Abstract
OBJECTIVE
To assess national trends in ectopic pregnancy incidence among assisted reproductive technology users and identify risk factors associated with ectopic pregnancy.
METHODS
We identified 553,577 pregnancies reported to the National ART Surveillance System between 2001 and 2011. Of those, 9,480 were ectopic, of which 485 were heterotopic. As a result of small numbers, ectopic and heterotopic pregnancies were combined for analysis. We assessed temporal trends in annual ectopic pregnancy rates using Poisson regression. We used log-binomial regression models with generalized estimating equations for correlated outcomes within clinics to calculate unadjusted and adjusted risk ratios for the association between ectopic pregnancy and selected patient characteristics and treatment factors.
RESULTS
The rate of ectopic pregnancy declined from 2.0% (n5735, 95% confidence interval [CI] 1.9–2.2) in 2001 to 1.6% (n=968, 95% CI 1.5–1.7) in 2011 (P for trend ,.001). The ectopic pregnancy rate ranged from 2.0% (n=7,469, 95% CI 1.9–2.0) for fresh, nondonor cycles to 1.0% (n=641, 95% CI 0.9–1.1) for fresh, donor cycles. Among fresh, nondonor cycles, the rate of ectopic pregnancy was 1.6% (95% CI 1.4–1.7) when one embryo was transferred compared with 1.7% (95% CI 1.7–1.8), 2.2% (95% CI 2.1–2.3), and 2.5% (95% CI 2.4–2.6) when two, three, or four or more embryos were transferred, respectively (adjusted risk ratios 1.11, 95% CI 0.94–0.30; 1.33, 95% CI 1.12–1.56; and 1.49, 95% CI 1.25–1.78).
CONCLUSION
Ectopic pregnancy incidence after assisted reproductive technology has decreased over time, but factors such as multiple embryo transfer increase the risk of ectopic pregnancy.
Ectopic pregnancy is a leading cause of maternal morbidity and mortality with a pregnancy-related mortality of 31.9 deaths per 100,000 pregnancies.1 The use of assisted reproductive technology (ART) has traditionally been thought to increase the risk of ectopic pregnancy compared with the general population,2–4 but reported rates among the ART population vary from 0.8% to 8.6%.4–16 In studies conducted in either individual U.S. clinics or in other countries, several factors were associated with increased risk of ectopic pregnancy after ART and include tubal factor infertility, use of assisted hatching, and intracytoplasmic sperm injection, fresh compared with frozen embryo transfers, day of embryo transfer, and the hormonal milieu specific to ovarian stimulation.4–7,9–13,15–18 Interpretation and generalizability of such results is difficult because ART practices vary between clinics and across different countries.
The field of ART is rapidly changing, and there have been no published reports on the trends and correlates of ectopic pregnancy over the past decade. Thus, the objectives of this study were to use data from the National ART Surveillance System to assess changes in the incidence of ectopic pregnancy between 2001 and 2011 and to identify risk factors for ectopic pregnancy among women undergoing ART.
MATERIALS AND METHODS
We used data from the Centers for Disease Control and Prevention's National ART Surveillance System. All U.S. fertility clinics performing ART are required to report annual data on all ART procedures to the Centers for Disease Control and Prevention. Data collected include patient demographic characteristics, medical and obstetric history, infertility diagnosis, and resultant pregnancies and births, if any. The Centers for Disease Control and Prevention estimates that National ART Surveillance System captures information on more than 95% of all ART procedures performed in the United States.19
This study included transcervical embryo transfer procedures performed from January 1, 2001, to December 31, 2011, that resulted in a clinical intrauterine pregnancy, an ectopic pregnancy, or a hetero-topic pregnancy. A clinical intrauterine pregnancy was reported when ultrasonography confirmed the presence of a gestational sac within the uterus. If there was missing ultrasound data, a clinical intrauterine pregnancy was confirmed by a documented birth, spontaneous abortion, or induced abortion. An ectopic pregnancy was reported when a gestational sac was confirmed to be outside the uterus by ultrasonography or by high serial serum β-human chorionic gonadotropin values in the absence of an intrauterine pregnancy on ultrasonography. A heterotopic pregnancy was reported when a clinical intrauterine pregnancy was confirmed in combination with an ectopic pregnancy. For this study, an ectopic pregnancy was defined as a clinical ectopic pregnancy or a clinical heterotopic pregnancy.
Rates of ectopic pregnancy were calculated by dividing the total number of ectopic and heterotopic pregnancies by the sum of intrauterine, ectopic, and heterotopic pregnancies and were reported as percentages. We evaluated the trend in ectopic pregnancy incidence during the study period by calculating the ectopic pregnancy rate by year. Statistically significant trends in ectopic pregnancy rate were determined using Poisson regression.
Ectopic pregnancy rates were classified on the basis of type of in vitro fertilization-embryo transfer (IVF-ET) procedure as either fresh or frozen cycles and by oocyte donor status. A fresh cycle was reported when the oocytes were retrieved and the resultant embryos transferred during the current IVF-ET cycle. A frozen–thawed cycle was reported when cryopreserved embryos resulting from a previous cycle were thawed and transferred during the current IVF-ET cycle. Nondonor cycles were reported when oocytes were retrieved from the patient; donor cycles were reported when oocytes or embryos were obtained from a donor, but the embryos were transferred to the patient. Stratification by these two categories resulted in four types of IVF-ET cycles: 1) fresh, nondonor; 2) fresh, donor; 3) frozen–thawed, nondonor; and 4) frozen–thawed, donor. Risk ratios were calculated to determine the relative risk of ectopic pregnancy for these four types of IVF-ET cycles using fresh, nondonor cycles as the referent.
All further analyses were restricted to fresh, nondonor cycles, the most common type of IVF-ET performed, accounting for 68% of all cycles. Risk factors evaluated were patient age, race or ethnicity, number of prior ART cycles, number of prior spontaneous abortions, number of prior live births, infertility diagnosis (male factor, tubal factor, endometriosis, uterine factor, ovulatory disorder, and diminished ovarian reserve), year of ART procedure, use of assisted hatching, use of intracytoplasmic sperm injection, day of embryo transfer (day 3 or day 5, typically corresponding to cleavage or blastocyst stage embryos, respectively, or other), number of embryos transferred, number of supernumerary embryos cryopreserved, ovarian hyperstimulation syndrome, and follicle-stimulating hormone (FSH) dosage. Infertility diagnoses were not mutually exclusive. The National ART Surveillance System does not include information on previous ectopic pregnancy. The amount of missing data was less than 0.5% for all variables except for FSH dosage (2.6%) and race or ethnicity (41.2%). As a result of the large proportion of missing data for race and ethnicity, this information should be interpreted with caution because it may be prone to bias.20 To assess whether clinic volume affected ectopic pregnancy rates, we compared rates of ectopic pregnancy according to quartiles of clinic volume based on the total number of cycles in each clinic during the most recent study year, 2011. We found no evidence of differences in rates of ectopic pregnancy by clinic volume (range 1.5–1.7%, X2 P=.24) and therefore did not include this information.
We compared the distribution of these risk factors between ectopic pregnancies and intrauterine pregnancies using the X2 test at a significance level of P<.05. We used log-binomial regression models with generalized estimating equations for correlated outcomes within clinics to calculate unadjusted and adjusted risk ratios (RRs) and 95% confidence intervals (CIs) for the association between ectopic pregnancy and selected risk factors. The final adjusted model included the variables listed except for race or ethnicity and FSH dosage. Race or ethnicity was excluded from the model because of the large amount of missing data. Follicle-stimulating hormone dosage was excluded because FSH was not used for stimulation in all cycles. Data were analyzed using SAS 9.3. This research was approved by the institutional review board at the Centers for Disease Control and Prevention.
RESULTS
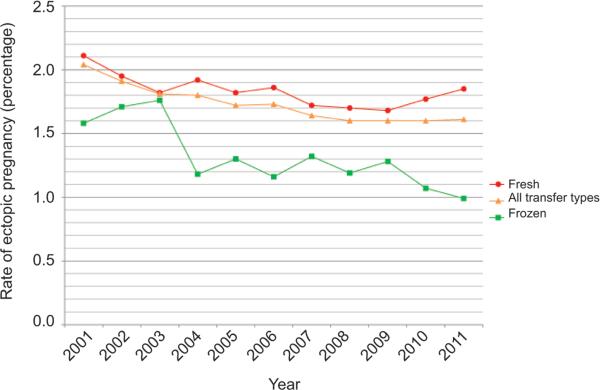
A total of 553,577 clinical intrauterine, ectopic, and heterotopic pregnancies were included in our study; of those, 9,480 (1.7%) were ectopic, including 485 (0.09%) that were heterotopic. Heterotopic pregnancy rates were 0.10% for fresh, nondonor cycles and 0.06% for all other cycle types. Heterotopic pregnancies were not further characterized as a result of small cell sizes and data suppression requirements. All further analyses included heterotopic pregnancies as ectopic pregnancies. For all transfer types combined, the ectopic pregnancy rate among women undergoing ART procedures declined from 2.0% (n5735, 95% CI 1.9–2.2) in 2001 to 1.6% (n=968, 95% CI 1.5–1.7) in 2011; decreases were noted for both frozen–thawed embryo transfer cycles and for fresh embryo transfer cycles (Fig. 1). P values for trend of ectopic pregnancy rate over time were 0.24 and 0.77 for cleavage-stage and blastocyst-stage embryo transfers, respectively, by Poisson regression (see the Appendix, available online at http://links.lww.com/AOG/A580). The ectopic pregnancy rate varied significantly by type of ART procedure performed (Table 1). Fresh, nondonor cycles had the highest ectopic pregnancy rate (2.0%, 95% CI 1.9–2.0), and fresh, donor cycles had the lowest ectopic pregnancy rate (1.0%, 95% CI 0.9–1.1).
Fig. 1.
Incidence of ectopic pregnancy by year and fresh compared with frozen embryo transfers, United States, 2001–2011. P<.001 for trend for all transfer types by Poisson regression.
Perkins. Risk of Ectopic Pregnancy After ART. Obstet Gynecol 2015.
Table 1.
Incidence of Ectopic Pregnancy by Type of In Vitro Fertilization-Embryo Transfer Procedure in the United States, 2001-2011
| IVF-ET Procedure | Total No. of Pregnancies | No. of Ectopic Pregnancies | Ectopic Pregnancy Rate (%) | RR | 95% CI |
|---|---|---|---|---|---|
| All IVF-ET procedures | 553,577 | 9,480 | 1.7 | — | — |
| Fresh, nondonor | 379,023 | 7,469 | 2.0 | Reference | Reference |
| Fresh, donor | 65,316 | 641 | 1.0 | 0.50 | 0.46–0.54 |
| Frozen-thawed, nondonor | 84,976 | 1,104 | 1.3 | 0.65 | 0.61–0.69 |
| Frozen-thawed, donor | 23,158 | 266 | 1.2 | 0.58 | 0.52–0.66 |
IVF-ET, in vitro fertilization-embryo transfer;RR, risk ratio;CI, confidence interval.
Among fresh, nondonor cycles, the proportions of various patient characteristics and ART treatment factors differed between ectopic pregnancies and intrauterine pregnancies (Table 2). Compared with intrauterine pregnancies, ectopic pregnancies were more common among women aged 35 years or older and among Asian and non-Hispanic black women. Women with ectopic pregnancies had a higher frequency of having had at least one prior ART cycle and a lower frequency of having had a prior birth compared with women with intrauterine pregnancies. Compared with intrauterine pregnancies, ectopic pregnancies were more common with tubal factor infertility, endometriosis, uterine factor infertility, and diminished ovarian reserve and less common with male factor infertility and ovulatory disorders. Assisted hatching was used more frequently and intracytoplasmic sperm injection was used less frequently among ectopic pregnancies than among intrauterine pregnancies. Ectopic pregnancies were more likely when three or more embryos were transferred per cycle and less likely when extra embryos were cryo-preserved. When FSH was used as the ovarian stimulation method, higher FSH doses were observed among ectopic pregnancies compared with intrauterine pregnancies, especially when FSH dosage was higher than 3,500 international units (38.5% compared with 30.1%, respectively; Table 2).
Table 2.
Characteristics of Ectopic Pregnancies Compared With Intrauterine Pregnancies by Patient and Assisted Reproductive Technology Treatment Factors for Fresh, Nondonor In Vitro Fertilization-Embryo Transfer Treatment Protocol, United States, 2001-2011
| Characteristic | Ectopic Pregnancy | Intrauterine Pregnancy | P (χ2) |
|---|---|---|---|
| Patient factors | |||
| Age (y) | <.001 | ||
| Younger than 30 | 938 (12.6) | 58,143 (15.7) | |
| 30-34 | 2,742 (36.7) | 141,717 (38.1) | |
| 35-37 | 1,817 (24.3) | 86,713 (23.3) | |
| 38-10 | 1,391 (18.6) | 61,370 (16.5) | |
| 41-13 | 543 (7.3) | 21,929 (5.9) | |
| 44 or older | 38 (0.5) | 1,682 (0.5) | |
| Race or ethnicity | <.001 | ||
| White (non-Hispanic) | 3,286 (44.0) | 169,290 (45.6) | |
| Black (non-Hispanic) | 313 (4.2) | 11,988 (3.2) | |
| Asian | 582 (7.8) | 19,346 (5.2) | |
| Hispanic | 356 (4.8) | 17,301 (4.7) | |
| Missing or unknown | 2,923 (39.1) | 153,208 (41.2) | |
| Prior ART cycles | <.001 | ||
| 0 | 4,253 (57.0) | 226,350 (60.9) | |
| 1 | 1,447 (19.4) | 68,721 (18.5) | |
| 2 or more | 1,765 (23.6) | 76,351 (20.6) | |
| Prior spontaneous abortions* | .01 | ||
| 0 | 5,176 (69.3) | 262,042 (70.5) | |
| 1 | 1,451 (19.4) | 71,430 (19.2) | |
| 2 or more | 842 (11.3) | 38,079 (10.3) | |
| Prior live births | |||
| 0 | 5,690 (76.4) | 264,169 (71.3) | <.001 |
| 1 | 1,392 (18.7) | 79,798 (21.5) | |
| 2 or more | 366 (4.9) | 26,415 (7.1) | |
| Infertility diagnosis | |||
| Male factor | 2,646 (35.4) | 146,582 (39.5) | <.001 |
| No male factor | 4,823 (64.6) | 224,972 (60.6) | |
| Tubal factor† | 1,682 (22.5) | 70,596 (19.0) | <.001 |
| No tubal factor | 5,787 (77.5) | 300,958 (81.0) | |
| Endometriosis | 1,094 (14.7) | 49,992 (13.5) | .003 |
| No endometriosis | 6,375 (85.4) | 321,562 (86.6) | |
| Uterine factor | 373 (5.0) | 16,676 (4.5) | .037 |
| No uterine factor | 7,096 (95.0) | 354,878 (95.5) | |
| Ovulatory disorder‡ | 1,191 (16.0) | 62,650 (16.9) | .036 |
| No ovulatory disorder | 6,278 (84.1) | 308,904 (83.1) | |
| Diminished ovarian reserve | 1,097 (14.7) | 46,683 (12.6) | <.001 |
| No diminished ovarian reserve | 6,372 (85.3) | 324,871 (87.4) | |
| Year of ART procedure | .001 | ||
| 2001-2003 | 1,862 (24.9) | 86,580 (23.3) | |
| 2004-2006 | 2,017 (27.0) | 99,264 (26.7) | |
| 2007-2009 | 2,128 (28.5) | 112,338 (30.2) | |
| 2010-2011 | 1,462 (19.6) | 73,372 (19.8) | |
| ART treatment factors | |||
| Use of assisted hatching | <.001 | ||
| No | 4,374 (58.6) | 230,295 (62.0) | |
| Yes | 3,095 (41.4) | 141,259 (38.0) | |
| Use of ICSI | .004 | ||
| No | 2,468 (33.1) | 116,900 (31.5) | |
| Yes | 4,993 (66.9) | 254,324 (68.5) | |
| Day of embryo transfer | <.001 | ||
| Cleavage stage | 4,436 (59.4) | 213,455 (57.5) | |
| Blastocyst stage | 2,397 (32.1) | 127,768 (34.4) | |
| Other | 636 (8.5) | 30,325 (8.2) | |
| No. of embryos transferred | <.001 | ||
| 1 | 410 (5.5) | 25,343 (6.8) | |
| 2 | 3,355 (44.9) | 189,084 (50.9) | |
| 3 | 2,261 (30.3) | 101,167 (27.2) | |
| 4 or more | 1,443 (19.3) | 55,958 (15.1) | |
| No. of supernumerary embryos cryopreserved | <.001 | ||
| 0 | 4,367 (58.7) | 204,354 (55.2) | |
| 1-2 | 1,088 (14.6) | 56,460 (15.3) | |
| 3-5 | 1,144 (15.4) | 62,979 (17.0) | |
| 6 or more | 847 (11.4) | 46,223 (12.5) | |
| Ovarian hyperstimulation syndrome | .019 | ||
| Absent | 7,383 (98.9) | 366,052 (98.5) | |
| Present | 86 (1.2) | 5,501 (1.5) | |
| FSH dosage (international units)§ | <.001 | ||
| 0-1,500 | 892 (12.3) | 58,640 (16.2) | |
| 1,501-2,000 | 769 (10.6) | 49,139 (13.6) | |
| 2,001-2,500 | 1,127 (15.5) | 61,360 (16.9) | |
| 2,501-3,000 | 1,037 (14.2) | 53,138 (14.7) | |
| 3,001-3,500 | 645 (8.9) | 30,776 (8.5) | |
| 3,501 or higher | 2,795 (38.5) | 109,090 (30.1) |
ART, assisted reproductive technology;ICSI, intracytoplasmic sperm injection;FSH, follicle-stimulating hormone.
Data are n (%) unless otherwise specified.
Pregnancy loss at less than 20 weeks of gestation.
Includes hydrosalpinx, tubal ligation (not reversed), and other tubal disease (not hydrosalpinx).
Includes polycystic ovary syndrome.
A total of 2.6% of data was missing because FSH was not used in all cycles.
In the adjusted analysis, being aged 30–43 years was associated with an increased risk for ectopic pregnancy compared with being younger than 30 years; although the highest rate was seen among the 41- to 43-year-olds (2.4%), the risk ratio was not significantly different from the risk ratios for women aged 30–34, 35–37, 38–40, and 44 years and older (Table 3). Having had more than one prior ART cycle was also associated with an increased risk for ectopic pregnancy (adjusted risk ratios 1.21, 95% CI 1.11–1.31). Of all infertility diagnoses, tubal factor infertility was the only one significantly associated with increased risk for ectopic pregnancy (adjusted RR 1.25, 95% CI 1.16–1.35). The rate of ectopic pregnancy also increased with increasing number of embryos transferred per cycle; the rate of ectopic pregnancy was 1.6% (95% CI 1.4–1.7) when one embryo was transferred compared with 1.7% (95% CI 1.7–1.8), 2.2% (95% CI 2.1–2.3), and 2.5% (95% CI 2.4–2.6) when two, three, or four or more embryos were transferred, respectively. The highest risk occurred when four or more embryos were transferred compared with only one embryo transferred (adjusted RR 1.49, 95% CI 1.25–1.78) and the risk ratio was significantly different compared with the risk ratios for cycles in which two or three embryos were transferred (P<.001). Having had one (adjusted RR 0.71, 95% CI 0.66–0.77) or two or more (adjusted RR 0.55, 95% CI 0.48–0.63) prior live births was negatively associated with ectopic pregnancy risk; the risk ratio for two or more prior births was statistically lower than having had one (P<.001). Finally, the risk of ectopic pregnancy was lower when male factor infertility was present compared with no male factor infertility (adjusted RR 0.85, 95% CI 0.79–0.92).
Table 3.
Risk of Ectopic Pregnancy by Patient and Assisted Reproductive Technology Treatment Factors for Fresh, Nondonor In Vitro Fertilization-Embryo Transfer, United States, 2001-2011
| Characteristic | Ectopic Pregnancy Rate (%) | Unadjusted RR (95% CI) | Adjusted RR* (95% CI) |
|---|---|---|---|
| Total | 2.0 | ||
| Patient factors | |||
| Age (y) | |||
| Younger than 30 | 1.6 | Reference | Reference |
| 30-34 | 1.9 | 1.20 (1.11-1.29) | 1.21 (1.10-1.33) |
| 35-37 | 2.1 | 1.29 (1.19-1.40) | 1.18 (1.06-1.31) |
| 38-10 | 2.2 | 1.40 (1.29-1.52) | 1.19 (1.06-1.34) |
| 41-13 | 2.4 | 1.52 (1.36-1.70) | 1.23 (1.04-1.45) |
| 44 or older | 2.2 | 1.39 (1.02-1.91) | 1.08 (0.68-1.70) |
| Prior ART cycles | |||
| 0 | 1.8 | Reference | Reference |
| 1 | 2.1 | 1.12 (1.05-1.19) | 1.05 (0.96-1.13) |
| 2 or more | 2.3 | 1.23 (1.15-1.30) | 1.21 (1.11-1.31) |
| Prior spontaneous abortions† | |||
| 0 | 1.9 | Reference | Reference |
| 1 | 2.0 | 1.03 (0.97-1.09) | 0.97 (0.90-1.05) |
| 2 or more | 2.2 | 1.12 (1.04-1.20) | 1.09 (0.99-1.20) |
| Prior live births | |||
| 0 | 2.1 | Reference | Reference |
| 1 | 1.7 | 0.77 (0.72-0.83) | 0.71 (0.66-0.77) |
| 2 or more | 1.4 | 0.63 (0.55-0.72) | 0.55 (0.48-0.63) |
| Infertility diagnosis | |||
| Male factor | 1.8 | 0.84 (0.80-0.89) | 0.85 (0.79-0.92) |
| No male factor | 2.1 | Reference | Reference |
| Tubal factor‡ | 2.3 | 1.23 (1.17-1.30) | 1.25 (1.16-1.35) |
| No tubal factor | 1.9 | Reference | Reference |
| Endometriosis | 2.1 | 1.10 (1.03-1.17) | 1.02 (0.94-1.11) |
| No endometriosis | 1.9 | Reference | Reference |
| Uterine factor | 2.2 | 1.12 (1.00-1.24) | 1.04 (0.91-1.20) |
| No uterine factor | 2.0 | Reference | Reference |
| Ovulatory disorder§ | 1.9 | 0.94 (0.88-1.00) | 0.97 (0.89-1.06) |
| No ovulatory disorder | 2.0 | Reference | Reference |
| Diminished ovarian reserve | 2.3 | 1.19 (1.11-1.28) | 1.08 (0.98-1.20) |
| No diminished ovarian reserve | 1.9 | Reference | Reference |
| Year of ART procedure | |||
| 2001-2003 | 2.1 | Reference | Reference |
| 2004-2006 | 2.0 | 0.95 (0.87-1.03) | 1.04 (0.95-1.14) |
| 2007-2009 | 1.9 | 0.88 (0.81-0.96) | 0.96 (0.87-1.05) |
| 2010-2011 | 2.0 | 0.93 (0.85-1.01) | 1.04 (0.94-1.16) |
| ART treatment factors | |||
| Use of assisted hatching | |||
| No | 1.9 | Reference | Reference |
| Yes | 2.1 | 1.15 (1.09-1.21) | 1.01 (0.94-1.08) |
| Use of ICSI | |||
| No | 2.1 | Reference | Reference |
| Yes | 1.9 | 0.93 (0.88-0.98) | 1.01 (0.94-1.09) |
| Day of embryo transfer | |||
| Cleavage stage | 2.0 | Reference | Reference |
| Blastocyst stage | 1.8 | 0.90 (0.86-0.96) | 1.07 (0.98-1.16) |
| Other | 2.1 | 1.01 (0.92-1.11) | 1.06 (0.95-1.18) |
| No. of embryos transferred | |||
| 1 | 1.6 | Reference | Reference |
| 2 | 1.7 | 1.10 (0.98-1.22) | 1.11 (0.94-1.30) |
| 3 | 2.2 | 1.37 (1.23-1.53) | 1.33 (1.12-1.56) |
| 4 or more | 2.5 | 1.58 (1.41-1.77) | 1.49 (1.25-1.78) |
| No. supernumerary embryos cryopreserved | |||
| 0 | 2.1 | Reference | Reference |
| 1-2 | 1.9 | 0.90 (0.84-0.96) | 0.95 (0.87-1.05) |
| 3-5 | 1.8 | 0.85 (0.80-0.91) | 0.96 (0.88-1.04) |
| 6 or more | 1.8 | 0.86 (0.80-0.93) | 0.99 (0.90-1.09) |
| Ovarian hyperstimulation syndrome | |||
| Absent | 2.0 | Reference | Reference |
| Present | 1.5 | 0.78 (0.61-0.99) | 0.90 (0.68-1.20) |
RR, risk ratio;CI, confidence interval;ART, assisted reproductive technology;ICSI, intracytoplasmic sperm injection.
Adjusted for all covariates in table using log-binomial regression models with generalized estimating equations for correlated outcomes within clinics.
Pregnancy loss at less than 20 weeks of gestation.
Includes hydrosalpinx, tubal ligation (not reversed), and other tubal disease (not hydrosalpinx).
Includes polycystic ovary syndrome.
DISCUSSION
The overall national ectopic pregnancy rate in our study was 1.7%, a rate similar to the general population of 2%2 and consistent with other recent studies.21–23 We found that ectopic pregnancy incidence declined over the study period with the most pronounced decline seen with frozen embryo transfers. One explanation for this decline may be that rates of tubal factor infertility are also decreasing in women undergoing ART. Findings from a recent national study suggest that diagnosis of tubal factor infertility among ART cycles declined from 26.0 to 14.8% between 2000 and 2010.24 Multiple studies have demonstrated that women with tubal factor infertility have an increased risk of ectopic pregnancy after ART compared with those with other types of infertility diagnoses,4,6,9,12,15,16,21 and we found that tubal factor infertility significantly increased the risk of ectopic pregnancy by 25%.
Declines in the transfer of three or more embryos during ART cycles may also contribute to decreasing rates of ectopic pregnancy.25 Similar to a previous report,21 we saw a dose–response relationship between the risk of ectopic pregnancy and the number of embryos transferred during an ART cycle. Although earlier studies have not demonstrated this trend,4,10,12,15 more recent investigations are consistent with our findings,21,26 likely as a result of improvements in IVF techniques resulting in improved implantation potential, and increased odds of extrauterine implantation. Although the use of ultrasound guidance during embryo transfer procedures has increased since the late 1990s, a recent meta-analysis showed no improvement in ectopic pregnancy rates with its use.27
Fresh, nondonor cycles had the highest ectopic rate in our study, a finding that is consistent with previous studies.10,18,22,28 Elevated hormone levels seen with ovarian stimulation used in fresh cycles may alter the uterine environment during embryo transfer, causing increased uterine contractility, and result in retrograde movement of the embryo into the fallopian tube. Women undergoing donor cycles and frozen–thawed cycles are less likely to have had controlled ovarian hyperstimulation and therefore less likely to have elevated hormone levels. Indeed, we found lower ectopic pregnancy rates among these cycles. Although we found a lower incidence of ovarian hyperstimulation syndrome among ectopic pregnancies than among intrauterine pregnancies, the number of observations was small and included a heterogeneous group of women. Although we did not have information on estrogen levels during each ART cycle, higher estrogen levels have been reported among ectopic pregnancies compared with intrauterine pregnancies after ART and among frozen cycles in which women received hormone replacement.5,16
In our study, older maternal age was associated with an increased risk of ectopic pregnancy in fresh, nondonor cycles. In the general population, older age is an unmodifiable risk factor for ectopic pregnancy with the highest incidence seen in the 35-year to 44-year age group. Explanations for this association include the accumulation of risk factors over time as a woman ages and changes in the anatomy and function of the fallopian tube that may predispose the embryo to extrauterine implantation.2
The primary strength of our study is that we used a large, population-based data set to analyze trends and risks for ectopic pregnancy in the ART population. It should be noted that, as a result of the large sample size of the National ART Surveillance System, even modest differences may be statistically significant although not clinically relevant. Our study is also subject to several limitations. First, the National ART Surveillance System does not collect information on patients’ history of prior ectopic pregnancy, a major risk factor for future ectopic pregnancy, and we could not link patients across the entire study period. In addition, the large amount of missing data on race and ethnicity prevented assessment of this variable. Next, data quality may have been heterogeneous during the study period because of improvements in the reporting system after 2004. Finally, our findings may not be generalizable to the general population of women because they may have different risk factors than women undergoing ART. Furthermore, pregnancies conceived using ART may be monitored more closely, which may result in more frequent identification of ectopic pregnancies than in spontaneously conceived pregnancies. It is also possible that some misclassification of ectopic pregnancies and miscarriages may have occurred.
Ectopic pregnancy can add to the emotional and financial burden of ART and further delay treatment success. Although characteristics such as maternal age and tubal factor infertility are unmodifiable risk factors for ectopic pregnancy, efforts to decrease the number of embryos transferred may further reduce ectopic pregnancy risk after ART.
Footnotes
Presented at the American Society for Reproductive Medicine Annual Meeting, October 18–22, 2014, Honolulu, Hawaii.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial Disclosure The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Grimes DA. Estimation of pregnancy-related mortality risk by pregnancy outcome, United States, 1991 to 1999. Am J Obstet Gynecol. 2006;194:92–4. doi: 10.1016/j.ajog.2005.06.070. [DOI] [PubMed] [Google Scholar]
- 2.Marion LL, Meeks GR. Ectopic pregnancy: history, incidence, epidemiology, and risk factors. Clin Obstet Gynecol. 2012;55:376–86. doi: 10.1097/GRF.0b013e3182516d7b. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583–91. doi: 10.1016/S0140-6736(05)67103-6. [DOI] [PubMed] [Google Scholar]
- 4.Strandell A, Thorburn J, Hamberger L. Risk factors for ectopic pregnancy in assisted reproduction. Fertil Steril. 1999;71:282–6. doi: 10.1016/s0015-0282(98)00441-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YL, Sun J, Su YC, Guo YH, Sun YP. Ectopic pregnancy in frozen-thawed embryo transfer: a retrospective analysis of 4,034 cycles and related factors. Syst Biol Reprod Med. 2013;59:34–7. doi: 10.3109/19396368.2012.731470. [DOI] [PubMed] [Google Scholar]
- 6.Malak M, Tawfeeq T, Holzer H, Tulandi T. Risk factors for ectopic pregnancy after in vitro fertilization treatment. J Obstet Gynaecol Can. 2011;33:617–9. doi: 10.1016/S1701-2163(16)34910-6. [DOI] [PubMed] [Google Scholar]
- 7.Jun SH, Milki AA. Assisted hatching is associated with a higher ectopic pregnancy rate. Fertil Steril. 2004;81:1701–3. doi: 10.1016/j.fertnstert.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Milki AA, Jun SH. Ectopic pregnancy rates with day 3 versus day 5 embryo transfer: a retrospective analysis. BMC Pregnancy Childbirth. 2003;3:7. doi: 10.1186/1471-2393-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesny P, Killick SR, Robinson J, Maguiness SD. Transcervical embryo transfer as a risk factor for ectopic pregnancy. Fertil Steril. 1999;72:305–9. doi: 10.1016/s0015-0282(99)00226-5. [DOI] [PubMed] [Google Scholar]
- 10.Ng EH, Yeung WS, So WW, Ho PC. An analysis of ectopic pregnancies following in vitro fertilisation treatment in a 10-year period. J Obstet Gynaecol. 1998;18:359–64. doi: 10.1080/01443619867137. [DOI] [PubMed] [Google Scholar]
- 11.Marcus SF, Brinsden PR. Analysis of the incidence and risk factors associated with ectopic pregnancy following in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:199–203. doi: 10.1093/humrep/10.1.199. [DOI] [PubMed] [Google Scholar]
- 12.Ribic-Pucelj M, Tomazevic T, Vogler A, Meden-Vrtovec H. Risk factors for ectopic pregnancy after in vitro fertilization and embryo transfer. J Assist Reprod Genet. 1995;12:594–8. doi: 10.1007/BF02212581. [DOI] [PubMed] [Google Scholar]
- 13.Pyrgiotis E, Sultan KM, Neal GS, Liu HC, Grifo JA, Rosenwaks Z. Ectopic pregnancies after in vitro fertilization and embryo transfer. J Assist Reprod Genet. 1994;11:79–84. doi: 10.1007/BF02215992. [DOI] [PubMed] [Google Scholar]
- 14.Nazari A, Askari HA, Check JH, O'Shaughnessy A. Embryo transfer technique as a cause of ectopic pregnancy in in vitro fertilization. Fertil Steril. 1993;60:919–21. [PubMed] [Google Scholar]
- 15.Dubuisson JB, Aubriot FX, Mathieu L, Foulot H, Mandelbrot L, de Jolière JB. Risk factors for ectopic pregnancy in 556 pregnancies after in vitro fertilization: implications for preventive management. Fertil Steril. 1991;56:686–90. doi: 10.1016/s0015-0282(16)54600-7. [DOI] [PubMed] [Google Scholar]
- 16.Karande VC, Flood JT, Heard N, Veeck L, Muasher SJ. Analysis of ectopic pregnancies resulting from in-vitro fertilization and embryo transfer. Hum Reprod. 1991;6:446–9. doi: 10.1093/oxfordjournals.humrep.a137356. [DOI] [PubMed] [Google Scholar]
- 17.Keegan DA, Morelli SS, Noyes N, Flisser ED, Berkeley AS, Grifo JA. Low ectopic pregnancy rates after in vitro fertilization: do practice habits matter? Fertil Steril. 2007;88:734–6. doi: 10.1016/j.fertnstert.2006.11.169. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro BS, Daneshmand ST, De Leon L, Garner FC, Aguirre M, Hudson C. Frozen-thawed embryo transfer is associated with a significantly reduced incidence of ectopic pregnancy. Fertil Steril. 2012;98:1490–4. doi: 10.1016/j.fertnstert.2012.07.1136. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. American Society for Reproductive Medicine. Society for Assisted Reproductive Technology . 2011 assisted reproductive technology fertility clinic success rates report. Atlanta (GA): U.S. Department of Health and Human Services. 2013. [Google Scholar]
- 20.Wellons MF, Fujimoto VY, Baker VL, Barrington DS, Broomfield D, Catherino WH, et al. Race matters: a systematic review of racial/ethnic disparity in Society for Assisted Reproductive Technology reported outcomes. Fertil Steril. 2012;98:406–9. doi: 10.1016/j.fertnstert.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006;107:595–604. doi: 10.1097/01.AOG.0000196503.78126.62. [DOI] [PubMed] [Google Scholar]
- 22.Decleer W, Osmanagaoglu K, Meganck G, Devroey P. Slightly lower incidence of ectopic pregnancies in frozen embryo transfer cycles versus fresh in vitro fertilization-embryo transfer cycles: a retrospective cohort study. Fertil Steril. 2014;101:162–5. doi: 10.1016/j.fertnstert.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Jun SH, Milki AA. Ectopic pregnancy rates with frozen compared with fresh blastocyst transfer. Fertil Steril. 2007;88:629–31. doi: 10.1016/j.fertnstert.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Kawwass JF, Crawford S, Kissin DM, Session DR, Boulet S, Jamieson DJ. Tubal factor infertility and perinatal risk after assisted reproductive technology. Obstet Gynecol. 2013;121:1263–71. doi: 10.1097/AOG.0b013e31829006d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni AD, Jamieson DJ, Jones HW, Jr, Kissin DM, Gallo MF, Macaluso M, et al. Fertility treatments and multiple births in the United States. N Engl J Med. 2013;369:2218–25. doi: 10.1056/NEJMoa1301467. [DOI] [PubMed] [Google Scholar]
- 26.Weigert M, Gruber D, Pernicka E, Bauer P, Feichtinger W. Previous tubal ectopic pregnancy raises the incidence of repeated ectopic pregnancies in in vitro fertilization-embryo transfer patients. J Assist Reprod Genet. 2009;26:13–7. doi: 10.1007/s10815-008-9278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abou-Setta AM, Mansour RT, Al-Inany HG, Aboulghar MM, Aboulghar MA, Serour GI. Among women undergoing embryo transfer, is the probability of pregnancy and live birth improved with ultrasound guidance over clinical touch alone? A systemic review and meta-analysis of prospective randomized trials. Fertil Steril. 2007;88:333–41. doi: 10.1016/j.fertnstert.2006.11.161. [DOI] [PubMed] [Google Scholar]
- 28.Ishihara O, Kuwahara A, Saitoh H. Frozen-thawed blastocyst transfer reduces ectopic pregnancy risk: an analysis of single embryo transfer cycles in Japan. Fertil Steril. 2011;95:1966–9. doi: 10.1016/j.fertnstert.2011.02.015. [DOI] [PubMed] [Google Scholar]