Abstract
Although Alzheimer’s and Parkinson’s diseases predominately affect elderly adults, the proteins that play a role in the pathogenesis of these diseases are expressed throughout life. In fact, many of the proteins hypothesized to be important in the progression of neurodegeneration play direct or indirect roles in the development of the central nervous system. The systems affected by these proteins include neural stem cell fate decisions, neuronal differentiation, cellular migration, protection from oxidative stress, and programmed cell death. Insights into the developmental roles of these proteins may ultimately impact the understanding of neurodegenerative diseases and lead to the discovery of novel treatments.
Recent studies implicating developmentally relevant proteins in the pathogenesis of neurodegenerative disease have led some investigators to suggest that neurodegenerative disease, including Alzheimer’s and Parkinson’s diseases, is the result of two “hits”: one that conveys susceptibility and one that results in the disease.1–4 The first “hit” is hypothesized to have a genetic or developmental component, whereas the second “hit” is environmental. Alternatively, it has been suggested that developmentally important processes that persist and remain focally relevant in adulthood play a role in neurodegenerative disease.5 In either case, it would be useful to know the functions of developmentally regulated genes and their protein products that have been implicated or proposed to play a role in neurodegenerative disease. This review focuses on the roles that genes and gene products suggested to be relevant for Alzheimer’s and Parkinson’s diseases play in normal neural development.
Alzheimer’s Disease
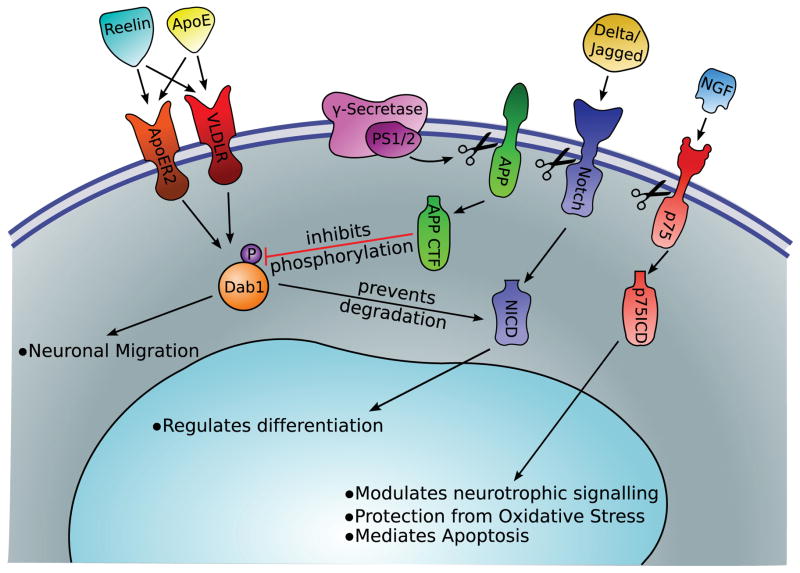
Alzheimer’s disease can be classified into two main subtypes: early-onset familial disease caused by specific genetic mutations and late-onset sporadic disease with various risk factors including genetic polymorphisms. The genes implicated in familial Alzheimer’s disease include the genes that code for Presenilin-1 (PS1), Presenilin-2 (PS2), and amyloid precursor protein (APP). PS1 and PS2 function as the catalytic subunit of the γ-secretase enzyme complex. This complex participates in the cleavage of APP into the pathogenic Aβ fragment that is found in amyloid plaques characteristic of Alzheimer’s disease. In addition, γ-secretase cleaves other transmembrane proteins, including Notch and the p75 neurotrophic receptor (p75NTR), to release important intracellular signaling peptides vital for neurodevelopment. Also, whereas the Aβ fragment of APP is toxic, full-length APP and the C-terminal fragment appear to regulate neurodevelopment pathways. As for sporadic Alzheimer’s disease, polymorphisms in the gene coding for apolipoprotein E (ApoE) have been linked to increased risk and earlier onset of disease. Although the mechanism behind the increased risk for disease is still unclear, the ApoE receptors (very low-density lipoprotein receptor [VLDLR], apolipoprotein E receptor 2 [ApoER2]) and the alternate ligand for these receptors (Reelin) have vital functions in the developing brain. Figure 1 summarizes the major signaling pathways in which proteins hypothesized to be implicated in the pathogenesis of Alzheimer’s disease participate, and Table 1 describes the model systems in which manipulation of these proteins has been characterized.
FIGURE 1.
Protein cleavage by γ-secretase results in downstream signaling purported to be of relevance for Alzheimer’s disease and development of the central nervous system. APP = amyloid precursor protein; ApoE = apolipoprotein E; NGF = nerve growth factor; NICD = Notch intracellular domain; PS1 = Presenilin-1; p75ICD = p75 intracellular domain; VLDLR = very-low-density lipoprotein receptor; ApoER2 = apolipoprotein E receptor 2; CTF = C-terminal fragment.
TABLE 1.
Models of Aberrant Developmentally Important Proteins Purported to Be of Relevance for Alzheimer’s Disease
| Gene Product | Type of Model | Characterization |
|---|---|---|
| Presenilin-1 | Humanized mutants, mice | Some combined with mutant APP; some have amyloid plaques, increased levels of Aβ, or both; many have no apparent cognitive impairment |
| Presenilin-2 | Humanized mutants, mice | Increased levels of Aβ |
| APP | Mutant mice | Some combined with mutant Presenilin-1; amyloid plaque burden is variable |
| ApoE | Knock-out and targeted mutation, mice | Alterations in immune system regulation, nerve regeneration, and muscle differentiation |
| p75NTR | Spontaneous mutants (negative) in PC12 cells; siRNA and shRNA downregulated in PC12 cells; exon III–deleted mice; exon IV–deleted (p75NTR knock-out) mice | PC12 cells abnormally susceptible to oxidant stress; exon III–deleted mice with subtle, variable changes in peripheral nerve regenerative capacity; exon IV–deleted mice with 28% increase in number of cholinergic neurons |
| Reelin | Autosomal recessive mutation, mice | Severe ataxia; inverted cortical lamination; abnormal positioning of neurons; aberrant orientation of cell bodies and fibers; cerebellar hypoplasia |
| VLDLR | Knock-out mice | Continued migration of cortical neurons past normal stop point; clinical phenotype like Reelin mutants but milder, unless combined with ApoER2 mutation |
| ApoER2 | Mutation, mice | Failure of migration of late-generated neocortical neurons; clinical phenotype like Reelin mutants but milder, unless combined with VLDLR mutation |
| Notch | Mice with null heterozygous mutations in Notch1 | Deficits in spatial learning and memory |
APP = amyloid precursor protein; ApoE = apolipoprotein E; p75NTR = p75 neurotrophic receptor; siRNA = small, interfering RNA; shRNA = short hairpin RNA; VLDLR = very-low-density lipoprotein receptor; ApoER2 = apolipoprotein E receptor 2.
Presenilins
PS1 and PS2 are membrane-bound proteins that form the active site of the γ-secretase complex. γ-Secretase is a membrane-bound complex of four proteins that cleaves the transmembrane region of its substrates. The majority of early-onset familial Alzheimer’s disease is attributed to mutations in the two genes, PSEN1 and PSEN2, that code for these proteins. Presenilin mutations not only reduce the efficiency with which the γ-secretase complex can cleave substrates, but they also increase the proportion of Aβ42, relative to other products, that is produced on cleavage of APP.6 Besides cleaving APP, γ-secretase also processes Notch and p75NTR proteins in crucial neurodevelopmental pathways.
Notch is a transmembrane protein expressed on the surface of neural stem cells and postmitotic cells in the brain. The association of Notch with its ligands (eg, Jagged and Delta) helps dictate survival, proliferation, self-renewal, and differentiation of neural stem cells, and regulates synaptic plasticity in differentiated neurons. When ligands bind to the Notch receptor, the γ-secretase complex cleaves the intracellular domain (ICD) from Notch; the Notch ICD is then translocated into the nucleus. After complexing with transcriptional regulators, such as C-promoter binding factor-1 (CBF-1), Notch influences the transcription of target genes primarily of the hairy and enhancer of split (HES) family. Most of these genes are transcription factors, and the overall effect of this pathway is to prevent the differentiation of neural stem cells. Thus, the absence of Notch, or presence of a Notch antagonist, results in premature neuronal maturation.7
p75NTR is also cleaved by γ-secretase after ligand binding, releasing the p75 intracellular domain (p75ICD). p75NTR is a transmembrane receptor with similarities to the tumor necrosis factor family of receptors. p75NTR expression is selectively high in the nucleus basalis of Meynert, a basal forebrain region pathologically associated with Alzheimer’s disease of both subtypes.8 The canonical ligand for p75NTR is nerve growth factor, but many other neurotrophins and proneurotrophins bind to p75NTR with high affinity. After binding of ligand, the ICD is cleaved from p75NTR and translocates to the nucleus.9 In normal development, p75NTR functions in diverse roles. One role is to activate cell death pathways similar to other members of the tumor necrosis factor receptor family. In sympathetic neurons, for example, brain-derived neurotrophic factor and neurotrophin-4 released at the cell body after distal axon stimulation promoted p75NTR-dependent cell death in neighboring neurons.10 In other studies, p75NTR has been shown to have important roles in modulating neurotrophic signals, promoting survival of developing neurons,11 and even protecting cells from oxidant stress and apoptosis.12 This “life-or-death” property of p75NTR has earned it and its family members the name “dependence receptors,” as they dictate cellular dependence on ligand for survival.13 Furthermore, signaling through p75ICD appears to require de novo cholesterol synthesis; p75ICD and its binding partner, neurotrophin receptor interacting factor (NRIF), regulate expression of the enzymes involved in this bio-synthetic pathway.14,15 It is tempting to hypothesize that a mutant PS1 that is less efficient at cleaving p75NTR to p75ICD could result in altered neuronal cholesterol synthesis, altered life-or-death decision making, and/or altered resistance to oxidant stress in patients with familial Alzheimer’s disease.
In addition to these roles as part of the γ-secretase complex, PS1 alone may be involved in other signaling pathways. These include the Wnt/β-catenin, phosphatidylinositol-3 kinase (PI3K)/Akt, mitogen-activated protein kinase, and epidermal growth factor receptor (EGFR) pathways, all of which are necessary in the developing brain.16
Apolipoprotein E
ApoE, an important component of chylomicrons and other lipoprotein particles, is expressed primarily by astrocytes in both the adult and the developing brain. As it does throughout the body, ApoE plays an important role in the trafficking of lipids in the brain. Building on studies that showed ApoE was a constituent in senile plaques, the ε4 isoform of ApoE was shown to be associated with increased risk for late-onset Alzheimer’s disease.17 The definitive cause for the increased risk is unknown, but recent evidence shows that ApoE may regulate the complex processing of APP, and that different isoforms have varying abilities to clear Aβ particles.18 Understanding the various functions of this system during development may give insights into the role of ApoE in Alzheimer’s disease.
ApoE receptors and their associated signaling cascades are critical for the proper migration and organization of neurons in the developing brain. Within the family of low-density lipoprotein receptors that are expressed in the brain, three are known to bind directly to ApoE. These receptors include ApoER2, VLDLR, and low-density lipoprotein receptor-related protein 1 (LRP1). Although ApoE can bind to all three receptors, Reelin, a large extracellular glycoprotein, is likely the major ligand for ApoER2 and VLDLR, especially in the developing brain.19 Reelin is crucial for coordinating the migration of neurons into organized layers in the cortex, cerebellum, and hippocampus.20 Although Reelin may not be of direct relevance for Alzheimer’s disease, alteration of the affinity of ApoE for its receptors may change the likelihood or temporal dynamics of Reelin binding to these receptors. Alternatively, it has been proposed that Reelin remains of relevance for neuronal migration during adulthood, as occurs in the olfactory system, a system frequently functionally abnormal early in the course of Alzheimer’s disease.5
In signaling through the ApoER2 and VLDLR, Reelin begins a signaling cascade as follows: Reelin binds to the receptors inducing the phosphorylation of Disabled-1 (Dab1) by the Src family kinases. Phosphorylated Dab1 has been shown to influence multiple cellular pathways. Dab1 interacts with Crk and CrkL to influence signaling through pathways such as C3G/Rap1 and PI3K/Akt.21 It can also bind to Notch ICD to prevent ubiquitination and degradation of Notch ICD before it reaches the nucleus.22
Reelin deficiency leads to a specific phenotype that includes disorganized development of the cerebral cortex and cerebellum. The disorganization is mainly attributed to the failure of neurons to migrate properly to the outer layers of the cortex. Similar phenotypes have been shown in mouse models that lack the Reelin receptors (VLDLR and ApoER2) or Dab1.23 In addition, targeted knockouts of Notch in terminally differentiated neurons and targeted knock-outs of Crk and CrkL show similar phenotypes.21 In humans, mutations in VLDLR are responsible for an inherited disorder of quadrupedal locomotion seen in two Turkish families and a Hutterite family. The affected subjects showed cerebellar hypoplasia and simplification of the cortex.24
Amyloid Precursor Protein
APP has a poorly defined role in neurodevelopment on its own, but it is involved in the pathways discussed for the Presenilins and ApoE. APP has been shown to be a substrate for intramembrane proteolysis by γ-secretase, similar to Notch and p75NTR. The C-terminal fragment of APP is able to bind to Dab1 and antagonize its functions by inhibiting phosphorylation. In Drosophila, this prevents the normal migration of eye neurons.25,26 APP binding to Dab1 inhibits neurite outgrowth in cultured hippocampal cells.27 In a mouse model, APP binding to Dab1 leads to a phenotype that is similar to, though less severe than, Reelin mutant mice.28 Perturbations of these interactions have been proposed to be of relevance for olfactory dysfunction in Alzheimer’s disease.5
Parkinson’s Disease
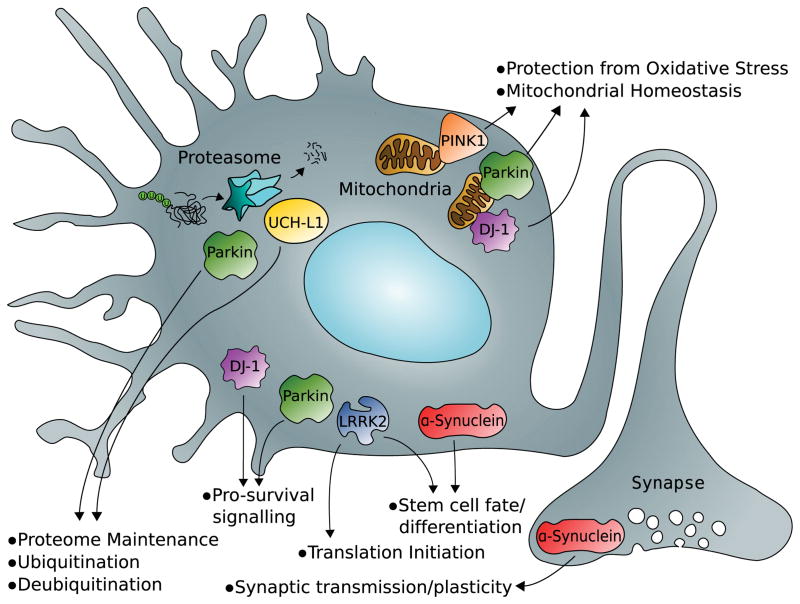
Parkinson’s disease can also be divided into heritable and sporadic forms. Large genetic studies focused on familial cases originally identified genomic loci that contained putative disease-related genes and named these loci PARK1-8. Further studies have identified specific genes at 7 of the 8 loci, pin-pointed specific causal mutations, and identified additional loci. The proteins encoded by the genes at the original loci (α-synuclein [PARK1, PARK4], Parkin [PARK2], ubiquitin C-terminal hydrolase L1 [UCH-L1 (PARK5)], PTEN-induced kinase 1 [PINK1 (PARK6)], DJ-1 [PARK7], and leucine-rich repeat kinase 2 [LRRK2 (PARK8)]) are pathogenic in heritable forms of Parkinson’s disease and have also proved to be important in sporadic forms of the disease. In addition, all six of the proteins have roles in normal neurodevelopment. Figure 2 summarizes the major signaling pathways in which proteins hypothesized to be implicated in Parkinson’s disease participate, and Table 2 describes the model systems in which manipulation of these proteins has been characterized. Note that only the mutant α-synuclein model demonstrates Lewy bodies inside of dopaminergic neurons.
FIGURE 2.
Developmentally important pathways involving cellular protein degradation, antioxidant reserve, and life-and-death decision making have been implicated in the pathogenesis of Parkinson’s disease. LRRK2 = leucine-rich repeat kinase 2; PINK1 = PTEN-induced kinase 1; UCH-L1 = ubiquitin C-terminal hydrolase L1.
TABLE 2.
Models of Aberrant Developmentally Important Proteins Purported to Be of Relevance for Parkinson’s Disease
| Gene Product | Inheritance Pattern in Human Disease | Type of Model | Characterization |
|---|---|---|---|
| α-Synuclein | Dominant point mutations; Parkinson’s disease | Knock-out mice; mutation, mice; cultured dopaminergic mesencephalic cells overexpressing wild-type or mutant | Knock-out mice: reduction in striatal dopamine and an attenuation of dopamine-dependent locomotor response to amphetamine, resistance to MPTP; overexpressing cells: death of dopaminergic neurons; mutant α-synuclein controlled by a prion promoter in mice: Lewy bodies in dopaminergic neurons |
| Parkin | Recessive; parkinsonian syndrome | Mutation, mice | Mutant mice: deficits in glutamate transmission, late-onset hypokinetic motor deficits; cultured cells from mutant mice: resistance of mesencephalic cells to nitric oxide toxicity |
| UCH-L1 | Dominant; polymorphism putatively inversely correlated with Parkinson’s disease | Mutation, mice | Isolated mutant UCH-L1 mice: sensory ataxia, posterior paralysis, death; overexpression of α-synuclein in UCH-L1 mutant mice: accelerated dopaminergic neuron loss in mesencephalon relative to overexpression of α-synuclein in UCH-L1 wild-type mice |
| PINK1 | Recessive; parkinsonian syndrome | Heterozygous deletion, Drosophila; conditional knock-out, mice | Drosophila: progressive loss of dopaminergic neurons and retinal neurons preventable with antioxidants; mice: decreases in evoked striatal slice dopamine release, reductions in dissociated chromaffin cell catecholamine quantal size and release frequency |
| DJ-1 | Recessive; parkinsonian syndrome | Mutant, Drosophila; deficiency, mice | Hypersensitivity to MPTP and oxidative stress |
| LRRK2 | Dominant; Parkinson’s disease | Mutant, Drosophila; mutant and knock-out mice | Loss of dopaminergic neurons in Drosophila; age-dependent and L-dopa–responsive hypokinesia, diminished dopamine release, nigrostriatal dopaminergic projection axonal pathology with Lewy bodies in mice |
MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; UCH-L1 = ubiquitin C-terminal hydrolase L1; PINK1 = PTEN-induced kinase 1; LRRK2 = leucine-rich repeat kinase 2.
α-Synuclein
The membrane-bound protein α-synuclein is a major constituent of Lewy bodies, pathological intracellular inclusions found in Parkinson’s disease. The normal function of α-synuclein has not been established definitively, but a few studies focused on Parkinson’s disease have given clues to its role in neurodevelopment. Based on localization studies in mammals and functional studies in yeast, it can be deduced that α-synuclein is important for normal synaptic activity. In rat brain, α-synuclein expression is strongest in areas of synaptic plasticity.29 A study looking at human brains from early fetal development through adulthood concluded that α-synuclein gross anatomic and subcellular localization of expression in brain varies as a function of age. Whereas early fetuses displayed staining in the cell bodies, older fetuses and adults almost exclusively showed α-synuclein staining in the neural processes.30 In yeast cells, α-synuclein was shown to affect lipid metabolism and vesicle trafficking,31 which, when combined with localization data, suggests involvement in synaptic development, plasticity, and transmission. Despite these findings and the fact that α-synuclein is expressed throughout the brain, knock-out mice display normal brain morphology, including number and structure of neurons. Under certain conditions, these mice do have defects in dopamine signaling, which may indicate a developmental role for α-synuclein in the nigrostriatal pathway that is functionally, but not structurally, apparent.32 α-Synuclein can also bind directly to the dopamine transporter in presynaptic terminals.33 In human neural progenitor cells, overexpression of wild-type α-synuclein altered the fate of these progenitors, resulting in a decreased number of glial cells.34 Overall, α-synuclein likely functions at the synapse of developing neurons, especially those in the dopamine system, but may also play a role in cell fate choices of neural stem cells.
Parkin
Parkin is an E3 ubiquitin ligase that, in cooperation with other ubiquitin-conjugating enzymes, can add ubiquitin molecules or chains to other proteins. Parkin mutations, which decrease its activity, are associated with juvenile Parkinson’s disease. Canonically, ubiquinated proteins are destined for degradation, but recent evidence proves that ubiquitination functions in many different signaling cascades.35 Studies focused on the disease have elucidated possible roles of Parkin in neurodevelopment including protein degradation, cellular metabolism, and growth factor signaling. When complexed with PINK1 and DJ-1, Parkin ubiquination targets substrates for proteasomal degradation.36 Parkin knock-out mice show dysregulation of multiple proteins related to oxidative phosphorylation and oxidative stress. These mice have impaired mitochondrial function and increased oxidative tissue damage.37 Parkin has been shown to interact with the EGFR and the EGFR interacting protein, Eps15. By ubiquitinating Eps15, Parkin modulates the trafficking of EGFR, leading to increased downstream signaling through the PI3K/Akt pathway.38 This is of particular interest in light of the recent demonstration of the role of the PI3K/Akt pathway in resistance to oxidant stress.39
Ubiquitin C-Terminal Hydrolase L1
UCH-L1 is a neuron-specific protein expressed in the brain, the peripheral nervous system, and even in the enteric nerves.40 UCH-L1 mutations and polymorphisms have been shown to modulate the risk for Parkinson’s disease. The main function of UCH-L1 is to maintain the levels of free ubiquitin in the cell by removing ubiquitin from proteins using a hydrolyzation reaction, termed de-ubiquitination. In addition to its hydrolase activity, UCH-L1 can also ligate hydrolyzed ubiquitin to other ubiquitin molecules forming polyubiquitin chains.41 Functionally, mice with truncated UCH-L1 protein (also known as gracile axonal dystrophy mice) have altered protein turnover because of the lack of free ubiquitin and eventually show axonal degeneration leading to sensory and motor ataxia. The activity of UCH-L1 may also be critical to neurogenesis and regulating the morphology of progenitor cells.42
PTEN-Induced Kinase 1
PINK1 is a ubiquitously expressed serine/threonine kinase, which mainly localizes to the mitochondria. PINK1 has been found to be mutated in some familial and sporadic cases of Parkinson’s disease. In neurodevelopment, PINK1 has critical importance in maintaining mitochondrial homeostasis. In a human cancer cell line, downregulation of PINK1 led to mitochondrial damage, which was morphologically comparable with that seen in fibroblasts of patients with known PINK1 mutations. Notably, upregulation of Parkin rescued the cell lines with PINK1 mutations.43
DJ-1
DJ-1 was originally discovered as an oncogene capable of transforming mouse embryonic fibroblasts and later shown to be expressed in breast, ovarian, and lung cancers. The finding that loss-of-function mutations of DJ-1 led to neurodegeneration in Parkinson’s disease indicates that DJ-1 expression must be tightly regulated, and DJ-1 function is critical in cell survival. DJ-1 is expressed in the brain during embryonic development, suggesting that it contributes to neuronal survival in the developing brain.40 After oxidative stress, DJ-1 localizes to the mitochondria, asserting a protective effect in a human neuroblastoma cell line.44 In cancer, survival through DJ-1 is mediated by increased Akt signaling; under hypoxia, DJ-1 is crucial for both the Akt activity and the mammalian target of rapamycin signaling that help stabilize the hypoxic response leading to survival.45
Leucine-Rich Repeat Kinase 2
Activating mutations in the gene coding for LRRK2 account for many of the monogenetic cases of both sporadic and familial Parkinson’s disease. LRRK2 is a large, multidomain protein with tyrosine kinase activity in addition to other protein interaction and enzymatic domains.46 Lrrk2 messenger RNA expression begins around postnatal day 8 in rats, peaking at 3 weeks and continuing into adulthood. Its homolog, Lrrk1, is expressed similarly both temporally and spatially except that it is not expressed in the striatum, where Lrrk2 is expressed strongly.47 A microarray study showed that alterations of LRRK2 expression in a human cell line led to differential expression of many genes involved in development and differentiation.48 In addition, LRRK2 can affect eukaryotic initiation factor 4E–dependent translation by phosphorylating eukaryotic initiation factor 4E binding protein leading to prosurvival effects in neurons.49
Conclusion
It has become increasingly clear that proteins implicated or suggested to be involved in neurodegenerative disease often play critical roles in normal, early-life development of the nervous system. This is not surprising; developmental and genetic factors have long been hypothesized to predispose certain individuals and families to particular neurodegenerative diseases. What may be surprising is the lack of apparent disease until late in life even though these genes are involved in so many critical pathways. It remains to be seen whether compensatory changes minimize the effects of these mutations early in life, subcellular injury is present but undetectable, accumulation of damage occurs slowly, or a second insult is required for neuronal injury. Understanding the roles and signaling pathways of these proteins during development may afford new drug targets for therapy and predictors of susceptibility useful for prevention of neurodegenerative disease.
References
- 1.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 2.Lim KL, Chew KC, Tan JM, et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci. 2005;25:2002–2009. doi: 10.1523/JNEUROSCI.4474-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Herrup K. Cell division in the CNS: protective response or lethal event in post-mitotic neurons? Biochim Biophys Acta. 2007;1772:457–466. doi: 10.1016/j.bbadis.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiner O, Shmueli A, Sapir T. Neuronal migration and neurodegeneration: 2 sides of the same coin. Cereb Cortex. 2009;19:142–148. doi: 10.1093/cercor/bhp039. [DOI] [PubMed] [Google Scholar]
- 6.Steiner H, Fluhrer R, Haass C. Intramembrane proteolysis by gamma-secretase. J Biol Chem. 2008;283:29627–29631. doi: 10.1074/jbc.R800010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lasky JL, Wu H. Notch signaling, brain development, and human disease. Pediatr Res. 2005;57:104R–109R. doi: 10.1203/01.PDR.0000159632.70510.3D. [DOI] [PubMed] [Google Scholar]
- 8.Salehi A, Ocampo M, Verhaagen J, Swaab DF. P75 neurotrophin receptor in the nucleus basalis of Meynert in relation to age, sex, and Alzheimer’s disease. Exp Neurol. 2000;161:245–258. doi: 10.1006/exnr.1999.7252. [DOI] [PubMed] [Google Scholar]
- 9.Jung KM, Tan S, Landman N, et al. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;278:42161–42169. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- 10.Deppmann CD, Mihalas S, Sharma N, et al. A model for neuronal competition during development. Science. 2008;320:369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamanoue M, Middleton G, Wyatt S, et al. p75-mediated NF-kappaB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol Cell Neurosci. 1999;14:28–40. doi: 10.1006/mcne.1999.0770. [DOI] [PubMed] [Google Scholar]
- 12.Tyurina YY, Nylander KD, Mirnics ZK, et al. The intracellular domain of p75NTR as a determinant of cellular reducing potential and response to oxidant stress. Aging Cell. 2005;4:187–196. doi: 10.1111/j.1474-9726.2005.00160.x. [DOI] [PubMed] [Google Scholar]
- 13.Bredesen DE, Mehlen P, Rabizadeh S. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol Rev. 2004;84:411–430. doi: 10.1152/physrev.00027.2003. [DOI] [PubMed] [Google Scholar]
- 14.Yan Ch, Mirnics ZK, Portugal CF, et al. Cholesterol biosynthesis and the pro-apoptotic effects of the p75 nerve growth factor receptor in PC12 pheochromocytoma cells. Brain Res Mol Brain Res. 2005;139:225–234. doi: 10.1016/j.molbrainres.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Korade Z, Mi Z, Portugal CF, Schor NF. Expression and p75 neurotrophin receptor dependence of cholesterol synthetic enzymes in adult mouse brain. Neurobiol Aging. 2007;28:1522–1531. doi: 10.1016/j.neurobiolaging.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 16.Wakabayashi T, De Strooper B. Presenilins: members of the gamma-secretase quartets, but part-time soloists too. Physiology (Bethesda, Md) 2008;23:194–204. doi: 10.1152/physiol.00009.2008. [DOI] [PubMed] [Google Scholar]
- 17.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Arcangelo G, Homayouni R, Keshvara L, et al. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 20.D’Arcangelo G, Curran T. Reeler: new tales on an old mutant mouse. Bioessays. 1998;20:235–244. doi: 10.1002/(SICI)1521-1878(199803)20:3<235::AID-BIES7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Park TJ, Curran T. Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J Neurosci. 2008;28:13551–13562. doi: 10.1523/JNEUROSCI.4323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto-Torii K, Torii M, Sarkisian MR, et al. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60:273–284. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borrell V, Pujadas L, Simó S, et al. Reelin and mDab1 regulate the development of hippocampal connections. Mol Cell Neurosci. 2007;36:158–173. doi: 10.1016/j.mcn.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Ozcelik T, Akarsu N, Uz E, et al. Mutations in the very low-density lipoprotein receptor VLDLR cause cerebellar hypoplasia and quadrupedal locomotion in humans. Proc Natl Acad Sci USA. 2008;105:4232–4236. doi: 10.1073/pnas.0710010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merdes G, Soba P, Loewer A, et al. Interference of human and Drosophila APP and APP-like proteins with PNS development in Drosophila. EMBO J. 2004;23:4082–4095. doi: 10.1038/sj.emboj.7600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pramatarova A, Ochalski PG, Lee CH, Howell BW. Mouse disabled 1 regulates the nuclear position of neurons in a Drosophila eye model. Mol Cell Biol. 2006;26:1510–1517. doi: 10.1128/MCB.26.4.1510-1517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoareau C, Borrell V, Soriano E, et al. Amyloid precursor protein cytoplasmic domain antagonizes reelin neurite outgrowth inhibition of hippocampal neurons. Neurobiol Aging. 2008;29:542–553. doi: 10.1016/j.neurobiolaging.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Pramatarova A, Chen K, Howell BW. A genetic interaction between the APP and Dab1 genes influences brain development. Mol Cell Neurosci. 2008;37:178–186. doi: 10.1016/j.mcn.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a pre-synaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 30.Raghavan R, Kruijff L, Sterrenburg MD, et al. Alpha-Synuclein expression in the developing human brain. Pediatr Dev Pathol. 2004;7:506–516. doi: 10.1007/s10024-003-7080-9. [DOI] [PubMed] [Google Scholar]
- 31.Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-Synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abeliovich A, Schmitz Y, Fariñas I, et al. Mice lacking alpha-Synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-Synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- 34.Schneider BL, Seehus CR, Capowski EE, et al. Over-expression of alpha-Synuclein in human neural progenitors leads to specific changes in fate and differentiation. 2007;16:651–666. doi: 10.1093/hmg/ddm008. [DOI] [PubMed] [Google Scholar]
- 35.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 36.Xiong H, Wang D, Chen L, et al. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009;119:650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palacino JJ, Sagi D, Goldberg MS, et al. Mitochondrial dysfunction and oxidative damage in Parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 38.Fallon L, Bélanger CM, Corera AT, et al. A regulated interaction with the UIM protein Eps15 implicates Parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- 39.Mi Z, Rogers D, Mirnics ZK, Schor NF. p75NTR modulation of cellular handling of reactive oxygen species. J Neurochem. 2009;110:296–306. doi: 10.1111/j.1471-4159.2009.06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galter D, Westerlund M, Belin AC, Olson L. DJ-1 and UCH-L1 gene activity patterns in the brains of controls, Parkinson and schizophrenia patients and in rodents. Physiol Behav. 2007;92:46–53. doi: 10.1016/j.physbeh.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Fallon L, Lashuel HA, et al. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-Synuclein degradation and Parkinson’s disease susceptibility. Cell. 2002;111:209–218. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai M, Ayukawa K, Setsuie R, et al. Ubiquitin C-terminal hydrolase L1 regulates the morphology of neural progenitor cells and modulates their differentiation. J Cell Sci. 2006;119:162–171. doi: 10.1242/jcs.02716. [DOI] [PubMed] [Google Scholar]
- 43.Exner N, Treske B, Paquet D, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by Parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canet-Avilés RM, Wilson MA, Miller DW, et al. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasseur S, Afzal S, Tardivel-Lacombe J, et al. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci USA. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mata IF, Wedemeyer WJ, Farrer MJ, et al. LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Westerlund M, Belin AC, Anvret A, et al. Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: implications for Parkinson’s disease. Neuroscience. 2008;152:429–436. doi: 10.1016/j.neuroscience.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 48.Häbig K, Walter M, Poths S, et al. RNA interference of LRRK2-microarray expression analysis of a Parkinson’s disease key player. Neurogenetics. 2008;9:83–94. doi: 10.1007/s10048-007-0114-0. [DOI] [PubMed] [Google Scholar]
- 49.Imai Y, Gehrke S, Wang HQ, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]