the psychological state of the animal greatly impact the subsequent behavior. If the solution is palatable and the animal is driven to consume the fluid, the ingestion continues. As the solution is ingested, however, the ingested liquid evokes postingestive signals that contribute to the termination of intake (i.e., satiety). Accordingly, the total amount ingested during a meal is a function of the activation of orosensory and postingestive signals that act in the brain to control ingestive behavior (Davis et al., 1998).
The study of ingestive behavior was greatly facilitated by the invention of the lickometer (originally named a “drinkometer”) by Stellar and Hill (1952). Analogous devices have been used many times since then to provide precise measures of drinking behavior. Of most relevance to the present report, these studies characterized rat drinking behavior, which occurs in bursts of licks separated by a brief pause. Indeed, work by several groups has shown that the size of each burst (i.e., the number of licks per burst) and the number of bursts in a bout of drinking are related to the orosensory and postingestive feedback, respectively, of the consumed substance (Davis and Smith, 1990; Davis and Perez, 1993; Baird et al., 2006). Although the earliest studies ascribing differences in licking patterns to the different types of feedback focused mainly on nutritive substances (e.g., sucrose), the studies by Davis et al. (2002) and Wirtshafter et al. (2012) suggest that the framework is equally applicable to studies of water and saline solutions.
The lateral parabrachial nucleus (LPBN) is an important brain structure for the control of water and sodium intake. The LPBN receives ascending projections from the area postrema and the medial portion of the nucleus of the solitary tract (AP/mNTS), which are in turn innervated by afferents from arterial baroreceptors, cardiopulmonary receptors, gustatory receptors and other visceral receptors that influence water and sodium intake (Norgren, 1981; Lanca and van der Kooy, 1985; Herbert et al., 1990; Johnson and Thunhorst, 1997; Johnson, 2007). Because the LPBN receives both orosensory and postingestive signals from the periphery, it is unclear how precisely the LPBN is involved in the control of intake.
A variety of approaches including blockade of serotonin (5-HT), cholecystokinin (CCK), corticotrophin release factor (CRF), or glutamate receptors, or activation of α2-adrenoceptors in the LPBN, increase sodium intake produced by the combination of the diuretic furosemide (FURO) and the angiotensin-converting enzyme (ACE) inhibitor captopril (CAP). This suggests the existence of important inhibitory mechanisms in the LPBN that control sodium intake (Menani et al., 1996, 1998; Menani and Johnson, 1998; Fratucci De Gobbi et al., 2001; Andrade et al., 2004; De Castro e Silva et al., 2006; Gasparini et al., 2009). In addition to the increase in intake, activation of LPBN α2-adrenoceptors by injection of moxonidine (an α2-adrenoceptorand imidazoline receptor agonist) reduces aversive responses and increases the ingestive responses to an intra-oral infusion of sodium (Andrade et al., 2011). It remains unknown, however, which signals involved in the control of sodium intake are modified by the activation of the α2-adrenoceptors in the LPBN. To address this open question, the present study activated α2-adrenoceptors by injections of moxonidine into the LPBN in FURO + CAP-treated rats and used lickometer recordings and microstructural analysis of licking behavior to test the hypotheses that changes in orosensory and/or post-ingestive feedback underlie the behavioral effects of the treatments.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague–Dawley rats (n = 10) weighing 280−320 g were purchased from Harlan Laboratories (Indianapolis, IN, USA). The animals were housed individually in hanging stainless steel cages in a room with controlled temperature (23 ± 2 °C) and humidity (55 ± 10%). Lights were on from 7:00 am to 7:00 pm. Harlan Teklad global rat chow (Harlan Teklad, Madison, WI, USA), tap water, and 1.8% NaCl were available ad libitum except where noted below. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo, and the handling and care of animals was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Brain surgery
Rats were anesthetized with ketamine (70 mg/kg of body weight i.p.) and xylazine (5 mg/kg of body weight i.m.) and placed in a stereotaxic instrument. The skull was positioned to have the bregma and lambda at the same horizontal level. Stainless steel cannulas (12 × 0.6 mm o.d.) were implanted bilaterally in the LPBN (coordinates: 9.7 mm caudal to bregma, 2.1 mm lateral to midline and 4.2 mm bellow dura mater). The tips of the cannulas were positioned at a point 2 mm above the LPBN. The cannulas were fixed to the cranium using dental acrylic resin and jeweler screws. At the end of the surgery, the animals received an intramuscular injection of the analgesic carprofen (5 mg/kg of body weight). After surgery, rats were allowed to recover for one week before starting water and NaCl intake tests.
Drugs and injections
All drugs were purchased from Sigma Chemical Co., St. Louis, MO, USA. Moxonidine (0.5 nmol/0.2 μl) or vehicle (propyleneglycol: water, 2:1) was injected into the LPBN. Furosemide (10 mg/kg of body weight) and captopril (5 mg/kg of body weight) were injected sc (subcutaneous). The doses of the drugs used were based on previous studies that injected these drugs centrally or peripherally (Menani and Johnson, 1998; Menani et al., 2000; Andrade et al., 2004). The injections into the LPBN were made using 5-μl Hamilton syringes connected by water-filled polyethylene tubing (PE-10) to injector cannulas (0.3 mm o.d.). The injector cannulas were 2 mm longer than the guide cannulas.
Lick measures and analyses
The cages used for lickometer analysis were modified hanging wire cages (Unifab Corp., Portage, MI, USA). Fluid was provided in glass bottles (237 ml) equipped with rubber stoppers and stainless steel drinking spouts (Girton Manufacturing Company, Inc., Millville, PA, USA). The cages were modified so that rats were required to lick through an opening in a stainless steel plate to minimize false licks (when a rat completes the circuit by touching the spout with something other than its tongue). Licks were recorded using a custom contact lickometer (Psychology electronics shop, University of Pennsylvania, Philadelphia, PA, USA). Data acquisition and post-acquisition processing was conducted in a MATLAB (MathWorks, Natick, MA, USA) software environment before being ported to Microsoft Excel for final analysis. All analyses defined a burst as a series of at least 2 licks with a maximum interlick interval (ILI) of 1 s.
Water and 1.8% NaCl intake tests
Water and 1.8% NaCl intake were induced by sc injections of FURO (10 mg/kg of body weight) + CAP (5 mg/kg of body weight). Immediately after the treatment with FURO + CAP, rats were maintained without water or 1.8% NaCl for 1 h. After this period, water and 1.8% NaCl were offered to the animals and the intake was recorded for 3 h. Vehicle or moxonidine were injected into LPBN 15 min before the animal access to water and 1.8% NaCl. During the tests, rats had no access to food.
Total intake was determined by weighing the bottles before and after the experiment. Rats then had access to chow, 1.8% NaCl and water for 48 h before starting another test.
Histology
At the end of the experiments, the animals received bilateral injections of 2% Evans Blue solution (0.2 μl) into LPBN. They then were anesthetized with ketamine (70 mg/kg of body weight i.p.) and xylazine (5 mg/kg of body weight i.p.) and perfused transcardially with 10% formalin. The brains were removed and fixed in 10% formalin for at least 2 days. Brains then were frozen, cut in 50-μm sections, stained with Cresyl Violet and analyzed by light microscopy to confirm the sites of the injections into the LPBN.
Statistical analysis
The results were analyzed by a one-way or two-way repeated measures ANOVA (analysis of variance) (counterbalanced design tests). Statistically significant (p < 0.05) main or interaction effects were further probed using Newman–Keuls post hoc tests. The results were reported as means ± S.E.M.
A total of six rats with injections correctly placed in the LPBN were used in the statistical analysis, except for the analyses of burst size and ILI. Rats that had no licks (and therefore no definable burst size or ILI) were excluded from these analyses.
Experimental protocol
Water and 1.8% NaCl intake and lick measures in rats treated with FURO + CAP combined with moxonidine into LPBN
Rats with bilateral stainless steel guide-cannulas implanted into LPBN were treated with FURO (10 mg/kg b. wt.) combined with CAP (5 mg/kg b. wt.) s.c. Moxonidine (0.5 nmol/0.2 μl) or vehicle was bilaterally injected into the LPBN 45 min after FURO + CAP-treatment. Water and 1.8% NaCl intake and licks were measured during 180 min, starting 15 min after the LPBN injections.
Rats were submitted to two tests. In the first test, after the treatment with FURO + CAP, half of rats received moxonidine injections into the LPBN and the remaining animals received vehicle injections into the LPBN. In the next test, rats received the same treatments into the LPBN in a counterbalanced design.
RESULTS
Histological analysis
LPBN injection sites were centered in the central lateral and dorsal lateral portions of the LPBN [see Fulwiler and Saper (1984) for definitions of the LPBN subnuclei] (Fig. 1). The sites of the injections into the LPBN in the present study were similar to those in previous studies using injections of methysergide, moxonidine, or noradrenaline into the LPBN (Menani and Johnson, 1995, 1998; Menani et al., 1996, 2000; Andrade et al., 2004; Gasparini et al., 2009).
Fig. 1.

Photomicrograph of a coronal section of a rat brain showing the injection sites into the LPBN. SCP, superior cerebellar peduncle.
In six rats, bilateral injections were correctly placed in the LPBN, whereas in four rats the injections did not reach the LPBN bilaterally. In two rats with misplaced injections, the injections were correctly placed into the LPBN in one side and in the other side the injection was just above the LPBN or a little lateral to the LPBN. In the two other rats the misplaced injections were located caudal to the LPBN. The ingestion of 1.8% NaCl and water by the rats with misplaced injections was also analyzed and presented.
Microstructural analyses of the ingestion of water and 1.8% NaCl in rats treated with FURO + CAP sc combined with injections of moxonidine into the LPBN
Bilateral injections of moxonidine (0.5 nmol/0.2 μl) into the LPBN increased FURO + CAP-induced 1.8% NaCl intake [F(1,5) = 26.94; p < 0.05] (Fig. 2A), without changing water intake [F(1,5) = 0.16; p > 0.05] (Fig. 2B). In rats with misplaced injections, moxonidine did not significantly change 1.8% NaCl intake (16 ± 7, vs. vehicle: 5 ± 2 ml/180 min, n = 4) [F(1, 3) = 3.51; p > 0.05] or water intake (6.5 ± 2.2, vs. vehicle: 7.1 ± 0.9 ml/180 min) [F(1, 3) = 0.05; p > 0.05], which confirms the specificity of the LPBN as the site in which moxonidine injections affect NaCl intake.
Fig. 2.
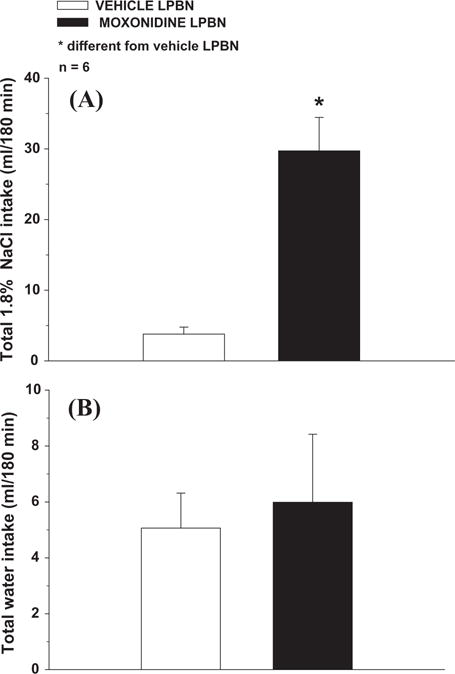
Total (A) 1.8% NaCl intake and (B) water intake induced by FURO + CAP in rats treated with bilateral injections of moxonidine (0.5 nmol/0.2 μl) or vehicle into the LPBN. The results are reported as means ± SEM. n = number of animals.
For 1.8% NaCl intake, the injections of moxonidine into the LPBN increased the number of licks/bin from 15 to 60 min of the test as indicated by the significant differences between treatments [F(1,5) = 20.83; p < 0.05] and interaction between treatment and time [F(11,55) = 6.67; p < 0.05] (Fig. 3A) and the number of bursts/bin from 15 to 75 min as indicated by the significant differences between treatments [F(1,5) = 15.60; p < 0.05] and interaction between treatment and time [F(11,55) = 6.04; p < 0.05] (Fig. 4A). However, moxonidine injections into the LPBN produced no effect on the number of licks/burst, in the period that was possible to perform this analysis (first 15 min of the test, the only period that rats treated with saline into the LPBN ingested NaCl) [F(1,9) = 0.16; p > 0.05] (Fig. 5A). No effect of this treatment on ILI was also found (0.20 ± 0.01, vs. vehicle: 0.18 ± 0.01 s) [F(1,9) = 2.73; p > 0.05] (n = 5 and 6 for vehicle and moxonidine into LBPN, respectively).
Fig. 3.

Number of licks/bin for (A) 1.8% NaCl intake and (B) water intake induced by FURO + CAP in rats treated with bilateral injections of moxonidine (0.5 nmol/0.2 μl) or vehicle into the LPBN. The results are reported as means ± SEM. n = number of animals.
Fig. 4.

Number of bursts/bin for (A) 1.8% NaCl intake and (B) water intake induced by FURO + CAP in rats treated with bilateral injections of moxonidine (0.5 nmol/0.2 μl) or vehicle into the LPBN. The results are reported as means ± SEM. n = number of animals.
Fig. 5.
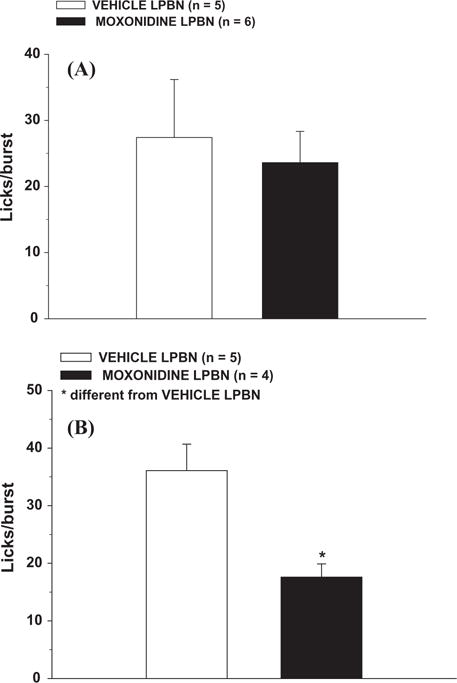
Number of licks/burst in the first 15 min of the test for (A) 1.8% NaCl intake and (B) water intake induced by FURO + CAP in rats treated with bilateral injections of moxonidine (0.5 nmol/0.2 μl) or vehicle into the LPBN. The results are reported as means ± SEM. n = number of animals.
For water intake, the injections of moxonidine into the LPBN did not affect the number of licks/bin [F(1,5) = 0.95; p > 0.05] (Fig. 3B), the number of bursts/bin [F(1,5) = 1.25; p > 0.05] (Fig. 4B), or the ILI (0.18 ± 0.03, vs. vehicle: 0.18 ± 0.01 s) [F(1,8) = 0.87 p > 0.05] (n = 5). However, moxonidine injections into LPBN decreased the number of licks/burst in the first 15 min of the test [F(1,7) = 10.85 p < 0.05] (Fig. 5B).
DISCUSSION
As previously demonstrated (Andrade et al., 2004), moxonidine injected into the LPBN strongly increased FURO + CAP-induced 1.8% NaCl intake, without significant changes in water intake. The present studies extend this finding to show that injections of moxonidine into the LPBN in rats treated with FURO + CAP increased the number of licks and the number of licking bursts for NaCl intake in the first hour of the test. However moxonidine injections into LPBN did not modify the size of licking bursts for NaCl. Concerning water intake, moxonidine did not change total water intake, licks, or the number of licking bursts, but it decreased burst size in the first 15 min of the test.
Previous studies provide evidence that burst size (licks per burst) is considered to be related to gustatory signals because it increases linearly with increases in the concentration of palatable solutions and decreases with conditioned aversive substances (Davis and Levine, 1977; Davis and Smith, 1990; Davis and Perez, 1993; Davis et al., 1998, 2002; Spector and St John, 1998; Smith, 2001; Baird et al., 2005, 2006; Boughter et al., 2007; Wirtshafter et al., 2012). On the other hand, treatments that modify post-ingestive feedback are associated with changes in the number of bursts within a drinking test. This is especially evident in tests of sham feeding. When the ingested solution is not allowed to accumulate in the gut, the observed increases in intake are a function of the rat taking more bursts (burst number) without any differences in the size of the bursts (Davis and Smith, 1992). Based on these findings, the present results suggest that injections of moxonidine into the LPBN affect postingestive signals involved in the control of sodium intake, but not orosensory signals. In contrast to the effect of moxonidine on sodium intake, that selectively affected burst number, we also observed a change in the size of licking bursts for water. This might suggest that this treatment changes orosensory signals for water intake, but we urge caution before drawing this conclusion because the effect of moxonidine on water intake patterns may be indirect. Indeed, the effect on water intake may be more directly related to the strong preference for 1.8% NaCl in these rats and this marked change in preference may cause the change in water intake microstructure. Nevertheless, the effect of moxonidine on sodium intake, likely due to an inhibition of postingestive signals of satiety, was clearly shown by the results.
Gustatory signals reach the brain through three main pathways: vagal innervation (X) of the pharynx, glossopharyngeal nerve fibers (IX) from the back of the tongue and facial nerve fibers (VII) from the palate and the front of the tongue. It is through the latter that the taste of NaCl reaches the CNS (Frank et al., 1983). The section of the chorda tympani branch of the facial nerve, but not the glossopharyngeal nerve, drastically interrupts the discrimination of NaCl and other chlorides (St John and Spector, 1998), whereas discrimination against other flavors remains intact (Breslin et al., 1993). A reduced sodium appetite with a decreased number of licks and reduced burst number was described in rats with the chorda tympani section and the absence of postingestive signals due to the presence of a fistula into the stomach. Even with the postingestive signal minimized, the reduction of the excitatory signals by the transection of the chorda tympani result in only a modest sodium appetite probably due to some remaining gustatory afferents (Frankmann et al., 1996). Although the authors did not discuss the interference of the chorda tympani nerve transection, the reduced number of bursts suggests that the transection of the chorda tympani may influence postingestive signals.
The fibers of the chorda tympani nerve synapse on neurons in the rostral NTS (rNTS) (Hamilton and Norgren, 1984). In rodents, cells in the rNTS transmit this information to the PBN, primarily in the waist and caudal medial PBN subnuclei, and also send efferents more rostrally to parts of the medial, external medial, ventral, and central lateral PBN (Norgren and Leonard, 1971; Norgren and Pfaffmann, 1975; Herbert et al., 1990; Karimnamazi et al., 2002). These projections are of particular importance for the present studies because the relevant subnuclei express α2-adrenoreceptors. Indeed, the areas of highest density α2-adrenoreceptors are found in the external and lateral portions of the PBN and caudally within the waist area (Herbert and Flugge, 1995). After reaching the PBN, gustatory neural signals are relayed to thalamic regions, such as the ventral posteromedial parvocellular thalamic nucleus (VPMpc), which projects to relevant cortical regions (Norgren, 1995). Lesions in the NTS, PBN or VPMpc prevent the changes in flavor observed in intact animals after sodium depletion (Flynn et al., 1991). PBN and NTS lesions also abolish sodium appetite induced by sodium depletion (Flynn et al., 1991). These previous studies suggest that the PBN is important for the changes in taste reactivity to NaCl that occur after sodium depletion.
The LPBN is also reciprocally connected to other forebrain areas such as the paraventricular nucleus of the hypothalamus, the central nucleus of the amygdala (CeA), and the median preoptic nucleus (Ciriello et al., 1984; Fulwiler and Saper, 1984; Lanca and van der Kooy, 1985; Shapiro and Miselis, 1985; Herbert et al., 1990; Jhamandas et al., 1992, 1996; Krukoff et al., 1993). Recent results showed that bilateral electrolytic lesions of the CeA abolished the increase of 1.8% NaCl intake produced by bilateral injections of moxonidine into LPBN in FURO + CAP-treated rats (Andrade-Franze et al., 2010). These connections certainly play an important role in taste/ingestive processing.
The present results suggest, however, that the activation of α2-adrenoreceptors in the LPBN may not modify the gustatory signals that reach the CNS facilitating sodium intake, at least in the first 15 min of the test, in FURO + CAP-treated animals. Prior study from Andrade et al. (Andrade et al., 2011) showed that moxonidine increased ingestive responses to oral infusion of hypertonic saline in rats treated with FURO + CAP just 30 min after animals had access to 1.8% NaCl solution. In the present study was not possible to analyze burst size beyond the first 15 min because most animals, especially in the control group, had stopped drinking. Nevertheless, the lack of an effect on burst size and the presence of an effect of burst number, strongly suggests that moxonidine selectively affects postingestive feedback with no more than a minimal effect on the orosensory component involved on sodium intake.
Postingestive signals, like those from visceral osmoreceptors and gastric stretch receptors, play an important role in the control of sodium intake (Levy and McCutcheon, 1974; Wolf et al., 1984). These signals activate vagal fibers that innervate the caudal medial NTS and also the AP, and both send projections to parts of the LPBN, including the inner portion of the external LPBN, the central and dorsolateral subnuclei, and the “waist” area (Herbert et al., 1990). These projections are likely involved in any changes in postingestive feedback that are suggested by the change in burst number after moxonidine treatment in the present studies. Accordingly, future research investigating the effect of moxonidine on these specific projections could provide insight into the mechanisms underlying the observed responses.
Intragastric infusion of NaCl is sufficient to satiate sodium appetite in rats that received formalin injections (Levy and McCutcheon, 1974). In addition, signals from the liver might also be involved in the control of sodium intake (Tordoff et al., 1987). Our current working hypothesis is that activation of α2-adrenoceptors in the LPBN removes inhibition of sodium intake, thereby increasing intake induced by FURO + CAP. The net result of this change is a reduction in the satiety normally brought about by the ingestion of sodium. Although we have not isolated orosensory factors from postingestive factors in the past, the present study that found an effect of moxonidine on burst number supports this hypothesis and indicates that moxonidine in the LPBN may reduce the postingestive feedback that contributes to sodium intake termination.
CONCLUSION
The present results suggest that postingestive signals are modified by the activation of α2 adrenoceptors into the LPBN contributing to a high intake of hypertonic NaCl.
Abbreviations
- 5-HT
serotonin
- ACE
angiotensin-converting enzyme
- ANG II
angiotensin II
- ANOVA
analysis of variance
- AP
area postrema
- CAP
captopril
- CCK
cholecystokinin
- CeA
central nucleus of the amygdale
- FURO
furosemide
- i.c.v.
intracerebroventricularly
- LPBN
lateral parabrachial nucleus
- mNTS
medial portion of the nucleus of the solitary tract
- NTS
nucleus of the solitary tract
- rNTS
rostral NTS
- sc
subcutaneous
- SCP
superior cerebellar peduncle
- SFO
subfornical organ
- VPMpc
ventral posteromedial parvocellular thalamic nucleus
References
- Andrade-Franze GM, Andrade CA, De Luca LA, Jr, De Paula PM, Menani JV. Lateral parabrachial nucleus and central amygdala in the control of sodium intake. Neuroscience. 2010;165:633–641. doi: 10.1016/j.neuroscience.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Andrade CA, Andrade-Franze GM, De Luca LA, Jr, Johnson AK, Menani JV. Changes in taste reactivity to intra-oral hypertonic NaCl after lateral parabrachial injections of an alpha2-adrenergic receptor agonist. Physiol Behav. 2011;104:702–708. doi: 10.1016/j.physbeh.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Andrade CA, Barbosa SP, De Luca LA, Jr, Menani JV. Activation of alpha2-adrenergic receptors into the lateral parabrachial nucleus enhances NaCl intake in rats. Neuroscience. 2004;129:25–34. doi: 10.1016/j.neuroscience.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Baird JP, Gray NE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci. 2006;120:937–951. doi: 10.1037/0735-7044.120.4.937. [DOI] [PubMed] [Google Scholar]
- Baird JP, St John SJ, Nguyen EA. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci. 2005;119:983–1003. doi: 10.1037/0735-7044.119.4.983. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, Baird JP, Bryant J, St John SJ, Heck D. C57BL/6J and DBA/2J mice vary in lick rate and ingestive microstructure. Genes Brain Behav. 2007;6:619–627. doi: 10.1111/j.1601-183X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Breslin PA, Kaplan JM, Spector AC, Zambito CM, Grill HJ. Lick rate analysis of sodium taste-state combinations. Am J Physiol. 1993;264:R312–318. doi: 10.1152/ajpregu.1993.264.2.R312. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Lawrence D, Pittman QJ. Electrophysiological identification of neurons in the parabrachial nucleus projecting directly to the hypothalamus in the rat. Brain Res. 1984;322:388–392. doi: 10.1016/0006-8993(84)90140-9. [DOI] [PubMed] [Google Scholar]
- Davis JD, Levine MW. A model for the control of ingestion. Psychol Rev. 1977;84:379–412. [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264:R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Davis JD, Riley PK, Peters CW, Rand KH. A comparison of ligase chain reaction to polymerase chain reaction in the detection of Chlamydia trachomatis endocervical infections. Infect Dis Obstet Gynecol. 1998;6:57–60. doi: 10.1002/(SICI)1098-0997(1998)6:2<57::AID-IDOG5>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. Am J Physiol. 1990;259:R1228–1235. doi: 10.1152/ajpregu.1990.259.6.R1228. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–228. [PubMed] [Google Scholar]
- Davis JD, Smith GP, McCann DP. The control of water and sodium chloride intake by postingestional and orosensory stimulation in water-deprived rats. Physiol Behav. 2002;75:7–14. doi: 10.1016/s0031-9384(01)00626-6. [DOI] [PubMed] [Google Scholar]
- De Castro e Silva E, Fregoneze JB, Johnson AK. Corticotropin-releasing hormone in the lateral parabrachial nucleus inhibits sodium appetite in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1136–R1141. doi: 10.1152/ajpregu.00075.2003. [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 1983;50:941–960. doi: 10.1152/jn.1983.50.4.941. [DOI] [PubMed] [Google Scholar]
- Frankmann SP, Sollars SI, Bernstein IL. Sodium appetite in the sham-drinking rat after chorda tympani nerve transection. Am J Physiol. 1996;271:R339–345. doi: 10.1152/ajpregu.1996.271.2.R339. [DOI] [PubMed] [Google Scholar]
- Fratucci De Gobbi JI, De Luca LA, Jr, Johnson AK, Menani JV. Interaction of serotonin and cholecystokinin in the lateral parabrachial nucleus to control sodium intake. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1301–1307. doi: 10.1152/ajpregu.2001.280.5.R1301. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Gasparini S, de Luca LA, Jr, Colombari DS, de Paula PM, Barbosa SP, Menani JV. Adrenergic mechanisms of the Kolliker-Fuse/A7 area on the control of water and sodium intake. Neuroscience. 2009;164:370–379. doi: 10.1016/j.neuroscience.2009.08.048. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Herbert H, Flugge G. Distribution of alpha 2-adrenergic binding sites in the parabrachial complex of the rat. Anat Embryol (Berl) 1995;192:507–516. doi: 10.1007/BF00187181. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Harris KH, Petrov T, Krukoff TL. Characterization of the parabrachial nucleus input to the hypothalamic paraventricular nucleus in the rat. J Neuroendocrinol. 1992;4:461–471. doi: 10.1111/j.1365-2826.1992.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Petrov T, Harris KH, Vu T, Krukoff TL. Parabrachial nucleus projection to the amygdala in the rat: electrophysiological and anatomical observations. Brain Res Bull. 1996;39:115–126. doi: 10.1016/0361-9230(95)02084-5. [DOI] [PubMed] [Google Scholar]
- Johnson AK. The sensory psychobiology of thirst and salt appetite. Med Sci Sports Exerc. 2007;39:1388–1400. doi: 10.1249/mss.0b013e3180686de8. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol. 1997;18:292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 1993;30:163–172. doi: 10.1016/0361-9230(93)90054-f. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, van der Kooy D. A serotonin-containing pathway from the area postrema to the parabrachial nucleus in the rat. Neuroscience. 1985;14:1117–1126. doi: 10.1016/0306-4522(85)90281-7. [DOI] [PubMed] [Google Scholar]
- Levy CJ, McCutcheon B. Importance of postingestional factors in the satiation of sodium appetite in rats. Physiol Behav. 1974;13:621–625. doi: 10.1016/0031-9384(74)90231-5. [DOI] [PubMed] [Google Scholar]
- Menani JV, Colombari DS, Beltz TG, Thunhorst RL, Johnson AK. Salt appetite: interaction of forebrain angiotensinergic and hindbrain serotonergic mechanisms. Brain Res. 1998;801:29–35. doi: 10.1016/s0006-8993(98)00530-7. [DOI] [PubMed] [Google Scholar]
- Menani JV, De Luca LA, Jr, Thunhorst RL, Johnson AK. Hindbrain serotonin and the rapid induction of sodium appetite. Am J Physiol Regul Integr Comp Physiol. 2000;279:R126–R131. doi: 10.1152/ajpregu.2000.279.1.R126. [DOI] [PubMed] [Google Scholar]
- Menani JV, Johnson AK. Lateral parabrachial serotonergic mechanisms: angiotensin-induced pressor and drinking responses. Am J Physiol. 1995;269:R1044–R1049. doi: 10.1152/ajpregu.1995.269.5.R1044. [DOI] [PubMed] [Google Scholar]
- Menani JV, Johnson AK. Cholecystokinin actions in the parabrachial nucleus: effects on thirst and salt appetite. Am J Physiol. 1998;275:R1431–1437. doi: 10.1152/ajpregu.1998.275.5.r1431. [DOI] [PubMed] [Google Scholar]
- Menani JV, Thunhorst RL, Johnson AK. Lateral parabrachial nucleus and serotonergic mechanisms in the control of salt appetite in rats. Am J Physiol. 1996;270:R162–168. doi: 10.1152/ajpregu.1996.270.1.R162. [DOI] [PubMed] [Google Scholar]
- Norgren R. Brain mechanisms of sensation. New York: Wiley Medical Publishers; 1981. The central organization of the gustatory and visceral systems in the nucleus of the solitary tract. [Google Scholar]
- Norgren R. Gustatory system. In: Paxinos G, editor. The rat nervous system. Australia: Academic Press; 1995. [Google Scholar]
- Norgren R, Leonard CM. Taste pathways in rat brainstem. Science. 1971;173:1136–1139. doi: 10.1126/science.173.4002.1136. [DOI] [PubMed] [Google Scholar]
- Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res. 1975;91:99–117. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol. 1985;234:344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- Smith GP. John Davis and the meanings of licking. Appetite. 2001;36:84–92. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- Spector AC, St John SJ. Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol. 1998;274:R1687–R1703. doi: 10.1152/ajpregu.1998.274.6.R1687. [DOI] [PubMed] [Google Scholar]
- St John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 1998;18:4353–4362. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar E, Hill JH. The rats rate of drinking as a function of water deprivation. J Comp Physiol Psychol. 1952;45:96–102. doi: 10.1037/h0062150. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Schulkin J, Friedman MI. Further evidence for hepatic control of salt intake in rats. Am J Physiol. 1987;253:R444–449. doi: 10.1152/ajpregu.1987.253.3.R444. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Covelo IR, Salija I, Stratford TR. Effects of muscimol in the nucleus accumbens shell on salt appetite and sucrose intake: a microstructural study with a comment on the sensitization of salt intake. Behav Neurosci. 2012;126:699–709. doi: 10.1037/a0029641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Schulkin J, Simson PE. Multiple factors in the satiation of salt appetite. Behav Neurosci. 1984;98:661–673. doi: 10.1037//0735-7044.98.4.661. [DOI] [PubMed] [Google Scholar]


