Abstract
Environmentally persistent free radicals (EPFRs) have been shown to form on the surfaces of various types of transition metal-containing particulate matter (PM), and it has been demonstrated they are capable of initiating adverse health impacts. Following sonification and solvent extraction for chemical analysis, they are partially converted to molecular species. Alcoholic solvents extracted the EPFRs with near 100% efficiency, while nonpolar hydrocarbon solvents exhibited <20% efficiency and dichloromethane exhibited 20–55% efficiency. The extracted radicals reacted in solution to form multiple molecular reaction products including catechol, hydroquinone, phenol, chlorinated phenols, dibenzo-p-dioxin, and dibenzofuran. This suggests that EPFRs in environmental samples are indistinguishable from molecular pollutants and are subject to misidentification as molecular adsorbates when traditional extraction and chemical analysis methods are employed. On the basis of these findings, the origin of the toxicity of particulate matter contaminated with toxic organic compounds should be considered for re-evaluation to include the possibility that EPFRs may be a significant contributor, and the impact of some molecular pollutants may have been overestimated.
Introduction
Research on the origins of human health impacts of fine and ultrafine particulate matter (PM) has increasingly focused on particle-induced oxidative stress in which the body “overresponds” to the inhaled particles and produces reactive oxygen species (ROS), including superoxide, hydrogen peroxide, and hydroxyl radicals, which can lead to cardiopulmonary disease and cancer (1–6). Most of this research has focused on the redox activity of iron contained in the particles, as it is known that iron(II) ions in solution can generate hydroxyl radical by disproportionation of hydrogen peroxide via the Fenton reaction (7, 8). So-called “quinones”, which actually consist of the family of hydroquinone and catechol (p-dihydroxy- and o-dihyroxybenzenes, respectively) derivatives as well as their quinones (p-diketo- and o-diketocyclohexadienes) have also been proposed as the causative agents (2, 9). These compounds are known to be formed from combustion of biomass (including cigarette tobacco) (10–12), and it is well established they are redox-active (2, 13–15).
Using electron paramagnetic resonance spectroscopy (EPR), we previously demonstrated that airborne and combustion-generated PM contain environmentally persistent free radicals (EPFRs) with spectra are consistent with that of highly redox-active semiquinone radicals (16, 17). We subsequently demonstrated that hydroquinone, catechol, and chlorinated benzenes and phenols form semiquinone-type EPFRs when the precursor is exposed to copper(II) oxide-containing particles under postflame, cool-zone combustion conditions through a mechanism of chemisorption followed by electron transfer from the adsorbate to the metal, which forms the organic radical and reduces the metal (18). The association of the radical with the particle stabilizes the radical toward decomposition and makes it resistant to reaction with molecular oxygen, that is, environmentally persistent. The particles containing these EPFRs generate DNA damage and induce pulmonary dysfunction consistent with free radical reactivity (2, 19, 20). This body of data implicates EPFRs in inducing the experimentally observed DNA damage and suggests they could be responsible for at least some of the oxidative stress-induced health impacts of exposure to airborne fine and ultrafine PM (2, 16).
A troubling issue is the determination of whether it is quinones, hydroquinones, or semiquinones actually associated with the PM. Unlike gas-phase pollutants typically collected by condensation or adsorption, which can be readily vaporized or desorbed for chemical analysis (21, 22), particle-associated pollutants can be difficult to extract and analyze (23, 24). Strong solvents, including mildly acidic or basic solutions, are typically employed to remove the chemicals most strongly bound to the PM (25, 26). This can result in chemical reactions that lead to new species and possible misidentification of the pollutant. Although this is a concern for multiple types of pollutants, quinones, hydroquinones, and semiquinones may be virtually indistinguishable by normal extraction techniques.
In this paper, we report the results of a laboratory study in which we attempted to determine how molecular hydroquinone, catechol, phenol, and chlorinated aromatics associate with metal-containing PM, whether they are extractable in various solvents, and what types of chemical reactions may be induced in the extraction/analytical process. These results suggest that EPFRs may be present in significant concentrations in environmental PM; that EPFRs may play a more significant role than molecular quinones in PM-induced oxidative stress; and that results of extraction and chemical analyses of organics in PM may be subject to misinterpretation.
Experimental Section
In order to remove the effects of varying composition of PM from different sources in the environment, this study was conducted with samples of surrogate PM prepared in our laboratory consisting of 5% CuO supported on silica. Copper is typically the second most prevalent transition metal in environmental samples after iron (27, 28). We chose copper rather than iron because we have previously established that reactions of substituted aromatic species with CuO form EPFRs (18). To facilitate detection of EPFRs and to be consistent with previous studies of the transition metal-induced formation of dioxins (29), we used 5% CuO supported on silica.
Particulate samples of 5% CuO/silica were prepared by impregnation of silica gel with copper oxide nitrate hemipentahydrate [Cu(NO3)2 · 2.5H2O] by the method of incipient wetness followed by calcination. Silica gel powder (Sigma–Aldrich, grade 923, 100–200 mesh size) was introduced into a 0.1 M solution of the copper nitrate [Sigma–Aldrich copper(II) nitrate hemipentahydrate, 99+%] in the amount for incipient wetness to occur. The solvent was evaporated by use of a Rotovap, initially dried for 24 h at room temperature, and finally dried at 120 °C for 12 h prior to calcination in air for 5 h at 450 °C. The resulting powder was ground to a mesh size of 100–120, corresponding to a mean particle diameter of 125–150 μm.
Six adsorbate precursors—monochlorobenzene (MCBz; Aldrich, 99.8% anhydrous), 1,2-dichlorobenzene (1,2-DCBz; Sigma–Aldrich, 99% HPLC grade), phenol (P; Sigma, 99.5% GC), 2-monochlorophenol (2-MCP; Aldrich, 99+%), hydroquinone (HQ; Sigma, 99+%), and catechol (CT; Sigma–Aldrich, 99.5+%) — were adsorbed onto 5% CuO/silica particles via a custom-made vacuum exposure system. Five solvents—methyl alcohol (MEA; EDM, 99.8% HPLC grade), isopropyl alcohol (IPA; Sigma–Aldrich, 99.8% HPLC grade), dichloromethane (DCM; EDM, 99.8% HPLC grade), toluene (TOL; EDM, 99.8% HPLC grade), and tert-butylbenzene (TBB; Aldrich, 99%)—were used for extraction of radicals.
The vacuum exposure system consisted of a vacuum gauge, dosing vial port, equilibration chamber, and two reactors (18). Each of the outlets from the equilibration chamber was equipped with a vacuum valve to control the adsorbate flow and vacuum. The system contained two bulb-shaped Pyrex reactors and a Suprasil quartz EPR tube as a side arm for EPR spectral measurements. The bottoms of the reactors, containing the particles to be dosed, were placed in a small tube furnace in a vertical orientation. The furnace controlled the temperature of sample pretreatment (surface cleaning and adsorbed water removal) and adsorption conditions. The lines between the sample and equilibration chamber were maintained at 80 °C to prevent condensation of the vapor on the glass walls.
Prior to chemisorption, the particle surrogates were in situ reoxidized in air at 450 °C for 1 h and evacuated to 10−4 Torr in order to remove organic contaminants. Vapors of the adsorbate were introduced at 230 °C for 5 min. Once the adsorption was completed, the system was evacuated for 2 h at 10−2 Torr to remove any residual physisorbed dosant, and the samples were cooled to room temperature. The EPR extraction cells were sealed under vacuum and subjected to EPR measurement. Solvent (1.5 mL) was then introduced into the EPR extraction cell, which was then placed in a sonicator for 1 h. The extract and residue were separated by centrifugation and subjected to EPR analysis.
Both the particulate and solvent extracts were analyzed for radicals by use of a Bruker model EMX 10/2.7 EPR spectrometer (Bruker Instruments, Billerica, MA) with dual cavities. The parameters for all solid sample spectra were microwave frequency of 9 GHz, microwave power of 2 mW, center field of 3250 G, sweep width of 2000 G, resolution of 2048 points, receiver gain of 1.0 × 104, modulation frequency of 100 kHz, modulation amplitude of 4.0 G, time constant of 163 ms, and sweep time of 167 s. Due to low concentration of radicals in the extract, the microwave power for the sample extracts was adjusted to 20 mW in order to detect any weak signal.
The extracts were also subjected to gas chromatography–mass spectrometry (GC-MS) analysis. The extract (1 μL) was injected into the helium carrier-gas stream of a chromatograph (Agilent Technologies, model 6890N) equipped with a 30 m long, 0.25 mm i.d., 0.25 μm film thickness capillary column (HP-5MS). The oven was temperature-programmed from 80 to 300 °C at a rate of 11 °C/min. The mass spectrometer (Agilent Technologies, model 9873) was operated in the range of 6–350 amu. The mass-spectral library (NIST/EPA/NIH 98, version 1.6) was used to qualitatively identify the molecular products. Semiquantitative yields of products were calculated from mass-selective detector (MSD) area counts.
Results
The chemisorption of aromatic compounds on CuO/silica surfaces, followed by electron transfer from the chemisorbed organic to the CuO, resulted in the appearance of a narrow EPR radical signal (total width ~10–20 G) on a broad Cu(II) background signal (total width ~300 G). Depending upon the precursor, these narrow lines exhibited g-factors of 2.0040–2.0065 and are indicative of formation of an organic radical. The signal was not detected on the surrogate particles that were not exposed to the adsorbate molecules. The radical signals were resistant to oxidation in air with half-lives ranging from 30 min to over an hour (18). Because the radicals were associated with the surface of the particle by at least a partial chemical bond, their extractability and chemical reaction by use of various solvents was unknown and was subjected to testing.
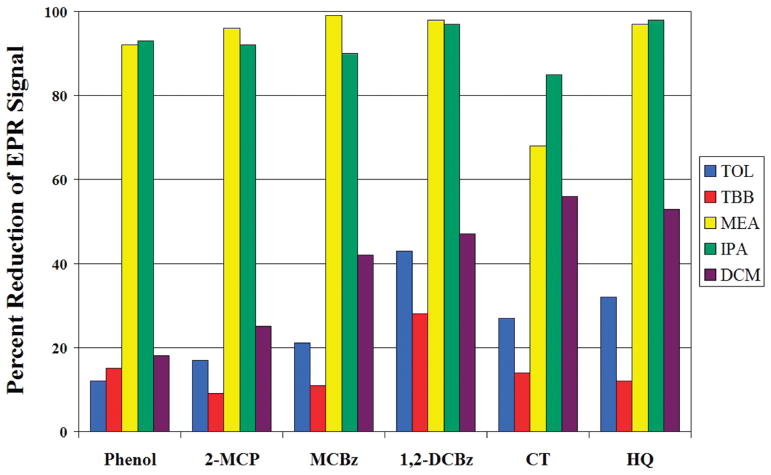
The extractabilities of the radicals were found to be dependent upon the extraction solvent (Figure 1). Extraction with polar, alcoholic solvents, IPA, and MEA resulted in >90% decrease in the radical signal in the particulate residue except for 70% for catechol. In contrast, the nonpolar hydrocarbon solvents TBB and TOL decreased the radical concentration by less than 10%, again with the exception of catechol, where the concentration decreased by 15–25%. For DCM, the decrease of the radical signal intensity in the residue after extraction varied with the radical precursor and ranged from ~20% to ~55%. Concomitant with the decrease of the radical signal in the residue after extraction (Figure 2), a weak, narrow EPR signal appeared in the extract when IPA and MEA were used as solvents that was not observed from extraction with TOL, TBB, or DCM (Supporting Information). The signal dissipated quickly, suggesting it was an extracted radical that reacted rapidly when disassociated from the particle.
FIGURE 1.
Extractability of radicals as percent reduction of EPR signal on the particle residue following solvent extraction.
FIGURE 2.
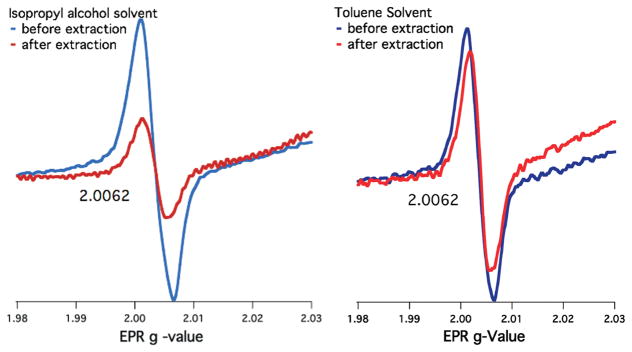
EPR spectra of particles with chemisorbed catechol before and after solvent extraction with isopropyl alcohol (example of a polar, alcoholic solvent) and toluene (example of a nonpolar, hydrocarbon solvent).
GC-MS analyses of the solvent extract identified molecular products (Table 1). In the case of the nonpolar hydrocarbon solvents, TOL and TBB, either no molecular species or only traces of parent adsorbate were detected. Multiple molecular species were detected in the IPA and MEA extract solutions. As was the case for radical extractability, DCM exhibited intermediate behavior.
TABLE 1.
Molecular Productsa Identified in Solvent Extracts by GC-MSD Analysis
| adsorbate | solvent
|
||||
|---|---|---|---|---|---|
| TOL | TBB | MEA | IPA | DCM | |
| phenol | phenol (trace) | phenol (trace) | phenol; dibenzofuran; 2-phenoxyphenol | phenol; dibenzofuran; 2-phenoxyphenol; dibenzo-p-dioxin | phenol; dibenzofuran |
| 2-MCP | 2-MCP (trace) | 2-MCP (trace) | phenol; 2-MCP; dibenzo-p-dioxin; 2-chlorodibenzo-p-dioxin | 2-MCP; phenol; dibenzo-p-dioxin; 2-chlorodibenzo-p-dioxin; 2,8-dichlorodibenzofuran; 2,4′-dichloro-5-hydroxydiphenyl ether; 1,2-DCBz | 2-MCP |
| MCBz | none | none | 1,2-DCBz; MCBz; 2-MCP; phenol; 2-chlorodiphenyl ether | MCBz; 2-chlorodiphenyl ether | MCBz; 1,2-DCBz; dibenzofuran |
| 1,2-DCBz | 1,2-DCBz (trace) | none | MCBz; 2-MCP; 1,2-DCBz | MCBz; 1,2-DCBz | 1,2-DCBz; 2-MCP; 1-chloronaphthalene; CT |
| CT | CT (trace) | none | o-benzoquinone; CT; phenol; 1,2-DCBz; 2-phenoxyphenol | CT; phenol; 2- dibenzofuran; 2-phenoxyphenol | CT; phenol |
| HQ | none | none | p-benzoquinone | p-benzoquinone | p-benzoquinone |
Only semivolatile products from reactions of EPFRs are included in the table. Other products, primarily lower molecular weight volatile species resulting from reactions of solvent radicals, were observed but were not fully identifiable or quantifiable with the HP-5MS capillary column.
The results are straightforward. The polar solvents (MEA, IPA, and DCM) extracted radicals while the nonpolar ones (TOL and TBB) did not. The mildly acidic alcohols, MEA and IPA, were more efficient than the nonprotonated polar solvent, DCM. These results are clearly evident from the reduction in EPR signal of particle residue after solvent extraction. However, EPR analyses of the extract solutions also demonstrated that the extracted radicals were converted to molecular products in solution.
Discussion
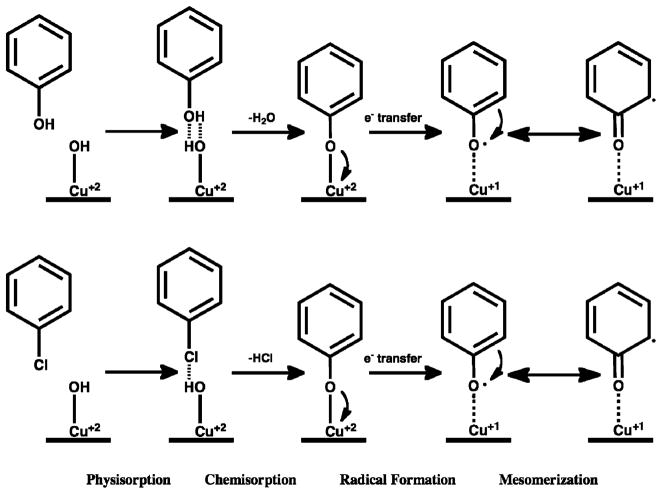
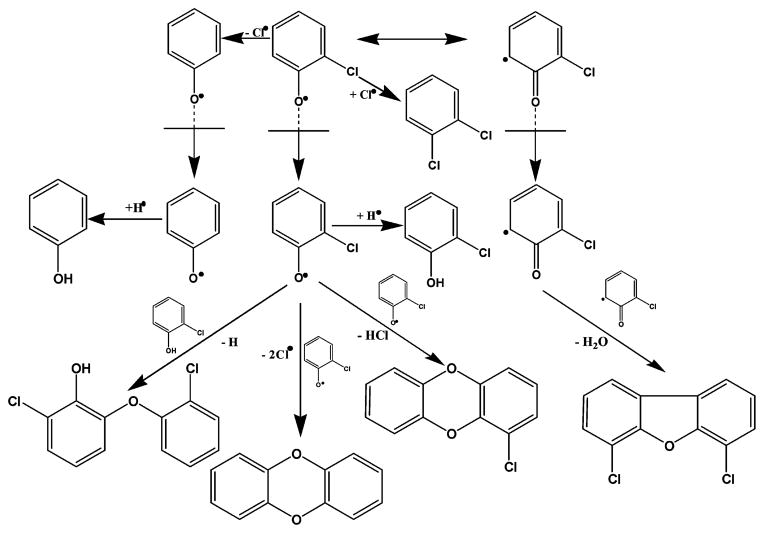
Our previous research indicated that the interaction of hydroxyl- and chlorine-substituted molecules with the CuO–silica surfaces resulted in their chemisorption followed by electron transfer to form a surface-associated radical (Scheme 1) (18, 30).
SCHEME 1.
Chemisorption and Radical Formation from Phenol and Monochlorobenzene on a CuO Surface
Due to the surface association, these radicals are stabilized and resistant to oxidation by O2, that is, environmentally persistent. The current experimental results support this contention. In the environment of the solvents that do not extract the radicals (TOL and TBB), the majority of the radicals survive on the surface of the particles despite sonication for 1 h (Figures 1 and 2). However, they are extractable under some conditions and then react in solution to form new molecular products. Both of these observations have implications concerning the fate of these radicals in the environment (vide infra).
Radical Extractability
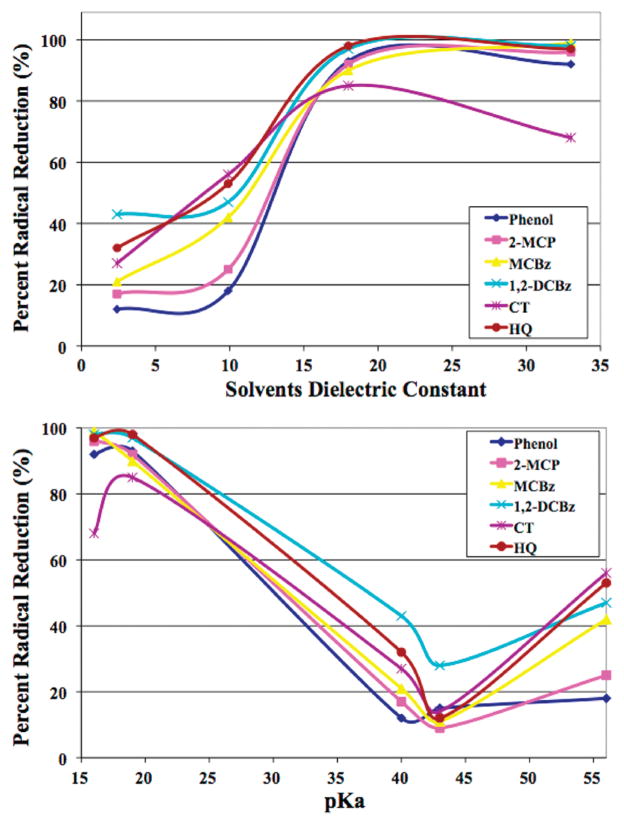
Table 2 summarizes the dielectric constants and pKas of the solvents used in this study. The polar alcoholic solvents, MEA and IPA, have a high dielectric constant, contain slightly acidic hydrogens, and efficiently extract the radicals. The nonpolar hydrocarbon solvents, TOL and TBB, have a low dielectric constant, no measurable acidity, and poorly extract the radicals. DCM, with a moderately high dielectric constant and no acidic hydrogens, extracts radicals with modest efficiency. A plot of solvent dielectric constant versus radical extractability and pKa (Figure 3) results in sigmoid-type curves with extraction efficiency correlating with both dielectric constant and pKa. DCM solvent and catechol adsorbate exhibit the greatest deviation from the trend.
TABLE 2.
Solvent Dielectric Constants and pKas
From comparison with benzene (pKa = 43).
From comparison with methane (pKa = 56).
FIGURE 3.
Extraction efficiency of various radicals as a function of solvent dielectric constant and pKa.
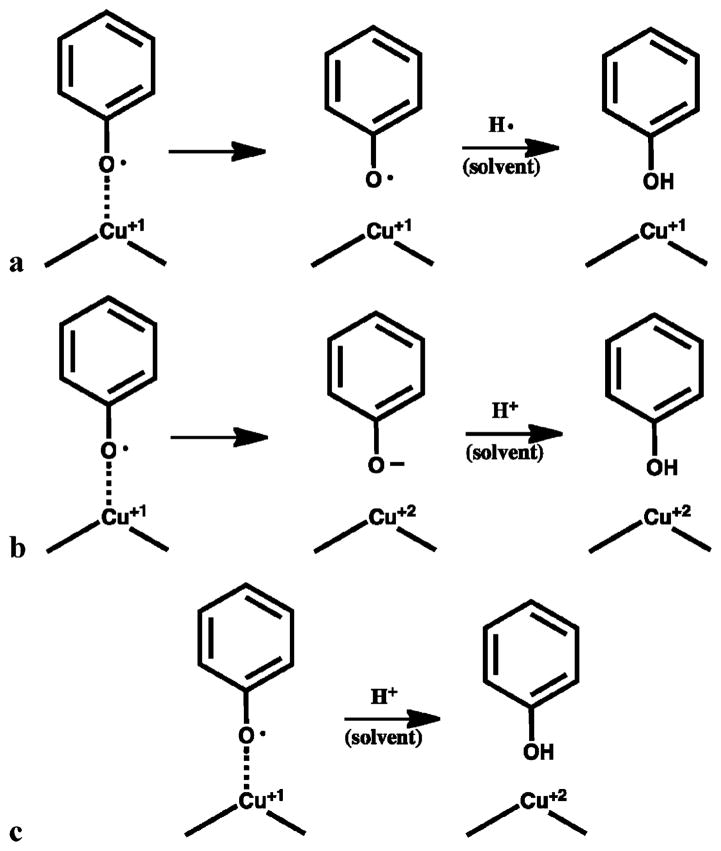
Three extraction scenarios can be envisioned: (1) The chemisorbed species is extracted as a radical via the solvation forces of the solvent, as expressed by the solvent dielectric constant (Scheme 2a). (2) The chemisorbed species is extracted as an anion and possibly stabilized by hydrogen bonding as well as the dispersion forces of the solvent, as expressed by both the dielectric constant and the pKa of the solvent (Scheme 2b). (3) The chemisorbed species is extracted as a molecular species via donation of an acidic hydrogen from the solvent, as expressed by the pKa of the solvent (Scheme 2c).
SCHEME 2. Possible Scenarios for Solvent Extraction of EPFRsa.
a(a) Extraction of the chemisorbed species as a radical due to simple solvation effects; (b) extraction as an anion assisted by H-bonding with the solvent; and (c) extraction as a molecular species by use of the solvent as a proton donor.
It is not clear which extraction scheme is dominant. The detection of a weak radical signal in solution indicates that pathway a proceeds to some extent and the radical is sufficiently nonreactive to be observable before H• is eventually abstracted from the solvent. The presence of the parent molecular species of the adsorbate suggests that either pathway c is occurring or the extracted anion is rapidly reacting with H+ from the solvent via pathway b. Pathway c is attractive in that it readily explains the increased extraction efficiency of the alcohol solvents, IPA and MEA, by their providing a proton to facilitate formation of a stable molecular species upon extraction.
There is precedent in the literature for the use of a proton donor (or acceptor) to facilitate extraction of pollutants from environmental particulate in the use of mild acid or base aqueous extraction solutions (18). Mild acid or base extraction of chlorinated phenols are in fact standard methods for their analysis, as they are considered “sticky” adsorbates (31). It is likely that they are actually chemisorbed and need acid-or base-catalyzed hydrolysis to remove them from the surfaces of the particles.
However, the presence of molecular reaction byproduct in solution indicates that there is initially a significant radical concentration in the extract solution that further reacts to form molecular products. Control experiments in which molecular species were dissolved in the solvents did not result in product formation. Clearly, the molecular products observed in the extract are the result of reactions of extracted radicals in solution.
Formation of Molecular Products in the Extract Solution
GC-MS analyses of the extracts revealed the presence of molecular products that are either products of radicals abstracting H• from the solvent, dimerization of the radicals, or more complex radical–radical recombination and radical–molecule displacement reactions. GC-MS analyses also revealed evidence of formation of low molecular weight products from reactions of secondary solvent radicals formed by reaction with extracted EPFRs.
Scheme 3 presents pathways of formation of observed products upon the extraction of 2-chlorophenoxyl radical formed by chemisorption of 2-MCP. The surface-associated 2-chlorophenoxyl radical may exist in both keto and enol forms (23). Dimerization results in formation of 1-monochlo-rodibenzo-p-dioxin and 1,2-dichlorodibenzofuran, respectively. Abstraction of a solvent hydrogen by phenoxyl forms 2-MCP. The radical–molecule reaction of 2-chlorophenoxyl with 2-MCP forms 1′,2-dichloro-1-hydroxydiphenyl ether. Traces of 1,2-DCBz were also observed due to surface-induced chlorination and possible simultaneous elimination from the surface. Dechlorination results in formation of phenoxyl radical, which then forms phenol by abstraction of a hydrogen atom from the solvent.
SCHEME 3.
Products of Solution Reaction of Radicals Extracted from Chemisorbed 2-MCP
The other EPFRs produced molecular products once extracted into solution by similar mechanisms. Pathways of product formation from the other radicals are presented in the Supporting Information.
Environmental Implications
We have previously demonstrated how EPFRs can be formed (29), and they are present in combustion-generated PM as well as airborne fine particles and coarse particles (1, 14). We have also demonstrated the potential for human health impacts of EPFRs by generation of ROS and the resulting oxidative stress (18). However, a logical question is whether the EPFRs in environmental PM are in high enough concentration to induce as great a health impact as molecular pollutants.
The conversion of EPFRs in solution back to the parent molecule and formation of other molecular products suggests EPFRs are subject to misidentification as molecular pollutants. Our samples were prepared to contain only free radicals, yet molecular products were identified by standard solvent extraction and analytical procedures. If one were not aware that these reactions can occur, then it would wrongly be assumed that the observed molecular pollutants were present on the particle rather than the EPFR.
Dibenzo-p-dioxins and dibenzofurans (PCDD/F) were identified in the solvent extracts when none existed on the particles. This raises the possibility that at least some of the PCDD/F observed in environmental samples may be due to solvent reactions of extracted chlorophenoxyl radical precursors or, at the very least, chemisorbed PCDD/F radicals. If this is true, then toxicity assessments of free PCDD/F molecules may not be valid for application to environmental particulate samples that, in actuality, contained chemisorbed EPFRs.
Quinones, hydroquinones/catechols, and phenols were also identified as solution reaction products, all of which have been reported in various types of combustion-generated and airborne particulate matter and implicated in the generation of oxidative stress (32, 33). It is possible that these pollutants reported as present in environmental samples were actually chemisorbed phenoxyl and semiquinone radicals. Because these radicals are highly redox-active and can induce oxidative stress in exposed populations, the biological activity of particles reported to contain quinones, catechols, and hydroquiones may actually be due to semiquinone-type radicals (11–13, 34).
Because Fe(II) ions in solution are know to form ROS by Fenton-type reactions, and the reported concentration of iron (~0.2–2 μg/m3) (2, 35) is much higher than that of quinones (150–1100 pg/m3) (36–38) in airborne PM, the “quinone hypothesis” of oxidative stress has received less attention. This raises the question of the relative chain length of ROS generating cycles by quinones, EPFRs, and environmental iron.
One might argue that catalytic cycles include molecular hydroquinones/catechols, quinones, and semiquinone radicals, and only one of these species needs to be present to initiate the catalytic cycle. However, this argument is incorrect since the rate of the overall catalytic reaction is equal to the rate of initiation multiplied by the chain length. If radicals react to initiate the chain at a higher reaction rate than their molecular counterparts, then they may be more important progenitors of damage than the molecular species, even if molecular species are present at higher concentrations. In addition, it is at best unclear if water-insoluble iron(III) oxides, which are the dominant oxidation state of iron in PM, can catalyze or even mediate ROS formation (34, 39).
These issues are complicated by the fate of EPFRs associated with ingested or inhaled particles. Since EPFR-containing PM in biological systems is not subjected to the same extraction procedure used for chemical analyses, EPFRs appear to remain associated with the PM where they continue to generate ROS and induce oxidative stress (40). Consequently, the biological fate of particle-associated EPFRs and their possible misidentification as related molecular species appear to be essential to understanding the toxicity of airborne particulate matter.
Supplementary Material
Acknowledgments
We gratefully acknowledge the partial support of this research under NSF Grants CTS-0625548 and CTS-0404314 as well as the Louisiana State University Patrick F. Taylor Chair.
Footnotes
Supporting Information Available
Additional mechanistic schemes. This information is available free of charge via the Internet at http://pubs.acs.org/.
Literature Cited
- 1.Squadrito GL, Cueto R, Dellinger B, Pryor WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radical Biol Med. 2001;31(9):1132–1138. doi: 10.1016/s0891-5849(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14(10):1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 3.Marsh JP, Mossman BT. Role of asbestos and active oxygen species in activation and expression of ornithine decarboxylase in hamster tracheal epithelial-cells. Cancer Res. 1991;51(1):167–173. [PubMed] [Google Scholar]
- 4.Kensler TW, Trush MA. Role of oxygen radicals in tumor promotion. Environ Mutagen. 1984;6(4):593–616. doi: 10.1002/em.2860060412. [DOI] [PubMed] [Google Scholar]
- 5.Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: Implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112(14):1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cormier SA, Lomnicki S, Backes W, Dellinger B. Origin and health impacts of emissions of toxic by-products and fine particles from combustion and thermal treatment of hazardous wastes and materials. Environ Health Perspect. 2006;114(6):810–817. doi: 10.1289/ehp.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valavanidis A, Salika A, Theodoropoulou A. Generation of hydroxyl radicals by urban suspended particulate air matter. The role of iron ions. Atmos Environ. 2000;34(15):2379–2386. [Google Scholar]
- 8.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Flickinger CW. Benzenediols - catechol, resorcinol and hydroquinone - review of industrial toxicology and current industrial exposure limits. Am Ind Hyg Assoc J. 1976;37(10):596–606. doi: 10.1080/0002889768507526. [DOI] [PubMed] [Google Scholar]
- 10.Sheesley RJ, Schauer JJ, Chowdhury Z, Cass GR, Simoneit BRT. Characterization of organic aerosols emitted from the combustion of biomass indigenous to South Asia. J Geophys Res, [Atmos] 2003;108(D9) [Google Scholar]
- 11.Carmella SG, Hecht SS, Tso TC, Hoffmann D. Roles of tobacco cellulose, sugars, and chlorogenic acid as precursors to catechol in cigarette smoke. J Agric Food Chem. 1984;32(2):267–273. [Google Scholar]
- 12.Simoneit BRT. Biomass burning - A review of organic tracers for smoke from incomplete combustion. Appl Geochem. 2002;17(3):129–162. [Google Scholar]
- 13.Leanderson P, Tagesson C. Cigarette smoke-induced DNA-damage - role of hydroquinone and catechol in the formation of the oxidative DNA-adduct, 8-hydroxydeoxyguanosine. Chem-Biol Interact. 1990;75(1):71–81. doi: 10.1016/0009-2797(90)90023-g. [DOI] [PubMed] [Google Scholar]
- 14.Hirakawa K, Oikawa S, Hiraku Y, Hirosawa I, Kawanishi S. Catechol and hydroquinone have different redox properties responsible for their differential DNA-damaging ability. Chem Res Toxicol. 2002;15(1):76–82. doi: 10.1021/tx010121s. [DOI] [PubMed] [Google Scholar]
- 15.Valavanidis A, Fiotakis K, Bakeas E, Vlahogianni T. Electron paramagnetic resonance study of the generation of reactive oxygen species catalysed by transition metals and quinoid redox cycling by inhalable ambient particulate matter. Redox Rep. 2005;10(1):37–51. doi: 10.1179/135100005X21606. [DOI] [PubMed] [Google Scholar]
- 16.Dellinger B, Pryor WA, Cueto R, Squadrito G, Deutsch WA. The role of combustion-generated radicals in the toxicity of PM2.5. Proc Combust Inst. 2000;28:2675–2681. [Google Scholar]
- 17.Maskos Z, Khachatryan L, Cueto R, Pryor WA, Dellinger B. Radicals from the pyrolysis of tobacco. Energy Fuels. 2005;19(3):791–799. [Google Scholar]
- 18.Lomnicki S, Truong H, Vajereno E, Dellinger B. A copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ Sci Technol. 2008;42(13):4982–4988. doi: 10.1021/es071708h. [DOI] [PubMed] [Google Scholar]
- 19.Maskos Z, Khachatryan L, Dellinger B. Precursors of radicals in tobacco smoke and the role of particulate matter in forming and stabilizing radicals. Energy Fuels. 2005;19(6):2466–2473. [Google Scholar]
- 20.Knaapen AM, Shi TM, Borm PJA, Schins RPF. Soluble metals as well as the insoluble particle fraction are involved in cellular DNA damage induced by particulate matter. Mol Cell Biochem. 2002;234(1):317–326. [PubMed] [Google Scholar]
- 21.Ying Q, Mysliwiec M, Kleeman MJ. Source apportionment of visibility impairment using a three-dimensional source-oriented air quality model. Environ Sci Technol. 2004;38(4):1089–1101. doi: 10.1021/es0349305. [DOI] [PubMed] [Google Scholar]
- 22.Allie S, Ranchoux R. Stack sampling of organic-compounds - application to the measurement of catalytic incinerator efficiency. J Air Pollut Control Assoc. 1980;30(7):792–794. [Google Scholar]
- 23.Martens D, Gfrerer M, Wenzl T, Zhang A, Gawlik BM, Schramm KW, Lankmayr E, Kettrup A. Comparison of different extraction techniques for the determination of poly-chlorinated organic compounds in sediment. Anal Bioanal Chem. 2002;372(4):562–568. doi: 10.1007/s00216-001-1120-y. [DOI] [PubMed] [Google Scholar]
- 24.Hawthorne SB, Miller DJ, Langenfeld JJ, Burford MD. Analytical-scale extraction of environmental samples using supercritical fluids. ACS Symp Ser. 1992;508:206–221. [Google Scholar]
- 25.Ross AB, Junyapoon S, Jones JM, Williams A, Bartle KD. A study of different soots using pyrolysis-GC-MS and comparison with solvent extractable material. J Anal Appl Pyrolysis. 2005;74(1–2):494–501. [Google Scholar]
- 26.Chawla B, Davis BH. Effect of temperature and solvent on coal extraction under mild conditions. Fuel Process Technol. 1989;23(2):133–148. [Google Scholar]
- 27.McGee JK, Chen LC, Cohen MD, Chee GR, Prophete CM, Haykal-Coates N, Wasson SJ, Conner TL, Costa DL, Gavett SH. Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ Health Perspect. 2003;111(7):972–980. doi: 10.1289/ehp.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lingard JJN, Tomlin AS, Clarke AG, Healey K, Hay AWM, Wild CP, Routledge MN. A study of trace metal concentration of urban airborne particulate matter and its role in free radical activity as measured by plasmid strand break assay. Atmos Environ. 2005;39(13):2377–2384. [Google Scholar]
- 29.Lomnicki S, Dellinger B. A detailed mechanism of the surface-mediated formation of PCDD/F from the oxidation of 2-chlorophenol on CuO/silica surface. J Phys Chem A. 2003;107(22):4387–4395. [Google Scholar]
- 30.Alderman SL, Dellinger B. FTIR investigation of 2-chlorophenol chemisorption on a silica surface from 200 to 500 °C. J Phys Chem A. 2005;109(34):7725–7731. doi: 10.1021/jp051071t. [DOI] [PubMed] [Google Scholar]
- 31.Siriwatwechakul W, Lafleur T, Prud’homme RK, Sullivan P. Effects of organic solvents on the scission energy of rodlike micelles. Langmuir. 2004;20(21):8970–8974. doi: 10.1021/la035853u. [DOI] [PubMed] [Google Scholar]
- 32.Balakrishna S, Lomnicki S, Dellinger B, Cormier SA. Resveratrol ameliorates the redox imbalances in human airway epithelial cells exposed to combustion generated nanoparticles. Free Radical Biol Med. 2008;45:S44–S44. [Google Scholar]
- 33.Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cyto-toxicity. Part Fibre Toxicol [Online] 2009;6:Article 11. doi: 10.1186/1743-8977-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valavanidis A, Fiotakis K, Vlahogianni T, Papadimitriou V, Pantikaki V. Corrigendum to: Determination of selective quinones and quinoid radicals in airborne particulate matter and vehicular exhaust particles. Environ Chem. 2006;3(3):233. [original article: Environ. Chem. 2006, 3 (2), 118–123] [Google Scholar]
- 35.Avakian MD, Dellinger B, Fiedler H, Gullet B, Koshland C, Marklund S, Oberdorster G, Safe S, Sarofim A, Smith KR, Schwartz D, Suk WA. The origin, fate, and health effects of combustion by-products: A research framework. Environ Health Perspect. 2002;110(11):1155–1162. doi: 10.1289/ehp.021101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurzau ES, Neagu C, Gurzau AE. Essential metals - case study on iron. Ecotoxicol Environ Saf. 2003;56(1):190–200. doi: 10.1016/s0147-6513(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 37.Espinosa AJF, Rodriguez MT, de la Rosa FJB, Sanchez JCJ. A chemical speciation of trace metals for fine urban particles. Atmos Environ. 2002;36(5):773–780. [Google Scholar]
- 38.Fernandez AJ, Ternero M, Barragan FJ, Jimenez JC. An approach to characterization of sources of urban airborne particles through heavy metal speciation. Chemosphere. 2000;2(2):123–136. [Google Scholar]
- 39.Cho AK, Stefano ED, You Y, Rodriguez CE, Schmitz DA, Kumagai Y, Miguel AH, Eiguren-Fernandez A, Kobayashi T, Avol E, Froines JR. Determination of four quinones in diesel exhaust particles, SRM 1649a, and atmospheric PM2.5. [of Aerosol Science and Technology on Findings from the fine particulate matter supersites program] Aerosol Sci Technol. 2004;38(12 Suppl 1):68–81. [Google Scholar]
- 40.Petrat F, Paluch S, Dogruoez E, Doerfler P, Kirsch M, Korth H-G, Sustmann R, De Groot H. Reduction of Fe(III) ions complexed to physiological ligands by lipoyl dehydrogenase and other flavoenzymes in vitro: implications for an enzymatic reduction of Fe(III) ions of the labile iron pool. J Biol Chem. 2003;278(47):46403–46413. doi: 10.1074/jbc.M305291200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.