Abstract
Importance
Positron emission tomography (PET) with 18-fluorodeoxyglucose (FDG) is recommended for the non-invasive diagnosis of pulmonary nodules suspicious for lung cancer. In populations with endemic infectious lung disease, FDG-PET may not accurately identify malignant lesions.
Objective
To estimate the diagnostic accuracy of FDG-PET for pulmonary nodules suspicious for lung cancer in regions where infectious lung disease is endemic and compare the test accuracy in regions where infectious lung disease is rare.
Data Sources and Study Selection
Databases of MEDLINE, EMBASE and the Web of Science were searched from October 1, 2000, through April 28, 2014. Articles reporting information sufficient to calculate sensitivity and specificity of FDG-PET to diagnose lung cancer were included. Only studies that enrolled more than 10 participants with benign and malignant lesions were included. Database searches yielded 1923 articles, of which 257 were assessed for eligibility. Seventy studies were included in the analysis. Studies reported on a total of 8511 nodules; 5105 (60%) were malignant.
Data Extraction and Synthesis
Abstracts meeting eligibility criteria were collected by a research librarian and reviewed by 2 independent reviewers. Hierarchical summary receiver operating characteristic curves were constructed. A random-effects logistic regression model was used to summarize and assess the effect of endemic infectious lung disease on test performance.
Main Outcome and Measures
The sensitivity and specificity for FDG-PET test performance.
Results
Heterogeneity for sensitivity (I2=87%) and specificity (I2=82%) was observed across studies. The pooled (unadjusted) sensitivity was 89% (95% CI, 86%-91%) and specificity was 75% (95% CI, 71%-79%). There was a 16% lower average adjusted specificity in regions with endemic infectious lung disease (61% [95% CI, 49%-72%]) compared with nonendemic regions (77% [95% CI, 73%-80%]). Lower specificity was observed when the analysis was limited to rigorously conducted and well-controlled studies. In general, sensitivity did not change appreciably by endemic infection status, even after adjusting for relevant factors.
Conclusions and Relevance
The accuracy of FDG-PET for diagnosing lung nodules was extremely heterogeneous. Use of FDG-PET combined with computed tomography was less specific in diagnosing malignancy in populations with endemic infectious lung disease compared with nonendemic regions. These data do not support use of FDG-PET to diagnose lung cancer in endemic areas unless an institution achieves test performance accuracy similar to that found in nonendemic regions.
Keywords: Lung cancer, diagnosis, FDG-PET, meta-analysis
Clinicians rely heavily on radiographic imaging to identify and noninvasively diagnose lung nodules between 3 and 30mm in diameter. The advent of lung cancer screening in high risk populations using low dose computed tomography (CT) scans will increase the number of lung nodules detected, requiring clinical evaluation and diagnosis.1 Depending on the risk for cancer, diagnostic guidelines suggest or recommend fluorodeoxyglucose F18 (FDG) combined with positron emission tomography (PET) as a noninvasive test to assess the risk of cancer or benign disease.2-4
In previously published meta-analyses.5,6 FDG-PET was reported to be 90% to 94% accurate in the characterization of malignant or benign lung nodules, with a sensitivity of 94% to 96% and specificity of 78% to 86%. Furthermore, combined FDG-PET and CT (FDG-PET/CT) scans have demonstrated a reduction in nontherapeutic resections (e.g. resection for benign lesions or metastatic disease) by 17% to 20%.7,8 For these reasons, FDG-PET/CT is widely accepted for the clinical diagnosis and staging of lung cancer in patients with suspicious lung nodules.2,9
Recent studies examining FDG-PET accuracy in diagnosing lung cancer in patients with lung lesions who reside in regions where fungal and other infectious lung diseases are endemic have shown mixed results.10,11 Histoplasmosis, coccidioidomycosis and blastomycosis are the most prevalent fungal lung diseases in the United States,12,13 and are common etiologies of lung granulomas.12 Histoplasmosis and blastomycosis are endemic across much of the Mississippi, Ohio and Missouri river valley regions through southern Ontario, Canada, whereas coccidioidomycosis is prevalent in the southwestern United States.12 Two international studies in areas with endemic tuberculosis found reduced FDG-PET/CT specificity of 25%14 and 21%.15
We undertook a systematic review and meta-analysis of the literature published after the 2001 meta-analysis by Gould et al6 describing FDG-PET accuracy to diagnose lung cancer among patients being evaluated with lung nodules or masses. This updated meta-analysis investigates the accuracy of FDG-PET to diagnose lung lesions in regions with locally endemic infectious lung diseases.
Methods
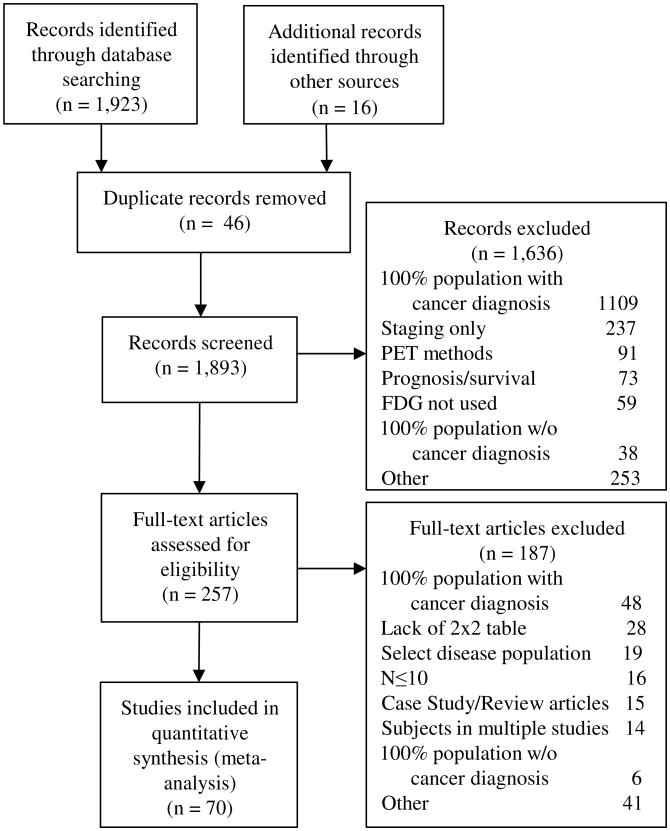
Studies evaluating individuals for possible lung cancer using FDG-PET, FDG-PET/CT or FDG-PET combined with another imaging modality were reviewed. We searched MEDLINE using the PubMed interface, EMBASE and the Web of Science for studies published between October 1, 2000 and April 28, 2014 (Appendix Table 1). The guidelines for Preferred Reporting Items for Systemic Reviews and Meta-Analyses were followed.16 Reasons for study exclusion are detailed in the statistical abstract and visually displayed in Figure 1.
Figure 1. Literature search PRISMA consort diagram.
PRISMA diagram of systematic review of eligible studies (Preferred Reporting Items for Systematic reviews and Meta-Analyses). The same study could be excluded for multiple reasons.
A study was classified as being from an endemic infectious region or population when the study reported presence of infectious lung diseases in the population from which participants were recruited or if granulomas arising from infectious lung diseases comprised at least 50% of reported benign etiologies. PET scan results were described by method of measuring FDG-PET avidity, the levels of risk or avidity, and the standardized uptake value threshold used to differentiate benign and cancerous diagnosis of disease. Details of study selection, data extraction, and synthesis are described in the statistical supplement.
Data Synthesis and Analysis
The sensitivity and specificity for FDG-PET test performance (pooled across studies) are displayed using forest plots. Study heterogeneity was assessed with an I2 statistic. Test performance in the presence of heterogeneity17 was summarized using hierarchical summary receiver operator curves (HSROC). Stability of test accuracy over time was assessed with diagnostic odds ratios and in the context of a random effects model.
Publication bias was visually inspected using a funnel plot and quantitatively measured (see statistical appendix).18 Study quality was measured using a modified Quality Assessment of Diagnostic Accuracy Studies questionnaire (see statistical appendix for methodology).19,20 Verification bias was defined as occurring when all diagnoses were determined pathologically or when a pathological diagnosis was coupled with a period of radiographic surveillance shorter than 12 months.21,22
A large I2 value indicates the data are not consistent with a simple pooling model; a more sophisticated model is needed to properly combine the data. Accordingly, a random-effects logistic regression was used to model test performance and account for heterogeneity among studies not attributable to observed study characteristics.
Study characteristics included in the model were endemic infectious lung disease in the study population, mean or median lesion diameter of less than 2 cm, scanner type as either PET only, combined PET and CT, or PET and another scanning modality (eg, time delayed PET, magnetic resonance imaging, volumetric CT), study quality score, and whether the study relied on only pathological determination of diagnosis. In studies with multiple imaging modalities, only the FDG-PET/CT portion of results were used (see Appendix Table 3).
Quality score was dichotomized with higher-quality studies (defined as those having at least 70% [≥11] affirmative quality questions).23 The final model equations, procedures used for model selection, methods of assessing model fit, and details on numerical fitting appear in the statistical appendix. Missing data were handled with multiple imputation performed using chained equations (10 imputations were used; details appear in the statistical appendix).
The regression model provides an estimate of sensitivity (or specificity) adjusted to a particular set of study characteristics (ie, a particular study profile). These adjusted estimates are then averaged together to yield a single, average estimate of sensitivity (or specificity). This estimate, which we refer to as the average adjusted estimate, is similar to a simple pooled unadjusted estimate of sensitivity or specificity, except that the average adjusted estimate now properly accounts for the various study profiles observed in our sample.
To maintain generalizability, the averaging of adjusted estimates occurs with respect to the observed frequency of study profiles in our sample. We report the average adjusted estimates of sensitivity and specificity because of the ease of their interpretation and general applicability. We also calculated certain adjusted estimates of sensitivity and specificity as a sensitivity analysis to ensure result robustness. We defined rigorously conducted and well-controlled studies as studies with high quality, lesion size of less than 2 cm, use of adiagnostic method that minimizes the likelihood of verification bias, and use of combined PET and CT.
In addition to using 95% confidence intervals to estimate population parameters, we used 95% prediction intervals (PIs) to estimate the anticipated performance of a single study randomly chosen from this population of studies. The PIs describe the population heterogeneity of test performance. All statistical tests were 2-sided with a type I error of .05. All analyses were performed with Stata version 12 (StataCorp) and R 2.15.3 (R Foundation for Statistical Computing).
Results
A total of 1923 articles were found; 16 articles were added from bibliography reviews along with an unpublished abstract. Forty-six articles were removed as duplicates and 1893 studies were screened. Upon initial abstract review, 1636 articles were excluded. An article could be excluded for multiple reasons, but the most common reason for exclusion during either portion of the review was inclusion of participants with 100% cancer prevalence (n=1025).
Two hundred and fifty-seven studies received full review, of which 187 were excluded upon secondary review. The remaining 70 studies met all inclusion criteria and were used for the final analysis (Appendix Table 2). The total number of reported nodules being evaluated by FDG-PET among the 70 studies was 8511 and median number of nodules per study was 83 (interquartile range, 56-140 nodules/study). Pooled cancer prevalence among nodules was 60% (n=5105 nodules). Individual study cancer prevalence varied from 21% to 86% across studies. Ten of 70 studies documented endemic infectious lung disease.10,11,14,15,24-29
The overall agreement for study eligibility between reviewers was 94.8%, and the K was 0.72 (using the method by Cohen), showing moderate agreement between reviewers. Consensus was used when reviewers disagreed; agreement was not reviewed quantitatively. Despite contacting corresponding authors, missing data on mean or median nodule size remained in 12 studies. Among the 49 studies reporting a mean or median lesion size, the median lesion size across studies was 2 cm (interquartile range, 1.7-2.8 cm) (Appendix Table 2).
Meta-analysis
An unadjusted pooled analysis of the 70 studies showed evidence of significant heterogeneity among studies in sensitivity, (I2 of 87% [95% CI, 85%-90%]) and specificity (I2 of 82% [95% CI, 78%-86%]). Pooled sensitivity of FDG-PET for diagnosing lung cancer was 89% (95% CI, 86%-91%) and pooled specificity was 75% (95% CI, 71%-79%).
Ten studies reporting endemic disease had an unadjusted pooled specificity of 54% (95% CI, 37%-69%)10,11,14,15,24-29 compared with 78% (95% CI, 74%-81%) in the remaining 60 studies. The asymmetry test (using the method by Deeks et al18) did not show evidence of publication bias (Ƥ = .14). No trend over time or between periods in diagnostic accuracy was observed (supplemental appendix).
A random-effects model that included a random intercept for each study and various fixed effects for the study characteristics (see statistical appendix for details) was used to account for the observed heterogeneity. The model yielded an average adjusted estimate of sensitivity of 89% (95% CI, 87%-91%) and specificity of 75% (95% CI, 71%-78%) (Figure 2)30-89.
Figure 2. Individual study estimates of sensitivity and specificity with average adjusted results.
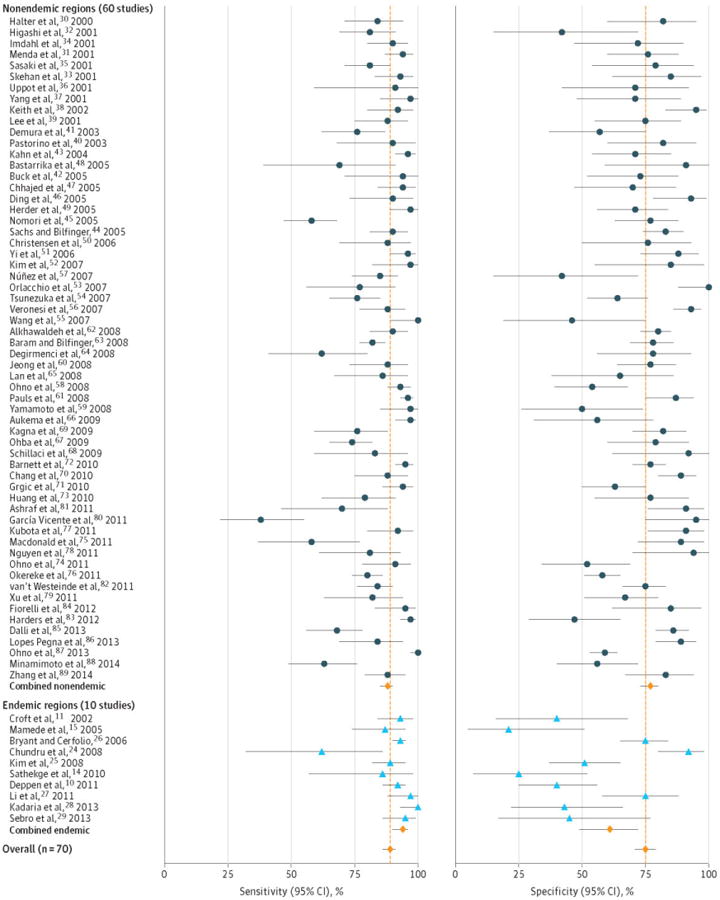
Forest plot reporting individual study sensitivity and specificity. Endemic infectious lung disease with are in blue and nonendemic studies are in black. Average adjusted results for endemic studies (n=10), non-endemic studies (n=60) and all studies combined (n=70) sensitivity and specificity are in red. Error bars are 95% confidence intervals for each study's corresponding test characteristic.
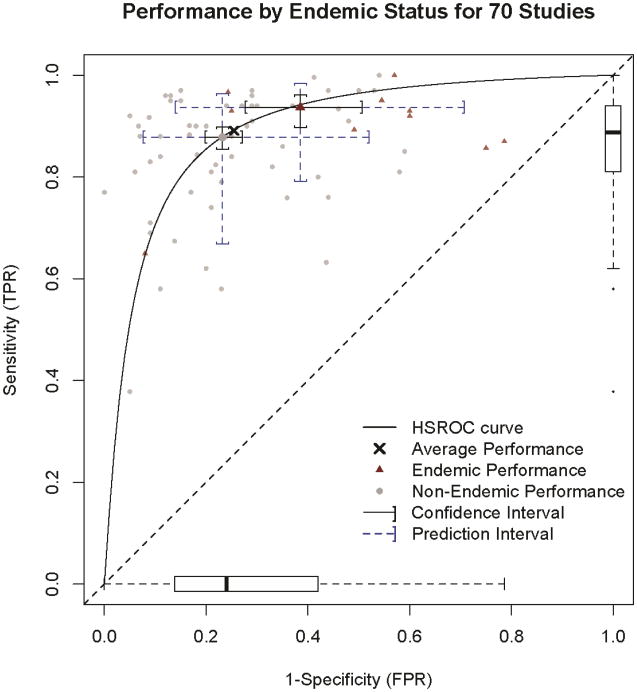
The area under the HSROC curve (Figure 3) was 0.90 (95% CI, 0.87-0.92). The PIs show the extreme amount of heterogeneity among studies that remains after adjusting for study characteristics. The sensitivity of a randomly chosen study was predicted as 89% (95% PI, 70%-97%) and specificity was 75% (95% PI, 45%-91%). Similar increases in PI length were observed for all analyses. The results presented in the following sections are adjusted results from the random-effects model using multiple imputation.
Figure 3. Performance by endemic status for 70 studies.
Hierarchical Summary Receiver Operator (HSROC) Curve with operating points for endemic and nonendemic infectious lung disease studies and 95% Confidence and Prediction Intervals for those operating points. The horizontal box and whiskers plot represents the distribution of study specificity, and the vertical box and whiskers plot represents the distribution of study sensitivity. The box limits are the closest data point to the interquartile range of 25 and 75% with the bar being the median (50%). Error bar whiskers represent the data point closest to 1.5 times the interquartile range and the dots outside the whiskers represent outlier study values.
Infectious Lung Disease
Ten studies reporting infectious lung disease endemic to the local population comprised 1431 individuals, of whom 1082 had cancer (76%). Granulomas as a percentage of benign diagnoses ranged from 45%26 to 75%.14 Studies of populations in China,27 South Africa14 and Japan15 reported tuberculosis as the common etiology for granulomatous disease.
The remaining North American studies found histoplasmosis, coccidioidomycosis, inflammation and unspecified granuloma as common etiologies for pathologically diagnosed benign disease. Four of the ten studies were retrospective10,25,28,29 and 7 studies10,11,14,15,25,26,29 determined diagnosis with pathology only.
The specificity was estimated to be 16% lower in populations with endemic infectious lung disease. This lower specificity persisted at 14% even for rigorously conducted and well-controlled studies. The average adjusted estimate of specificity in regions with endemic disease was 61% (95% CI, 49%-72%) compared with 77% (95% CI, 73%-80%) for nonendemic populations (Figure 3). For rigorously conducted and well-controlled studies, the estimates of specificity in endemic and nonendemic regions were 66% (95% CI, 51%-78%) and 80% (95% CI, 74%-85%), respectively.
The average adjusted sensitivity did not significantly differ by endemic status (94% [95% CI, 90%-96%] vs 88% [95% CI, 85%-90%] in nonendemic regions). The adjusted estimate of sensitivity in rigorously conducted and well-controlled studies was slightly higher in endemic regions (96% [95% CI, 92%-98%] vs 90% [95% CI, 86%-93%] in nonendemic regions).
Size of Lesion
Among the 34 studies reporting average or median lesion diameters of less than or equal to 2 cm, the average adjusted sensitivity was 87% (95% CI, 84%-90%).14,24,26-29,32,39,45,46,48,50,52-54,58,60,62,64,67-69,72,74,75,77,80-83,85-88 In comparison, 23 studies with an average or median diameter greater than 2 cm had a slightly higher average adjusted sensitivity (91% [95% CI, 89%-93%]).10,11,15,25,30,31,34,35,37,41,43,44,55-57,61,63,73,76,78,79,84,86 Specificity of FDG-PET to diagnose lung cancer was not significantly influenced by lesion size (74% for studies with lesions ≤2 cm and 75% for studies with larger average lesion size).
Type of FDG-PET Scan
The 40 studies using FDG-PET/CT demonstrated slightly better average adjusted sensitivity (90% [95% CI, 88%-92%]) compared with the 19 studies using only FDG-PET (89% [95% CI, 84%-92%]) or the studies combining FDG-PET with another method of imaging (82% [95% CI, 75%-89%]).11,15,25,30,32-37,39,40,47,49-51,54,57,75,90 Scanner type had little association with specificity. The average adjusted specificity for studies that used a FDG-PET or FDG-PET/CT in combination with another imaging modality (75%) was similar to studies using only FDG-PET/CT (76%).38,41-43,53,62,75,79,80,87
PET not combined with CT or with other imaging modalities had a slightly worse average adjusted specificity of 70% (95% CI, 62%-77%) compared with FDG-PET/CT or FDG-PET plus another imaging modality. Among the other imaging modalities reported, 2 studies used single-photon emission CT as the alternative secondary scanning modality.41,43 Two studies used dynamic, 3-dimensional CT scanning.53,87 Two studies reported using F18-fluorothyminidine in conjunction with FDG.42,79 One study used a sodium iodide detector38 and 3 studies created algorithms of staggered PET scans and changes in standard uptake values (dual-time point).28,62,80
Study Quality
Study quality scores ranged from 3 to 14 (median score was 10 of 15 possible points). The quality metric that most studies failed to meet was patients receiving the same reference standard regardless of index test result (67%). Studies often lacked sufficient numbers of benign cases (29 studies had <25 benign cases). Most studies (81%) emulated the use of the FDG-PET scan in current clinical practice.
Lower-quality studies had reduced average adjusted sensitivity (87%[95% CI, 85%-90%]) compared with higher-quality studies (91%[95% CI, 88%-93%]) after controlling for other study characteristics in the regression model. Average adjusted specificity was similar across lower-quality studies (75%) compared with higher-quality studies (74%). Studies that relied on either pathological diagnosis or less than 1 year of follow-up had similar average adjusted sensitivity (87% [95% CI, 83%-90%) compared with those that did not (90% [95% CI, 88%-92%]).
The average adjusted specificity among studies that relied on a combination of prolonged surveillance and pathological diagnosis had higher average adjusted specificity (77% [95% CI, 73%-81%]) than those that exhibited possible verification bias (69% [95% CI, 61%-75%]). Additional study quality results are provided in the statistical supplement.
Sensitivity Analysis for the Effect of Individual Studies on Pooled Estmates
Removal of the largest study by Bryant and Cerfolio26 (n=585), which reported endemic infectious lung disease, reduced the average adjusted specificity from 61% to 56% in studies with endemic infectious lung disease. Its removal had little influence on the sensitivity of the test in either average adjusted results or in the endemic infectious lung disease populations. A sensitivity analysis using the distance method by Cook identified 1 study (Garcia Vicente et al80) as potentially over influential. However, its exclusion did not noticeably change results. No individual study unduly influenced the estimated sensitivity or specificity of FDG-PET.
Discussion
For the last decade, molecular imaging with FDG-PET has become part of the diagnostic arsenal of tests considered for the evaluation of suspicious lung nodules. This method of imaging is suggested based on low-quality evidence (grade 2C) for the diagnosis of solid nodules larger than 8mm.2 The limitation of FDG-PET in the diagnosis of smaller lesions is well documented and this meta-analysis also found studies reporting lesions smaller than 2 cm had lower sensitivity compared with studies reporting on larger nodules.3,91,92 Previous meta-analyses found FDG-PET to be highly sensitive (94% to 96%) and reasonably specific (78% to 86%) in the diagnosis of lung cancer.5,6 Compared to prior studies, the sensitivity and specificity in our meta-analysis was lower. The HSROC was 0.9, which is similar to that reported by Gould et al6, and our study also exhibited heterogeneity across studies.
In the 2001 meta-analysis,6 727 of the 1474 (49%) lesions were from Japanese or European populations.6 Also, a portion of the studies in the meta-analysis were populations from the northeast or other areas of the United States where granulomatous disease is less common. Similarly in the study by Cronin et al,5 860 of the 1190 lesions (72%) reported in the 22 studies reviewed were from geographic areas where infectious lung disease is rare.
In regions where infectious lung disease is highly prevalent, the specificity of FDG-PET scans to diagnose lung nodules suspicious for lung cancer in our study was approximately 61%. However, the best specificity in endemic regions (from either the average adjusted or adjusted results) was 66%. Therefore, in individuals being evaluated for a suspicious lung lesion, and who reside in a region with significant endemic infectious lung disease, FDG-PET/CT scan does not reliably distinguish benign disease from lung cancer.
We have shown that the specificity of FDG-PET/CT for the diagnosis of lung cancer was overstated in regions with endemic infectious lung disease and could lead to unnecessary biopsies or thoracotomies for indeterminate lung nodules. Knowledge of this limitation in such regions is especially important should low-dose CT screening for lung cancer is widely adopted and should be reflected in current nodule management guidelines.2,3
Our review included more studies and had greater heterogeneity in both sensitivity and specificity when compared to earlier meta-analyses by Gould et al6 and Cronin et al.5 Some heterogeneity across studies arises from the scanning method, the size of the lesion examined, whether the study relied only on pathological verification of cancer, and the prevalence of endemic infectious lung disease in the study population. However, there remained substantial variability among studies in test performance that was not accounted for by these factors.
We observed a transition in the literature from scanners using FDG-PET only to FDG-PET/CT since their introduction into clinical practice in 2001.93,94 Recently, radiologists have undertaken significant efforts to find a complement or replacement for the FDG radionuclide or the positron emission image-generating scanner.42,95-98
We attempted to include the breadth of research in PET for lung nodule diagnosis by searching for studies that compared new modalities or radio nuclides to existing FDG-PET or PET plus CT. Multimodality studies collectively had a higher specificity (80%) compared to studies using either FDG-PET or CT alone, but as a group they may be susceptible to publication bias that potentially decreases the accuracy of FDG-PET compared with the newer imaging methods.
To date, no replacement for FDG has been suggested for the diagnosis of lung nodules suspicious for lung cancer.91,99 In addition, a majority of participants (n=4615) were in studies in which the mean or median lesion size was less than or equal to 2 cm. The lower sensitivity observed in this analysis arises, in part, from the application of this diagnostic modality to a broader clinical population with both smaller lesions and a greater likelihood of infectious disease.
After adjusting for study characteristics in our model, the precision of estimated sensitivity and specificity is quite good (shown by the narrow 95% CIs in Figure 3). However, variability remains even after adjusting for known study characteristics as shown in the distribution of individual study sensitivity and specificity estimates and combined PIs.
The range of test performance observed in practice is quite large (shown by the wide PIs in Figure 3). These reflect a lack of consistency in the application of FDG-PET diagnosis for lung nodules that is concerning and this meta-analysis suggests significant variability in practice patterns. Accordingly, technical standards and consistent adherence to imaging protocols and image interpretation should be strictly followed to reduce these inconsistencies. This is especially important in smaller lesions (<2cm) and in regions with endemic infectious lung disease to prevent false-positive and negative test results that could cause harm to patients.
The limitations of this analysis are those common to meta-analyses (eg, publication bias, selection bias, limited information from study reports, and potential for ecological fallacy). Even though we did not find evidence of significant publication bias, this does not exclude its possibility. Because FDG-PET was recommended for the diagnosis of lung cancer, a publication bias to report poor FDG-PET accuracy or negative results may exist.
Studies reporting results from scanners with FDG-PET only may no longer reflect clinical practice and arguably should not be included in this analysis. However, we controlled for the shortcomings of scanners with FDG-PET only in the regression model so that additional studies reporting results from smaller lesions and from regions with endemic infectious lung disease could be explored.
Although the accuracy of FDG-PET/CT is superior to the accuracy of FDG-PET only, we included both modalities because they are still in use in the United States and elsewhere, and, as previously stated, we did not find a significant difference in specificity based on these 2scanner types. To avoid selection bias, this meta-analysis attempted to broadly review studies reporting use of FDG-PET to characterize lung nodules and examined studies in which FDG-PET/CT was compared with other imaging modalities for the diagnosis and staging of lung cancer. We controlled for study heterogeneity using a random-effects regression model with a number of clinically important covariates; however, residual confounding may still be present.
In this large meta-analysis, the observed association between lower specificity and endemic infectious lung disease appeared robust across sensitivity analyses. We found that studies that fully used the metabolic and anatomic information from a FDG-PET/CT scan in a semiquantitative interpretation (rather than a simplified dichotomizing of a standard uptake value) demonstrated improved test accuracy. Even in areas of endemic disease, robust reading methods by experienced readers generated accurate scans.26,27
Until this expertise and method is more uniformly applied among scan readers, FDG-PET for the diagnosis of lung cancer in patients who reside in a region with significant endemic infectious lung disease should be recognized as having lower specificity (approximately 61%) than previously reported. Knowledge of this reduction in specificity should limit the use of FDG-PET to diagnose lung cancer unless substantial institutional expertise in FDG-PET interpretation has been proven. Should low-dose CT screening for lung cancer become the diagnostic standard, knowledge of FDG-PET/CT performance is even more critical because the vast majority of indeterminate lung nodules detected through screening are benign.100
Conclusions
The accuracy of FDG-PET for diagnosing lung nodules was extremely heterogeneous. Use of FDG-PET/CT was less specific in diagnosing malignancy in populations with endemic infectious lung disease compared with nonendemic regions. These data do not support the use of FDG-PET to diagnose lung cancer in endemic regions unless an institution achieves test performance accuracy similar to that found in nonendemic regions.
Supplementary Material
Acknowledgments
Authors wish to thank Rebecca Jerome, MLS, MPH research librarian Vanderbilt University Medical Center, for her uncompensated assistance in the literature review. We also wish to thank the reviewers for their insight and assistance in making our manuscript stronger. Drs. Grogan and Deppen had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
This work has been supported by R03HS021554-01 from AHRQ (E.L.G.). This work was also supported by: Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service Career Development Award 10-024 (E.L.G.), NIH/NCI K07 CA172294 (M.C.A.), Vanderbilt Institute for Clinical and Translational Research grant, UL1TR000011 from NCATS/NIH (REDCap database), the lung SPORE CA90949 (P.P.M.), and a EDRNU01 CA152662 (P.P.M.).
References
- 1.Humphrey LL, Deffebach M, Pappas M, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Annals of Internal Medicine. 2013;159(6):411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 2.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer?: diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. CHEST Journal. 2013;143(5_suppl):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Lung Cancer Screening v2.2014. [Accessed 05-20-2014];NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2014 http://www.nccn.org/professionals/physician_gls/recently_updated.asp.
- 4.MacMahon H, Austin J, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 5.Cronin P, Dwamena B, Kelly A, Carlos R. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology. 2008;246:772–782. doi: 10.1148/radiol.2463062148. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001 Feb 21;285(7):914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 7.Herder GJ, Golding RP, Hoekstra OS, et al. The performance of 18F-fluorodeoxyglucose positron emission tomography in small solitary pulmonary nodules. Euro J Nucl Med Mol Imaging. 2004;31(9):1231–1236. doi: 10.1007/s00259-004-1552-7. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines. Chest. (2nd) 2007 Sep;132(3 Suppl):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 9.Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. CHEST Journal. 2013;143(5_suppl):e191S–e210S. doi: 10.1378/chest.12-2354. [DOI] [PubMed] [Google Scholar]
- 10.Deppen S, Putnam JB, Jr, Andrade G, et al. Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg. 2011 Aug;92(2):428–432. doi: 10.1016/j.athoracsur.2011.02.052. discussion 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft DR, Trapp J, Kernstine K, et al. FDG-PET imaging and the diagnosis of non-small cell lung cancer in a region of high histoplasmosis prevalence. Lung Cancer. 2002;36(3):297–301. doi: 10.1016/s0169-5002(02)00023-5. [DOI] [PubMed] [Google Scholar]
- 12.Mason R, Broaddus V, Martin T, Murray J, Nadel J, et al., editors. Murray and Nadel's Textbook of Respiratory Medicine. 5th. Vol. 2 Saunders; 2010. [Google Scholar]
- 13.Bradsher RW. Histoplasmosis and Blastomycosis. Clin Inf Dis. 1996;22:S102–S111. doi: 10.1093/clinids/22.supplement_2.s102. [DOI] [PubMed] [Google Scholar]
- 14.Sathekge M, Maes A, Pottel H, Stoltz A, van de Wiele C. Dual time-point FDG-PET-CT for differentiating benign form malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J. 2010;100(9):598–601. doi: 10.7196/samj.4082. [DOI] [PubMed] [Google Scholar]
- 15.Mamede M, Higashi T, Kitaichi M, et al. 18F-Fdg Uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancer and inflammatory lesions of the lung. Neoplasia. 2005;7(4):369–379. doi: 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–2884. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 18.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology. 2005;58(9):882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Fontela PS, Pant Pai N, Schiller I, Dendukuri N, Ramsay A, Pai M. Quality and Reporting of Diagnostic Accuracy Studies in TB, HIV and Malaria: Evaluation Using QUADAS and STARD Standards. PLoS One. 2009;4(11):e7753. doi: 10.1371/journal.pone.0007753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiting P, Rutjes A, Reitsma J, Bossuyt P, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(1):25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003 Jun 19;348(25):2535–2542. doi: 10.1056/NEJMcp012290. [DOI] [PubMed] [Google Scholar]
- 22.Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: Part 2: pretest probability and algorithm. CHEST Journal. 2013;143(3):840–846. doi: 10.1378/chest.12-1487. [DOI] [PubMed] [Google Scholar]
- 23.Gould M, Kuschner W, Rydzak C. Test performance of Positron Emission Tomography and Computed Tomography for mediastinal staging in patients with non small cell lung cancer: a meta analysis. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 24.Chundru S, Wong CY, Wu D, et al. Granulomatous disease: is it a nuisance or an asset during PET/computed tomography evaluation of lung cancers? Nucl Med Commun Jul. 2008;29(7):623–627. doi: 10.1097/MNM.0b013e3282fdc979. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Machac J, Krynyckyi B, et al. Fluoro-deoxy-glucose positron emission tomography for evaluation of indeterminate lung nodules: assigning a probability of malignancy may be preferable to binary readings. Ann Nucl Med. 2008;22(3):165–170. doi: 10.1007/s12149-007-0112-1. [DOI] [PubMed] [Google Scholar]
- 26.Bryant AS, Cerfolio RJ. The Maximum Standardized Uptake Values on Integrated FDG-PET/CT Is Useful in Differentiating Benign From Malignant Pulmonary Nodules. Ann Thorac Surg. 2006;82(3):1016–1020. doi: 10.1016/j.athoracsur.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Su M, Li F, Kuang A, Tian R. The value of 18F-FDG-PET/CT in the differential diagnosis of solitary pulmonary nodules in areas with a high incidence of tuberculosis. Ann Nucl Med. 2011;25(10):804–811. doi: 10.1007/s12149-011-0530-y. [DOI] [PubMed] [Google Scholar]
- 28.Kadaria D, Archie DS, SultanAli I, Weiman DS, Freire AX, Zaman MK. Dual Time Point Positron Emission Tomography/Computed Tomography Scan in Evaluation of Intrathoracic Lesions in an Area Endemic for Histoplasmosis and With High Prevalence of Sarcoidosis. The American Journal of the Medical Sciences. 2013;346(5):358–362. doi: 10.1097/MAJ.0b013e31827b9b6d. [DOI] [PubMed] [Google Scholar]
- 29.Sebro R, Aparici C, Hernandez-Pampaloni M. FDG PET/CT evaluation of pathologically proven pulmonary lesions in an area of high endemic granulomatous disease. Ann Nucl Med. 2013;27(4):400–405. doi: 10.1007/s12149-013-0695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halter G, Storck M, Guhlmann A, Frank J, Grosse S, Liewald F. FDG positron emission tomography in the diagnosis of peripheral pulmonary focal lesions. Thorac Cardiovasc Surg. 2000 Apr;48(2):97–101. doi: 10.1055/s-2000-9875. [DOI] [PubMed] [Google Scholar]
- 31.Menda Y, Bushnell DL, Madsen H, McLaughlin K, Kahn D, Kernstine KH. Evaluation of various corrections to the standardized uptake value for diagnosis of pulmonary malignancy. Nucl Med Commun. 2001;22(10):1077–1081. doi: 10.1097/00006231-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Higashi K, Ueda Y, Sakuma T, et al. Comparison of [(18)F]FDG PET and (201)Tl SPECT in evaluation of pulmonary nodules. J Nucl Med. 2001 Oct;42(10):1489–1496. [PubMed] [Google Scholar]
- 33.Skehan S, Coates G, Otero C, O'Donovan N, Pelling M, Nahmias C. Visual and semiquantitative analysis of 18F-fluorodeoxyglucose positron emission tomography using a partial-ring tomograph without attenuation correction to differentiate benign and malignant pulmonary nodules. Can Assoc Radiol J. 2001;52(4):259–265. [PubMed] [Google Scholar]
- 34.Imdahl A, Jenkner S, Brink I, et al. Validation of FDG positron emission tomography for differentiation of unknown pulmonary lesions. Eur J Cardiothorac Surg. 2001;20(2):324–329. doi: 10.1016/s1010-7940(01)00800-4. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki M, Kuwabara Y, Yoshida T, et al. Comparison of MET-PET and FDG-PET for differentiation between benign lesions and malignant tumors of the lung. Ann Nucl Med. 2001;15(5):425–431. doi: 10.1007/BF02988346. [DOI] [PubMed] [Google Scholar]
- 36.Uppot R, Conde K, Sagar V, Manzone T. Positron emission tomography (PET) imaging for solitary pulmonary nodules--review of the Delaware experience. Del Med J. 2001;73(10):381–385. [PubMed] [Google Scholar]
- 37.Yang SN, Liang JA, Lin FJ, Kwan AS, Kao CH, Shen YY. Differentiating benign and malignant pulmonary lesions with FDG-PET. Anticancer Res. 2001 Nov-Dec;21(6A):4153–4157. [PubMed] [Google Scholar]
- 38.Keith C, Miles K, Griffiths M, Wong D, Pitman A, Hicks R. Solitary pulmonary nodules: accuracy and cost-effectiveness of sodium iodide FDG-PET using Australian data. Eur J Nucl Med Mol Imaging. 2002;29(8):1016–1023. doi: 10.1007/s00259-002-0833-2. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Aronchick JM, Alavi A. Accuracy of F-18 fluorodeoxyglucose positron emission tomography for the evaluation of malignancy in patients presenting with new lung abnormalities: a retrospective review. Chest. 2001 Dec;120(6):1791–1797. doi: 10.1378/chest.120.6.1791. [DOI] [PubMed] [Google Scholar]
- 40.Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003 Aug 23;362(9384):593–597. doi: 10.1016/S0140-6736(03)14188-8. [DOI] [PubMed] [Google Scholar]
- 41.Demura Y, Tsuchida T, Ishizaki T, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med. 2003 Apr;44(4):540–548. [PubMed] [Google Scholar]
- 42.Buck A, Hetzel M, Schirrmeister H, et al. Clinical relevance of imaging proliferative activity in lung nodules. Euro J Nucl Med Mol Imaging. 2005;32(5):525–533. doi: 10.1007/s00259-004-1706-7. [DOI] [PubMed] [Google Scholar]
- 43.Kahn D, Menda Y, Kernstine K, et al. The Utility of 99mTc Depreotide Compared With F-18 Fluorodeoxyglucose Positron Emission Tomography and Surgical Staging in Patients With Suspected Non-small Cell Lung Cancer*. Chest. 2004;125(2):494–501. doi: 10.1378/chest.125.2.494. [DOI] [PubMed] [Google Scholar]
- 44.Sachs S, Bilfinger TV. The impact of positron emission tomography on clinical decision making in a university-based multidisciplinary lung cancer practice*. Chest. 2005;128(2):698–703. doi: 10.1378/chest.128.2.698. [DOI] [PubMed] [Google Scholar]
- 45.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Visual and Semiquantitative Analyses for F-18 Fluorodeoxyglucose PET Scanning in Pulmonary Nodules 1 cm to 3 cm in Size. Ann Thorac Surg. 2005;79(3):984–988. doi: 10.1016/j.athoracsur.2004.07.072. [DOI] [PubMed] [Google Scholar]
- 46.Ding QY, Hua YQ, Zhang GZ, et al. A controlled study of positron-emission-tomography and positron-emission-tomography/computed tomography in differential diagnosis of solitary pulmonary nodules--report of 60 cases. Chin Med J (Engl) 2005 Sep 20;118(18):1572–1576. [PubMed] [Google Scholar]
- 47.Chhajed P, Bernasconi M, Gambazzi F, Bubendorf L, Rasch H, Kneifel S. Combining bronchoscopy and positron emission tomography for the diagnosis of the small pulmonary nodule < or = 3 cm. Chest. 2005;128:3558–3564. doi: 10.1378/chest.128.5.3558. [DOI] [PubMed] [Google Scholar]
- 48.Bastarrika G, Garcia-Velloso MJ, Lozano MD, et al. Early Lung Cancer Detection using Spiral Computed Tomography and Positron Emission Tomography. Am J Respir Crit Care Med. 2005 Mar 24; doi: 10.1164/rccm.200411-1479OC. [DOI] [PubMed] [Google Scholar]
- 49.Herder GJ, van Tinteren H, Golding RP, et al. Clinical Prediction Model To Characterize Pulmonary Nodules*. Chest. 2005;128(4):2490–2496. doi: 10.1378/chest.128.4.2490. [DOI] [PubMed] [Google Scholar]
- 50.Christensen JA, Nathan MA, Mullan BP, Hartman TE, Swensen SJ, Lowe VJ. Characterization of the Solitary Pulmonary Nodule: 18F-FDG PET Versus Nodule-Enhancement CT. Am J Roentgenol. 2006 Nov 1;187(5):1361–1367. doi: 10.2214/AJR.05.1166. 2006. [DOI] [PubMed] [Google Scholar]
- 51.Yi CA, Lee KS, Kim BT, et al. Tissue Characterization of Solitary Pulmonary Nodule: Comparative Study Between Helical Dynamic CT and Integrated PET/CT. J Nucl Med. 2006;47(3):443–450. [PubMed] [Google Scholar]
- 52.Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in Characterization of Solitary Pulmonary Lesions. J Nucl Med. 2007;48(2):214–220. [PubMed] [Google Scholar]
- 53.Orlacchio A, Schillaci O, Antonelli L, D'Urso S, Sergiacomi G, Nicoli P. Solitary pulmonary nodules: morphological and metabolic characterisation by FDG-PET-MDCT. Radiol Med (Torino) 2007;112:157–173. doi: 10.1007/s11547-007-0132-x. [DOI] [PubMed] [Google Scholar]
- 54.Tsunezuka Y, Shimizu Y, Tanaka N, Takayanagi T, Kawano M. Positron Emission Tomography in Relation to Noguchi's Classification for Diagnosis of Peripheral Non-Small-Cell Lung Cancer 2 cm or Less in Size. World J Surg. 2007;31(2):314–317. doi: 10.1007/s00268-006-0475-9. [DOI] [PubMed] [Google Scholar]
- 55.Wang F, Wang Z, Yao W, Xie H, Xu J, Tian L. Role of 99mTc-Octreotide Acetate Scintigraphy in Suspected Lung Cancer Compared with 18F-FDG Dual-Head Coincidence Imaging. J Nucl Med. 2007;48(9):1442–1448. doi: 10.2967/jnumed.107.040824. [DOI] [PubMed] [Google Scholar]
- 56.Veronesi G, Bellomi M, Veronesi U, et al. Role of Positron Emission Tomography Scanning in the Management of Lung Nodules Detected at Baseline Computed Tomography Screening. Ann Thorac Surg. 2007;84(3):959–966. doi: 10.1016/j.athoracsur.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 57.Núñez R, Kalapparambath A, Varela J. Improvement in sensitivity with delayed imaging of pulmonary lesions with FDG-PET. Revista Española de Medicina Nuclear (English Edition) 2007;26(4):196–207. doi: 10.1157/13107971. [DOI] [PubMed] [Google Scholar]
- 58.Ohno Y, Koyama H, Takenaka D, et al. Dynamic MRI, dynamic multidetector-row computed tomography (MDCT), and coregistered 2-[fluorine-18]-fluoro-2-deoxy-D-glucose–positron emission tomography (FDG-PET)/CT: Comparative study of capability for management of pulmonary nodules. Journal of Magnetic Resonance Imaging. 2008;27(6):1284–1295. doi: 10.1002/jmri.21348. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto Y, Nishiyama Y, Ishikawa S, et al. 3′-Deoxy-3′-18F-Fluorothymidine as a Proliferation Imaging Tracer for Diagnosis of Lung Tumors: Comparison With 2-Deoxy-2-18F-Fluoro-D-Glucose. J Cardiovasc Comput Tomogr. 2008;32(3):432–437. doi: 10.1097/RCT.0b013e3180980db9. [DOI] [PubMed] [Google Scholar]
- 60.Jeong SY, Lee KS, Shin KM, et al. Efficacy of PET/CT in the characterization of solid or partly solid solitary pulmonary nodules. Lung Cancer. 2008;61(2):186–194. doi: 10.1016/j.lungcan.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 61.Pauls S, Buck A, Halter G, et al. Performance of Integrated FDG-PET/CT for Differentiating Benign and Malignant Lung Lesions -Results from a Large Prospective Clinical Trial. Mol Imaging Biol. 2008;10(2):121–128. doi: 10.1007/s11307-007-0129-9. [DOI] [PubMed] [Google Scholar]
- 62.Alkhawaldeh K, Bural G, Kumar R, Alavi A. Impact of dual-time-point 18F-FDG PET imaging and partial volume correction in the assessment of solitary pulmonary nodules. Euro J Nucl Med Mol Imaging. 2008;35(2):246–252. doi: 10.1007/s00259-007-0584-1. [DOI] [PubMed] [Google Scholar]
- 63.Baram D, Bilfinger T. Interaction of clinical suspicion and PET in the diagnosis of suspected thoracic malignancy. Med Sci Monit. 2008;14(7):CR379–383. [PubMed] [Google Scholar]
- 64.Degirmenci B, Wilson D, Laymon CM, et al. Standardized uptake value-based evaluations of solitary pulmonary nodules using F-18 fluorodeoxyglucose-PET/computed tomography. Nucl Med Commun. 2008;29(7):614–622. doi: 10.1097/MNM.0b013e3282f9b5a0. [DOI] [PubMed] [Google Scholar]
- 65.Lan XL, Zhang YX, Wu ZJ, Jia Q, Wei H, Gao ZR. The value of dual time point 18F-FDG PET imaging for the differentiation between malignant and benign lesions. Clin Radiol. 2008;63(7):756–764. doi: 10.1016/j.crad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Aukema TS, Valdés Olmos RA, Klomp HM, et al. Evaluation of 18F-FDG PET-CT for Differentiation of Pulmonary Pathology in an Approach of Outpatient Fast Track Assessment. J Thorac Oncol. 2009;4(10):1226–1230. doi: 10.1097/JTO.0b013e3181b2b782. [DOI] [PubMed] [Google Scholar]
- 67.Ohba Y, Nomori H, Shibata H, et al. Evaluation of Semiquantitative Assessments of Fluorodeoxyglucose Uptake on Positron Emission Tomography Scans for the Diagnosis of Pulmonary Malignancies 1 to 3 cm in Size. Ann Thorac Surg. 2009;87(3):886–891. doi: 10.1016/j.athoracsur.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 68.Schillaci O, Travascio L, Bolacchi F, et al. Accuracy of early and delayed FDG PET-CT and of contrast-enhanced CT in the evaluation of lung nodules: a preliminary study on 30 patients. Radiol med. 2009;114(6):890–906. doi: 10.1007/s11547-009-0400-z. [DOI] [PubMed] [Google Scholar]
- 69.Kagna O, Solomonov A, Keidar Z, et al. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer. Eur J Nucl Med Mol Imaging. 2009 Jun;36(6):997–1004. doi: 10.1007/s00259-009-1061-9. [DOI] [PubMed] [Google Scholar]
- 70.Chang CY, Tzao C, Lee SC, et al. Incremental Value of Integrated FDG-PET/CT in Evaluating Indeterminate Solitary Pulmonary Nodule for Malignancy. Mol Imaging Biol. 2010;12(2):204–209. doi: 10.1007/s11307-009-0241-0. [DOI] [PubMed] [Google Scholar]
- 71.Grgic A, Yüksel Y, Gröschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using 18F-fluorodeoxyglucose and SUV quantification. Euro J Nucl Med Mol Imaging. 2010;37(6):1087–1094. doi: 10.1007/s00259-010-1387-3. [DOI] [PubMed] [Google Scholar]
- 72.Barnett PG, Ananth L, Gould MK VA-SNAP Group. Cost and Outcomes of Patients With Solitary Pulmonary Nodules Managed With PET Scans. Chest. 2010 Jan 1;137(1):53–59. doi: 10.1378/chest.08-0529. 2010. [DOI] [PubMed] [Google Scholar]
- 73.Huang YE, Pu YL, Huang YJ, et al. The utility of the nonattenuation corrected 18F-FDG PET images in the characterization of solitary pulmonary lesions. Nucl Med Commun. 2010;31(11):945–951. doi: 10.1097/MNM.0b013e32833ed57d. [DOI] [PubMed] [Google Scholar]
- 74.Ohno Y, Koyama H, Matsumoto K, et al. Differentiation of Malignant and Benign Pulmonary Nodules with Quantitative First-Pass 320–Detector Row Perfusion CT versus FDG PET/CT. Radiology. 2011;258(2):599–609. doi: 10.1148/radiol.10100245. [DOI] [PubMed] [Google Scholar]
- 75.MacDonald K, Searle J, Lyburn I. The role of dual time point FDG PET imaging in the evaluation of solitary pulmonary nodules with an initial standard uptake value less than 2.5. Clin Radiol. 2011;66(3):244–250. doi: 10.1016/j.crad.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Okereke IC, Gangadharan SP, Kent MS, Nicotera SP, DeCamp MM. [18F]Fluorodeoxyglucose positron emission tomography-computerized tomography and lung cancer: a significant referral bias exists. European Journal of Cardio-Thoracic Surgery. 2011;39(4):560–564. doi: 10.1016/j.ejcts.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 77.Kubota K, Murakami K, Inoue T, Saga T, Shiomi S. Additional effects of FDG-PET to thin-section CT for the differential diagnosis of lung nodules: a Japanese multicenter clinical study. Ann Nucl Med. 2011;25(10):787–795. doi: 10.1007/s12149-011-0528-5. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen NC, Kaushik A, Wolverson MK, Osman MM. Is there a common SUV threshold in oncological FDG PET/CT, at least for some common indications? A retrospective study. Acta Oncologica. 2011;50(5):670–677. doi: 10.3109/0284186X.2010.550933. [DOI] [PubMed] [Google Scholar]
- 79.Xu B, Guan Z, Liu C, et al. Can multimodality imaging using 18F-FDG/18F-FLT PET/CT benefit the diagnosis and management of patients with pulmonary lesions? Euro J Nucl Med Mol Imaging. 2011;38(2):285–292. doi: 10.1007/s00259-010-1625-8. [DOI] [PubMed] [Google Scholar]
- 80.García Vicente AM, Castrejón AS, León Martín AA, García BG, Pilkington Woll JP, Muñoz AP. Value of 4-Dimensional 18F-FDG PET/CT in the Classification of Pulmonary Lesions. Journal of Nuclear Medicine Technology. 2011;39(2):91–99. doi: 10.2967/jnmt.110.082719. [DOI] [PubMed] [Google Scholar]
- 81.Ashraf H, Dirksen A, Loft A, et al. Combined use of positron emission tomography and volume doubling time in lung cancer screening with low-dose CT scanning. Thorax. 2011;66(4):315–319. doi: 10.1136/thx.2010.136747. [DOI] [PubMed] [Google Scholar]
- 82.van't Westeinde SC, de Koning HJ, Thunnissen FB, et al. The Role of the (18)F-Fluorodeoxyglucose-Positron Emission Tomography Scan in the Nederlands Leuvens Longkanker Screenings Onderzoek Lung Cancer Screening Trial. Journal of Thoracic Oncology. 2011;6(10):1704–1712. doi: 10.1097/JTO.0b013e3182286d0b. [DOI] [PubMed] [Google Scholar]
- 83.Harders SW, Madsen HH, Hjorthaug K, et al. Characterization of pulmonary lesions in patients with suspected lung cancer: computed tomography versus [(1)(8)F] fluorodeoxyglucose-positron emission tomography/computed tomography. Cancer Imaging. 2012;12(3):437–446. doi: 10.1102/1470-7330.2012.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fiorelli A, Rizzo A, Messina G, et al. Correlation between matrix metalloproteinase 9 and 18F-2-fluoro-2-deoxyglucose-positron emission tomography as diagnostic markers of lung cancer. European Journal of Cardio-Thoracic Surgery. 2012;41(4):852–860. doi: 10.1093/ejcts/ezr117. [DOI] [PubMed] [Google Scholar]
- 85.Dalli A, Selimoglu Sen H, Coskunsel M, et al. Diagnostic value of PET/CT in differentiating benign from malignant solitary pulmonary nodules. Balkan Journal of Oncology. 2013;18(4):935–941. [PubMed] [Google Scholar]
- 86.Lopes Pegna A, Picozzi G, Falaschi F, et al. Four-Year Results of Low-Dose CT Screening and Nodule Management in the ITALUNG Trial. Journal of Thoracic Oncology. 2013;8(7):866–875. doi: 10.1097/JTO.0b013e31828f68d6. [DOI] [PubMed] [Google Scholar]
- 87.Ohno Y, Nishio M, Koyama H, et al. Comparison of Quantitatively Analyzed Dynamic Area-Detector CT Using Various Mathematic Methods With FDG PET/CT in Management of Solitary Pulmonary Nodules. American Journal of Roentgenology. 2013;200(6):W593–W602. doi: 10.2214/AJR.12.9197. [DOI] [PubMed] [Google Scholar]
- 88.Minamimoto R, Senda M, Jinnouchi S, et al. Detection of Lung Cancer by FDG-PET Cancer Screening Program: A Nationwide Japanese Survey. Anticancer Research. 2014;34(1):183–189. [PubMed] [Google Scholar]
- 89.Zhang J, Cui LB, Tang X, et al. DW MRI at 3.0 T versus FDG PET/CT for detection of malignant pulmonary tumors. International Journal of Cancer. 2014;134(3):606–611. doi: 10.1002/ijc.28394. [DOI] [PubMed] [Google Scholar]
- 90.Menda Y, Kahn D. Somatostatin receptor imaging of non-small cell lung cancer with 99mTc depreotide. Semin Nucl Med. 2002;32(2):92–96. doi: 10.1053/snuc.2002.31564. [DOI] [PubMed] [Google Scholar]
- 91.Acker MR, Burrell SC. Utility of 18F-FDG PET in Evaluating Cancers of Lung. J Nucl Med Tech. 2005;33(2):69–74. [PubMed] [Google Scholar]
- 92.Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure Guideline for Tumor Imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47(5):885–895. [PubMed] [Google Scholar]
- 93.Beyer T, Antoch G, Müller S, et al. Acquisition Protocol Considerations for Combined PET/CT Imaging. J Nucl Med. 2004;45(1 suppl):25S–35S. [PubMed] [Google Scholar]
- 94.Beyer T, Townsend DW, Brun T, et al. A Combined PET/CT Scanner for Clinical Oncology. J Nucl Med. 2000;41(8):1369–1379. [PubMed] [Google Scholar]
- 95.Yang W, Zhang Y, Fu Z, Sun X, Mu D, Yu J. Imaging proliferation of (1)(8)F-FLT PET/CT correlated with the expression of microvessel density of tumour tissue in non-small-cell lung cancer. Euro J Nucl Med Mol Imaging. 2012 Aug;39(8):1289–1296. doi: 10.1007/s00259-012-2126-8. [DOI] [PubMed] [Google Scholar]
- 96.Dittman H, Dohmen B, Paulsen F. 18-FLT PET for diagnosis and staging of thoracic tumors. Eur J Nucl Med Mol Imag. 2003;30:1407–1412. doi: 10.1007/s00259-003-1257-3. [DOI] [PubMed] [Google Scholar]
- 97.Baylin SB, Jackson RD, Goodwin G, Gazdar AF. Neuroendocrine-related biochemistry in the spectrum of human lung cancers. Exp Lung Res. 1982 Nov;3(3-4):209–223. doi: 10.3109/01902148209069654. [DOI] [PubMed] [Google Scholar]
- 98.Zhuang H, Pourdehnad M, Lambright ES, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001 Sep;42(9):1412–1417. [PubMed] [Google Scholar]
- 99.Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines. Chest. (2nd) 2007 Sep;132(3 Suppl):108S–130S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 100.The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. New England Journal of Medicine. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.