Abstract
Background
Low high-density lipoprotein-cholesterol (HDL-C) constitutes a major risk factor for atherosclerosis. Recent studies from our group reported a genetic association between the WW domain-containing oxidoreductase (WWOX) gene and HDL-C levels. Here, through next-generation resequencing, in vivo functional studies and gene microarray analyses, we investigated the role of WWOX in HDL and lipid metabolism.
Methods and Results
Using next-generation resequencing of the WWOX region, we first identified 8 variants significantly associated and perfectly segregating with the low-HDL trait in two multi-generational French Canadian dyslipidemic families. To understand in vivo functions of WWOX, we used liver-specific Wwoxhep−/− and total Wwox−/− mice models, where we found decreased ApoA-I and ABCA1 levels in hepatic tissues. Analyses of lipoprotein profiles in Wwox−/−, but not Wwox hep−/− littermates, also showed marked reductions in serum HDL-C concentrations, concordant with the low-HDL findings observed in families. We next obtained evidence of a gender-specific effect in female Wwoxhep−/− mice, where an increase in plasma triglycerides and altered lipid metabolic pathways by microarray analyses were observed. We further identified a significant reduction in ApoA-I and LPL, and upregulation in Fas, Angptl4 and Lipg, suggesting that the effects of Wwox involve multiple pathways, including cholesterol homeostasis, ApoA-I/ABCA1 pathway, and fatty acid biosynthesis/triglyceride metabolism.
Conclusions
Our data indicate that WWOX disruption alters HDL and lipoprotein metabolism through several mechanisms and may account for the low-HDL phenotype observed in families expressing the WWOX variants. These findings thus describe a novel gene involved in cellular lipid homeostasis, which effects may impact atherosclerotic disease development.
Keywords: high-density lipoprotein cholesterol, gene, lipids and lipoprotein metabolism, gene expression/regulation, cardiovascular disease, WWOX, ApoA-I, ABCA1
Introduction
Low plasma high-density lipoprotein cholesterol (HDL-C) constitutes a major and coherent cardiovascular risk factor1. HDL-C levels are regulated by a combination of environmental and genetic factors, with heritability estimates of 40–60%2, emphasizing the need to characterize novel genetic regulators of HDL metabolism.
We previously identified a region-wide significant association between low HDL-C and the WW-domain containing oxidoreductase (WWOX) gene on chromosome 16q23–24, in dyslipidemic families and low HDL-C cases and controls3. Genome-wide association studies4,5 have also shown that WWOX is strongly associated with HDL-C, triglyceride (TG) levels6 and left ventricular thickness7. Its specific role in cellular lipid homeostasis and lipoprotein metabolism remains however unknown.
The WWOX gene spans 1.1 Mb at the common fragile site FRA16D (chr16q23)8,9. It encodes a 46-kDa tumor suppressor10,11, the expression of which is altered in several types of human malignancies10–12. Wwox disruption in mice results in metabolic abnormalities, impaired growth, and postnatal lethality, implying an indispensable role for Wwox in metabolism10,13. Its interactions are believed to be largely driven by binding to proline-rich PPxY motifs found within an array of potential ligands, such as p73, RUNX, c-Jun, AP2, and NF-κB transcription factors, as well as several other cellular proteins including SIMPLE, ErbB4 and Ezrin14–18. Furthermore, Wwox is expressed across various tissues regulating a wide variety of cellular functions such as protein degradation, transcription, cellular trafficking and metabolic reactions19. The highest Wwox expression was detected in hormonally regulated tissues (testis, ovary, prostate and liver). This expression pattern, coupled with the presence of a short chain dehydrogenase (SRD) domain, suggests a role for WWOX in steroid metabolism. Moreover, it was recently observed that Wwox knock-out (KO) mice exhibit marked reductions in serum lipid levels and display impaired gene expression of key stereoidogenic enzymes10,20.
We therefore sought to characterize WWOX as a novel genetic determinant involved in HDL-C regulation. Using a combination of next-generation resequencing in HDL-deficient families, in vivo functional studies by means of total Wwox KO (Wwox−/−) and Wwox liver-specific KO (Wwoxhep−/−) mouse models, along with conventional and modern gene microarray analyses, we demonstrated the role of WWOX in HDL and lipid metabolism.
Materials and Methods
Ethics protocols
Mice were maintained in a clean, modified-barrier animal facility. Animals were fed a standard rodent chow diet (Harlan Lab, Indianapolis, IN) and water ad libitum, unless otherwise mentioned, under controlled light (12L:12D) and temperature (68–74°F). Animal research was approved by the University of Texas M. D. Anderson Cancer Center Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3343-01), the Hebrew University-Hadassah Medical School and the McGill University Animal Care Committee. Procedures followed were in accordance with institutional guidelines.
Generation of total Wwox-deficient mice and C57BL/6-Wwoxflox;AlbCre KO/transgenic mice
Total Wwox KO (Wwox−/−) mice were generated as previously described10. Wwox liver-specific KO mice (Wwoxhep−/−) were produced by crossing female Wwoxflox/flox (129SV/C57Bl/6 background, previously generated11 with male Alb-Cre transgenic mice having the Cre-recombinase gene under the control of the Albumin promoter (pure C57Bl6/J inbred from Jackson Labs21). The Wwoxflox/WT;AlbCre+/− mice were intercrossed to obtain Wwoxflox/flox;AlbCre+/− mice (Wwoxhep−/−). Genotypes were determined by PCR using the oligonucleotide primers: Cre F: 5′-GCCTGCATTACCGGTCGATGCAACG-3′ Cre R: 5′-GTGGCAGATGGCGCGGCAACACCAT-3′ Wwox-N1: 5′-ATGGGCCGAAACTGGAGCTCAGAA-3′ Wwox-N2: 5′-TCAGCAACTCACTCTGGCTTCAAC-3′ Wwox-L: 5′-GCATACATTATACGAAGTTATTCGAG-3′.
Lipid measurements and lipoprotein separation assays
Serum was isolated by centrifugation (3000 rpm, 15 min, 4°C) from blood extracted from mice fasted for 4–6 hours. For lipid measurements, serum samples (150 µl) were shipped on dry ice to LipoScience Inc. (Raleigh, North Carolina) where levels of total cholesterol, HDL-C, LDL-C, TG, ApoA-I, apoB and glucose were measured using an Olympus AU400e immunoautoanalyzer. Liposcience measurement assays were previously validated in humans and lipid values falling below human range linearity were excluded from analyses (apoB, ApoA-I, LDL-C). For lipoprotein profiles separation, 250 µl of serum was separated by high-performance liquid chromatography equipped with a Superose 6 10/300 GL column (GE Healthcare) attached to a Beckman Coulter System Gold™ apparatus. A 150 mM NaCl mobile phase with a flow rate of 0.4 ml/ml was used for separation of samples into 72×400 ul-HPLC fractions that were collected in a 96-well plate using the ProteomeLabTM automated fraction collector (Beckman Coulter). Cholesterol and triglycerides content in each fraction was determined enzymatically (Infinity™ kit; Thermo Scientific) according to manufacturer’s instructions. ApoA-I-containing particles were separated by two-dimensional polyacrylamide gradient gel (2D-PAGGE) electrophoresis as described previously22. Briefly, serum samples (25 µl) were separated in the first dimension (according to their charge) by 0.75% agarose gel electrophoresis (100 V, 3 h, 4°C) and in the second dimension (according to the size) by 5–23% polyacrylamide concave gradient gel electrophoresis (125 V, 24 h, 4°C). Iodinated high molecular weight protein mixture (7.1–17.0 nm; Pharmacia) was run as a standard on each gel. Electrophoretically separated samples were electrotransferred (30 V, 24 h, 4°C) onto nitrocellulose membranes (Hybond ECL; Amersham). ApoA-I-containing particles were detected with immunopurified polyclonal anti-ApoA-I antibody (Biodesign) labeled with 125I. The presence of labeled 125I-ApoA-I was detected directly by autoradiography using Kodak XAR-2 film.
RNA isolation and analysis of gene expression by RT-PCR
Total RNA was isolated from liver tissues using the RNeasy mini RNA extraction kit (Qiagen), according to manufacturer's instructions. Total RNA (200 ng) was reverse-transcribed using the QuantiTect Reverse Transcription kit (Qiagen). Real-time quantitative PCR was carried out using the Quantitect SYBR Green PCR kit and QuantiTect Primer assays (Qiagen): Wwox, (QT00147735), ApoA-I (QT00110663), and Abca1 (QT00165690). All reactions were performed on an ABI PRISM 7300 Sequence Detection System (Applied Biosystems). Amplifications were carried out in a 96-well plate with 50 µl reaction volumes and 40 amplification cycles (94°C, 15s; 55°C, 30s; 72°C, 34s). All samples were run in triplicate and mRNA expression was taken as mean of three separate experiments. The relative abundance (fold change relative to control) of target mRNA was determined using the ΔΔCt method where the expression of each gene was normalized to Gapdh (QT01020908) loading control.
Immunoblotting
Using a Tissue Tearor (Biospec Products), liver tissues were homogenized on ice in RIPA buffer (20mM Tris-HCl (pH 8), 150mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, and 4mM EDTA) containing complete protease inhibitors (Roche Diagnostics). The homogenate was sonicated three times 10 sec each before centrifugation for 3 min at 5000 rpm, 4°C. The supernatant was used as total liver protein extracts and protein concentrations were measured with Bradford reagent (Bio-Rad) according to manufacturer’s instructions. Equal amounts of protein were separated by SDS-PAGE, transferred to a nitrocellulose membrane, subsequently blocked with 5% skim milk and incubated with various primary antibodies (anti-ABCA1 (Novus Biologicals), -ApoA-I (Biodesign), -ANGPTL4 (Novus Biologicals) or -WWOX (rabbit anti-Wwox antibody8 or obtained from Cell Signaling) and horseradish peroxidase-conjugates secondary antibodies (Jackson Biolabs). Chemiluminescence detection was performed using Western lighting plus ECL reagents (Pierce Thermo Scientific) as described by the manufacturer. Density of target bands was quantified using BioRad Imager software.
Microarray data analysis
Output from the Agilent Feature Extraction software were read into R, preprocessed and tested for differential expression using functions from the Bioconductor23 package Limma24. Specifically, the function read.maimages was employed to read raw intensities into R. Quality control was performed by inspecting various diagnostic plots of the intensity distribution and correlation structure of control and regular probes. One array, JGT059, did not pass quality control and was discarded from the dataset prior to preprocessing. The normexp method with an offset value of 16 was used for global background adjustment, followed by quantile normalization and a log2 transformation (functions backgroundCorrect and normalizeBetweenArrays). Within-array duplicate spots were summarized by averaging using the function avereps. The annotation for probes was retrieved from the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/), platform id GPL10787.
Using the appropriate Limma functions, a linear model was fit to each gene separately. This linear model included the mice gender and the Wwoxhep−/− status as independent categorical variables. Moderated t-tests25 on various coefficients of interest were used to test the differences between Wwoxhep−/− and WT mice, or male and female mice. The differential response and the male/female average response to the Wwoxhep−/− were also tested. False discovery rate (FDR) estimation was carried out using the Benjamini-Hochberg method.
IPA (Ingenuity® Systems, www.ingenuity.com) was utilized for pathway, network and functional analysis. For this purpose, gene-level statistical significance for differential expression was reached at P<0.05 and |Fold Change (FC)|>1.5. The over-representation of pathways and biological functions in the lists of significantly differentially expressed genes was tested using Fisher’s exact test with the Benjamini-Hochberg method used for FDR estimation.
Weighted gene co-expression network analysis (WGCNA)
The conventional differential expression data analysis was used to identify genes that are differentially expressed between different groups of mice. We also utilized the weighted gene co-expression network analysis (WGCNA) to identify tightly connected subsets of genes in biologically meaningful modules26,27. WGCNA is known to alleviate the multiple testing problems associated with conventional microarray data analysis, as it focuses on the relationship between modules and the clinical traits28 instead of relating thousands of genes to clinical traits.
The weighted gene co-expression network was constructed using the blockwise Modules function26,27. The dynamic tree-cutting algorithm was used to identify modules of co-expressed genes that were correlated to five binary traits: the knockout status (KO versus WT), gender (female versus male), gender of the knockout mice (KO females versus KO males), knockout status of male mice (male KO versus male WT), and knockout status of female mice (female KO versus female WT). Genes not classified to any given module were assigned to the grey module. The maximum block size was set at 5,000 genes; a power (β) was set to 17 as this was found to satisfy the scale-free topology criteria 26; the minimum module size was set at 50; and the maximum height at which the tree could be cut was set to 0.995. All the other parameters were left at default settings29. Subsequent analyses focused on modules that included Wwox probes. The blue module included three Wwox probes (A_51_P110371 corresponding the Wwox isoform ENSMUST00000004756; A_55_P2015892 corresponding the Wwox isoform ENSMUST00000160862; and A_55_P2015882 corresponding the Wwox isoform ENSMUST00000109108, respectively). The turquoise module included one Wwox probe (A_55_P2152387 corresponding the Wwox isoform ENSMUST00000004756).
Enrichment Analysis
In the WGCNA, to systematically search for enrichments in biological gene ontology (GO) functions, the DAVID database was used30. DAVID can sort a large number of genes in a given module into relevant biological annotation terms (e.g. GO Terms). The ~2,200 gene expression probes (fold change [FC]>1.5) were used as background in the DAVID analyses. DAVID further determined the enrichment scores and nominal p-values (Fisher’s Exact test) of the underlying biological term30.
Human study samples
The human study samples consist of two French Canadian families collected at the Cardiovascular Genetics Laboratory of the McGill University Health Centre, Royal Victoria Hospital, Montreal, Canada, as described previously31. The affection status in all families was determined using the 5th age-sex specific population percentiles of HDL-C31. Lipids and lipoproteins were measured using standardized techniques as described previously32. The research protocol was approved by the Research Ethics Board of the McGill University Health Center, and all subjects gave informed consent.
Library construction and resequencing
We selected two closely related family members per family with HDL-C levels <5th age-sex percentile and 4 unrelated controls with HDL-C levels >30th percentile for targeted sequencing. The selected families also demonstrated both shared haplotypes and linkage to low HDL-C on chromosome 16q23-q243,33. We performed targeted re-sequencing +/− 100kb of WWOX, as well as two other regional genes previously known to influence HDL-C levels (LCAT and CETP +/−100kb) to search for other possible regional causes of low HDL-C. We used Agilent SureSelect Target Enrichment System, and masked the repeat regions to avoid read alignment errors.
Resequencing data analysis
Each sample was sequenced on one lane of Illumina GA2 Analyzer. We used Burroughs-Wheeler Aligner (BWA)34 to align sequence reads to the reference sequence (hg19) and SAMtools to make SNP calls. Duplicates were removed and a pileup file was generated using SAMtools35. After quality control the mean coverage was 110X. In the sequenced HDL-C cases and controls, we found 1965 SNPs, 1.02% of which were not present in the HapMap36, the 1000 Genomes Project37, and the dbSNP135 databases. Next, we filtered those variants that were shared only by the affected subjects of the two low HDL-C families and not present in the normal controls resequenced. Annovar was used for functional annotation38. In these families, we did not identify any exonic or intronic CETP or LCAT variants that would have been shared by all of the sequenced low HDL-C family members and absent from the sequenced individuals with normal HDL-C levels. Association analysis in the extended low HDL-C families was performed using a measured genotype approach utilizing the ‘Polygenic-QTL’ option of Mendel39, using continuous HDL-C levels with age and sex as covariates.
Statistical analysis
Statistical analyses were performed with GraphPad statistical software 5.0 (GraphPad Softwares Inc). Data were expressed as mean ± SEM, as indicated in figure legends. Two-tailed Student’s t-test was used for comparisons between two groups as applicable, where indicated, and a level of significance of P<0.05 was considered statistically significant. For microarray analyses, the LIMMA R package25 was used to fit a linear model to each gene and to perform moderated t-tests on the differences between WT and Wwoxhep−/− mice, or male and female mice. Enrichment analysis for the weighted gene co-expression network study was performed using the David software30, as described in its corresponding section. The over-representation of pathways and biological functions in the lists of significantly differentially expressed genes was tested using Fisher’s exact test with the Benjamini-Hochberg method used for FDR estimation, as described in Microarray data analyses.
Results
Resequencing of WWOX in low HDL-C families
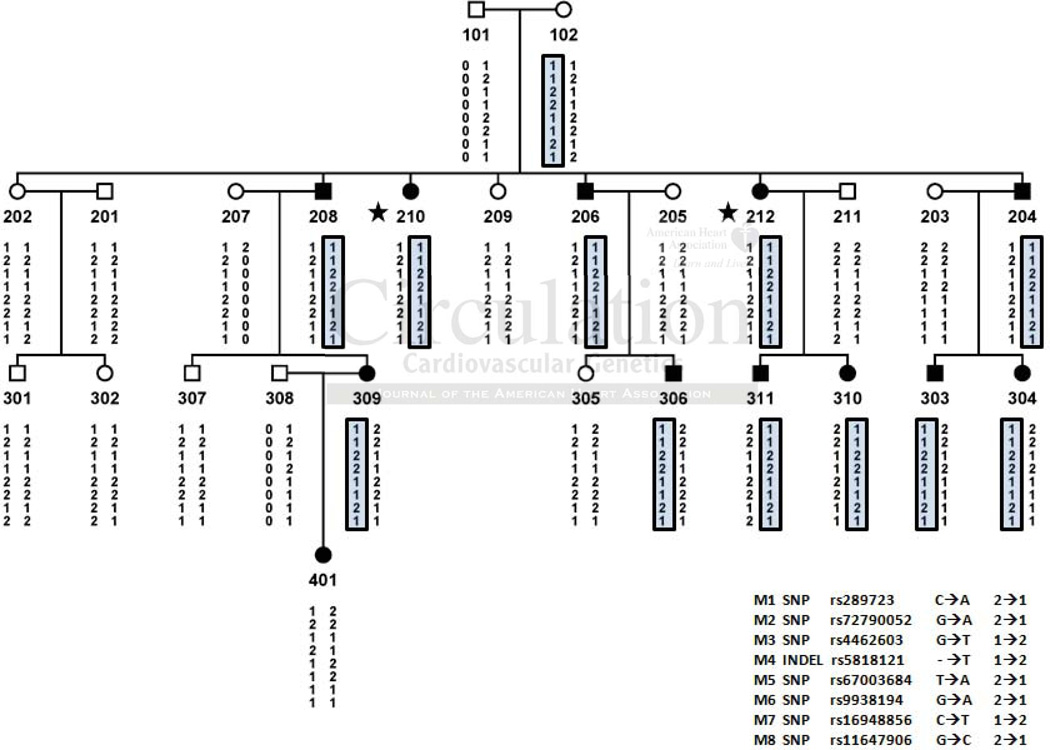
We first examined the genetic association between low HDL-C and the WWOX gene. We selected four family members (HDL-C ≤5th age-sex percentile, Table 1) from two French Canadian families with linkage on chr16q23–24 where the WWOX gene is located40, and four unrelated controls (HDL-C ≥30th percentile, Table 1) for targeted resequencing of the entire 1.1 Mb WWOX region +/− 100kb, in search for regional variants causing low HDL-C. We did not identify any exonic or intronic CETP or LCAT variants in these families that would have been shared by all of the sequenced low HDL-C family members and not present in the sequenced individuals with normal HDL-C levels. Lipid levels and other clinical characteristics of all members from both French Canadian families are indicated in Suppl. Table 1. We identified 19 variants that were shared by all sequenced cases in family 1 and 2 and absent in normal controls. We selected 7 SNPs and an INDEL based on their frequency and linkage disequilibrium to avoid typing redundant SNPs (Table 2). These variants were then investigated for co-segregation with the low HDL-C affection status in the extended low HDL-C families. All 16 affected family members except one shared the variant allele haplotype of rs72790052, rs4462603, rs5818121, rs67003684, rs9938194, rs16948856, and rs11647906 (Figure 1A, B). The rs67003684 and rs9938194 variants are less likely candidates however, as the haplotype co-segregating to the affected subjects in family 2 contains the WT allele of these two variants. Using the Mendel software, we observed statistically significant evidence for association between rs72790052, rs4462603, and rs5818121 and serum HDL-C levels (ranges of P=0.0005-0.0013; Beta=−0.3544-−0.3333; and SE=0.1016-0.1035) in the combined analysis of these two families (Table 2). These three variants are located in intron 5 and are rare (MAF=~0.1–9.0%), whereas the variants rs16948856 and rs11647906 located in intron 8 are relatively common (MAF=18% and 27% respectively). Additionally, we observed a borderline gender effect in females (P=0.059) between these segregating WWOX variants and low HDL. Preliminary results further indicate that rs72790052 was also significantly associated with WWOX expression in human adipose microarray analyses (192 subjects, data not shown). Finally, among the 8 variants, there is only one nonsynonymous variant (rs289723) in the NLRC5 gene which did not co-segregate with low HDL-C in these families (Figure 1A, B). Taken together, we observed a strong genetic co-segregation between the WWOX gene and the low HDL-C trait in two multi-generational families, confirming and complementing previous genetic associations at the chr16q23–24 locus41.
Table 1.
Lipid levels and other clinical characteristics of the four affected and four controls that were resequenced
| FAM | Ind_ID | Sex | Status | Age | HDL-C | HDL% | BMI | TG | TC | LDL-C |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 210 | F | 2 | 69 | 0.67 | <5% | 23.8 | 5.31 | 5.71 | 2.65 |
| 1 | 212 | F | 2 | 48 | 0.66 | <5% | 21.5 | 3.78 | 6.22 | 3.86 |
| 2 | 201 | F | 2 | 40 | 0.47 | <5% | 29.2 | 3.67 | 3.98 | 1.86 |
| 2 | 301 | F | 2 | 13 | 0.46 | <5% | 25.6 | 1.34 | 3.82 | 2.76 |
| Control | 101 | F | 1 | 50 | 1.41 | 33 | 27.6 | 1.41 | 6.2 | 4.15 |
| Control | 102 | F | 1 | 50 | 1.58 | 49 | S18.9 | 1.35 | 4.84 | 2.64 |
| Control | 103 | F | 1 | 28 | 1.44 | 52 | 27 | 0.54 | 3.73 | 2.05 |
| Control | 104 | M | 1 | 39 | 1 | 33 | 19 | 0.66 | 5.03 | 3.73 |
Lipid levels are in mmol/L.
FAM-family, Ind ID-individual ID, F-female, M-male, HDL-C-high-density lipoprotein-cholesterol, BMI-body mass index, TG-triglycerides, TC-total cholesterol, LDL-C-low-density lipoprotein-cholesterol, Status 2-Affected individual (HDL<5th age-sex percentile), Status 1-Non affected individual (HDL >30th age-sex percentile)
Table 2.
Association analysis in the two families with low HDL-C
| SNP | Position | Ref Allele (Ref Allele code) |
Var allele (Var allele code) |
MAF | Gene | Location | Effect* | SE | P-value |
|---|---|---|---|---|---|---|---|---|---|
| rs289723 | Chr16:57080528 | C (=2) | A (=1) | 0.267 | NLRC5 | Exon21 | −0.1973 | 0.1956 | 0.3131 |
| rs72790052 | Chr16:78305527 | G (=2) | A (=1) | 0.025 | WWOX | Intron5 | −0.3544 | 0.1016 | 0.0005 |
| rs4462603 | Chr16:78311633 | G (=1) | T (=2) | 0.092 | WWO X | Intron5 | −0.3544 | 0.1016 | 0.0005 |
| rs5818121 | Chr16:78320704 | -- (=1) | T(=2) | -- | WWOX | Intron5 | −0.3333 | 0.1035 | 0.0013 |
| rs67003684 | Chr16:78321698 | T(=2) | A (=1) | 0.175 | WW O X | Intron5 | −0.3213 | 0.1099 | 0.0035 |
| rs9938194 | Chr16:78322348 | G (=2) | A (=1) | 0.158 | WWOX | Intron5 | −0.3213 | 0.1099 | 0.0035 |
| rs16948856 | Chr16:78946587 | C (=1) | T (=2) | 0.183 | WWOX | Intron8 | −0.2801 | 0.1208 | 0.0205 |
| rs11647906 | Chr16:79084889 | G (=2) | C (=1) | 0.275 | WWOX | Intron8 | −0.5195 | 0.2229 | 0.0198 |
Effect size shown is beta-coefficient per copy of the rare allele after adjustment for age and sex.
SE-standard error, Ref-reference; Var-variant, MAF-minor allele frequency (all MAFs were obtained from the Europeans of National Heart, Lung, and Blood Institute (NHLBI) project)
Figure 1.
Haplotypes of the variants identified in targeted sequence analysis in two HDL-C-deficient French Canadian families. (A and B) Fifteen of the 16 affected subjects with HDL-C<5th age-sex specific population percentile have the risk alleles for the WWOX variants rs72790052, rs4462603, and rs5818121. The subjects whose samples were resequenced are indicated by a star. M, marker; Completely filled symbols <5% HDL-C; Unfilled symbols >10% HDL-C.
Targeted ablation of Wwox in mouse livers alters triglycerides serum levels
We were next interested in investigating whether the human genetic phenotype could translate to a physiological effect of WWOX on HDL metabolism in a mouse model. Given that hepatocytes play a major role in the formation of nascent HDL particles, we first generated liver-specific Wwox KO (Wwoxhep−/−) mice to further delineate the role of Wwox in lipid metabolism. Wwoxhep−/− mice were healthy, with a normal body weight, and presented histologically normal livers throughout their lifespan.
Serum levels of total cholesterol, HDL-C and TG were measured in young and old Wwoxhep−/− mice. While targeted deletion of Wwox in hepatocytes did not appear to result in a substantial change in total and HDL-C serum concentrations (Suppl. Figure 1A–D), a significant increase in TG levels was observed in older female mice, as compared to WT (P=0.0025, Suppl. Figure 1F). These data suggest that the Wwoxhep−/− mouse model harbors a significant sex-effect on circulating TG levels and warrants more elaborate HDL-related investigations.
Gene expression profiling of Wwoxhep−/− mice predicts roles for Wwox in HDL and lipid metabolic pathways
To determine the effect of hepatic Wwox ablation on global gene expression in the liver, we performed microarray analyses on RNA from ~100 days old mice (8 males and 8 females, 4 WT and 4 Wwoxhep−/− per group). Statistical analysis of Agilent genechip expression profiling data identified 699 probes differentially expressed (P<0.05, |fold change (FC)|>1.5) between Wwoxhep−/− and WT females and 424 for males (P<0.05 with |FC|>1.5), corresponding to 473 and 311 annotated genes, respectively. Wwox levels were reduced by 2.85 fold in females (P=1.46×10−4) and decreased by 2.34 fold in males (P=4.97×10−4). Hepatic deletion of Wwox resulted primarily in a significantly different gender-specific gene expression pattern, with only 24 commonly regulated genes between males and females (Suppl. Figure 2A). Heatmap cluster analyses of the top 100 differentially expressed genes in both genders are shown in Suppl. Figure 2B, where significant genotype differences were also observed. In order to control for the gender effect, a comparison between Wwoxhep−/− male versus Wwoxhep−/− female was also performed by substracting the baseline WT from the Wwoxhep−/− female vs male difference (Suppl. Figure 3C). The Ingenuity resource was employed for pathways, network and functional analyses of significantly regulated probes (699 in females and 424 in males with P<0.05, |FC|>1.5) between WT and Wwoxhep−/− mice. We identified the lipid metabolism function as top statistically significant annotated molecular and cellular function in females (P=1.17×10−7; Benjamini-Hochberg FDR adjusted P=4.86×10−4, Figure 2A), containing 64 genes (Suppl. Table 2A) associated with 31 different sub-cellular functions (range P=1. 17×10−7 - 2.32×10−2; Benjamini-Hochberg FDR adjusted range P=4.86×10−4 - 1.61×10−1, Suppl. Table 3A). In males, the lipid metabolism function was ranked as 7th significant function (P=2.32×10−5; Benjamini-Hochberg FDR adjusted P=2.74×10−3, Figure 2C) associated with the Wwoxhep−/− differential expression profile, containing 56 genes (Suppl. Table 2B) linked to 16 subcellular functions (range P=2.32×10−5 – 7.48×10−3; Benjamini-Hochberg FDR adjusted range P=2.74×10−3 – 9.24×10−2, Suppl. Table 3B). Importantly, top canonical pathways differentially regulated between WT and Wwoxhep−/− female mice included pathways involved in FXR/RXR activation (P=0.0126), atherosclerosis signaling (P=0.0147), glycerolipid metabolism (P=0.019), fatty acid (FA) biosynthesis (P=0.0173) and LXR/RXR (P=0.0477) activation (Figure 2B), among others. The latter two pathways were also common to male canonical pathways, which were additionally found to be enriched in acute-phase response signaling (Figure 2D).
Figure 2.
Top 15 Functions and Canonical Pathways associated with Wwox hepatic deletion in mice. Lipid metabolism was the top most significant molecular and cellular function associated with differentially expressed genes (P<0.05, |FC|>1.5) in females (A, green) and seventh in males (C, green). Overall in females, there were more lipid- and cardiovascular-associated canonical pathways (B, red) than in males (D, red). Fatty acid metabolism and LXR/RXR activation were pathways found in both males and females. Ingenuity Systems Inc. software was used to clusters genes by function or pathways. Significance threshold P<0.05 was calculated using Fisher's exact test.
To better represent differential gene expression between WT and Wwoxhep−/− mice, all genes found to be associated with the lipid metabolism function in females (64 genes, Suppl. Table 2A) and males (56 genes, Suppl. Table 2B) were displayed in heatmaps (Figure 3A, B). We obtained evidence that the following key lipid genes, among others, where significantly deregulated in females: ApoA-I (P=0.0028, FC= −1.80), neutral cholesterol ester hydrolase 1 (Nche1, P=0.003, FC=1.59), FA synthase (Fas, P=0.003, FC=1.98), glycerol-3-phosphate acyltransferase (Gpam, P=0.00426, FC=1.51), endothelial lipase (Lipg, P=0.0045, FC=1.94), SREBP chaperone (Scap, P=0.0093, FC= −1.80), lipoprotein lipase (Lpl, P=0.014, FC= −2.08), phospholipid transfer protein (Pltp, P=0.028, FC=1.93), angiopoietin-like 4 (Angptl4, P=0.032, FC=1.64), choline kinase-α (P=0.042, FC= −1.70) and insulin induced gene 2 (Insig2, P=0.049, FC= −1.75). While AbcA1 levels did not seem statistically significant at the mRNA level, a trend towards an AbcA1 decrease and an Angptl4 increase was observed in males. Further classification of lipid metabolism genes by subcellular functions in both genders confirmed a greater number of lipid-related functions in females than in males (31 versus 16 functions, Suppl Table 3A versus B). These include regulation of cholesterol, TG and FA concentration, synthesis and transport, suggesting that the differentially expressed lipid-related genes between WT and Wwoxhep−/− females were strongly associated with different lipid metabolic pathways, including HDL, TG and FA metabolism (Suppl. Table 3A and B). These results were validated by network pathways analyses of the 64 and 56 genes involved in lipid metabolism, for females and males respectively (Suppl. Figure 3A, B). As such, we observed through the several altered metabolic pathways a predicted overall decrease in HDL liver metabolism in both sexes, and a predicted increase in TG-related genes in Wwoxhep−/− females, consistent with previous serum observations (Suppl. Figure 1F).
Figure 3.
Hierarchical cluster analysis of genes involved in lipid metabolism. Among the differentially expressed genes (P<0.05, |FC|>1.5) between WT and Wwox hep−/− mice, 64 genes in females (A) and 56 genes in males (B) were significantly associated with the lipid metabolism (top function in females). Heat map representations of these genes are shown, with expression values being standardized within-gene and mapped to a color scale going from green (significantly down-regulated) to red (significantly up-regulated). The cluster correlation is shown on the right of the heatmap.
Weighted gene co-expression network analysis (WGCNA)
To identify co-expression networks correlated with the KO status, we utilized the WGCNA method. WGCNA clustered the ~2,200 gene expression probes (FC>1.5) from the mice microarrays into 7 distinct gene co-expression modules and a grey module that represents the non-co-expressed genes (Suppl. Figure 4). We observed associations with a nominal P-value of 0.03-3×10−9 for 5 of the 7 modules. The turquoise, black, green, blue, and red coexpression module eigengenes (i.e. the average module expression values for an individual mouse) were significantly associated with the hepatic knockout status (KO versus WT), gender (female versus male), gender of the knockout mice (KO females versus KO males) and knockout status of male (male KO versus WT) and female mice (female KO versus WT) (Suppl. Figure 4).
We focused our subsequent analysis on the blue and turquoise modules that included the Wwox probes. We observed that the blue co-expression module showed a trend towards a negative correlation with the knockout status (cor = −0.48 [p = 0.07]) which became significant with the knockout status of the female mice (KO versus WT) (cor = −0.81[p = 3 × 10−4]) and to a lesser extent with the knockout status of the male mice (KO versus WT, cor = −0.62 [p = 0.01], Suppl. Figure 4). The turquoise module also demonstrated a similar statistically significant trend in female mice. Taken together, the WGCNA analyses also suggest that gender strongly affects the genes co-expressed with Wwox.
Enrichment Analysis
We used the DAVID database30 to determine if any of the underlying biological terms were enriched within the blue and turquoise co-expression modules including the Wwox probes. The enrichment analysis of the blue modules showed genes significantly enriched in biological processes, acute-phase and acute phase response (Bonferroni corrected P=0.05–4.0×10−5). The genes in the turquoise coexpression module did not show evidence for functional enrichment (P>0.05). When we further investigated the significant enrichment categories of the blue co-expression module genes for overlap with the conventional differential microarray gene expression data, we noticed that the statistically significant enrichment categories (acute phase and acute phase response) of the blue module contain the serum amyloids (Saa) Saa1, Saa2, and Saa3 genes also implicated in Figure 3, Suppl. Table 2, and Suppl. Figure 2B. HDL is the major carrier of these Saa1 and Saa3, and Saa is associated with a pro-inflammatory HDL particle42. Altogether, these findings suggest significant functional enrichment related to acute-phase and acute phase response underlying the blue co-expression module that included the Wwox probes.
Comparison with the conventional differential gene expression data
When comparing the WGCNA data with the conventional differential gene expression results, we observed that the blue module included 6 of the significant genes (Cgn, Hsd17b6, Insig2, Me1, Ostb, and Wwox) detected in the conventional microarray analysis between female Wwoxhep−/− and WT mice (Suppl. Table 2, Figure 3, Suppl. Figure 2B). Similarly, the blue module included 36 of the genes detected in the conventional differential gene expression analysis between male Wwoxhep−/− and WT mice, such as Saa1, Saa2, Socs3 and Stat3. Of the blue module genes that were found to be significantly enriched for acute-phase and acute phase response categories using the David enrichment analysis, Apcs, Clec3b, Cp, F13a1, Hpx, Prg4, S100a9, Saa1, Saa2, Saa3, and Stat3 were negatively correlated with the knockout status of the male mice (KO versus WT) in accordance with conventional differential analyses data (Suppl. Table 2, Figure 3, Suppl. Figure 2B). Importantly, we also observed that the turquoise co-expression module included 50 of the genes detected in the conventional differential gene expression analysis including the key lipid genes Apoa1, Apoc2, and Lipg genes (Suppl. Table 2, Figure 3, Suppl. Figure 2B). Taken together, the WGCNA and functional enrichment analyses provide a novel network of genes co-expressed with the Wwox gene and enriched for functions in acute phase response and lipid metabolism in a sex-dependent manner.
Validation of gene expression changes on HDL- and TG-regulators in Wwoxhep−/− mice
We next sought to validate findings from microarray and WGCNA analyses by assessing protein levels of key regulators of HDL and TG metabolism, in addition to qRT-PCR validation (data not shown). One of the significantly down-regulated lipid-related genes in Wwoxhep−/− female mice was ApoA-I (P=0.0028, FC= −1.8), which interaction with ABCA1 is essential for nascent HDL formation. In both male and female tissues, a significant reduction in ApoA-I protein levels was found (males 55% reduction, *P=0.0068, Figure 4A, D; females 50% reduction, ***P=0.00073, Figure 4B, E). There was also a concomitant decrease in males ABCA1 (50% reduction, *P=0.035, Figure 4A, D), but no significant change in females (Figure 4B, E). We also examined Angptl4, recently established as a potent modulator of blood plasma TG and HDL levels43,44 and found to be significantly upregulated in Wwoxhep−/− mice microarray data (Suppl. Table 2A). Consistent with mRNA levels, Angptl4 protein expression was higher in Wwoxhep−/− mice compared to the WT counterpart (60% increase in males and 35% increase in females, Figure 4C, F). In concordance with microarray data, these protein results thus point towards involvement of Wwox in both HDL and TG metabolisms.
Figure 4.
Validation of gene expression changes on HDL- and TG-related proteins and serum lipoproteins separation in Wwoxhep−/− mice. Total protein extracts were isolated from liver samples from male (A) and female (B) WT and Wwoxhep−/− mice as described in Methods. Equivalent quantities of lysate were analyzed by western blot, using antibodies against ABCA1, ApoA-I and GAPDH (loading control). Band intensities were quantified, and relative levels of ABCA1 and ApoA-I were graphed for both male (D) and female (E) samples. Graphs represent the average values of three different mice samples, with error bars representing the SEM. Blots shown are representative of 5 independent experiments. (C) Lysates were prepared as described in panel A and subjected to western blot, using antibodies against ANGPTL4 and GAPDH (loading control). Relative levels were quantified and values graphed represent means ± SEM of three independent samples (F). Statistical analyses were performed using Student's t-test (G-I) Serum (250 ul) from Wwox WT and Wwoxhep−/− (hep KO) mice was separated by HPLC. Total lipoprotein cholesterol (panels G and H) and TG (panels I and J) content was determined as described in Methods. Profiles shown are averages of total HPLC cholesterol and TG profiles for females (n=10/genotype, panels H and J) and males (n=5/genotype, panels G and I). The elution position of VLDL, LDL, HDL and free glycerol are shown. VLDL-associated TG levels were significantly elevated in females Wwox hep KO mice as compared to WT.
Given these findings, we next assessed by HPLC serum lipid levels in 10 Wwoxhep−/− females and 5 Wwoxhep−/− males. Lipoprotein fraction separation confirmed previous lipid measurements (Suppl. Figure 1) where sole deletion of Wwox in hepatocytes did not result in significant differences in HDL-C plasma concentrations in mice of both genders (Figure 4G–H). VLDL-associated TG levels were however significantly increased in Wwoxhep−/− females (Figure 4I–J), consistent with direct serum lipid measurements (Suppl. Figure 1F) and most importantly, with an elevated Angptl4 expression (Figure 4C, F). These data thus suggest that while hepatic Wwox deletion reduces HDL liver production, it may not be sufficient to lower overall circulating HDL-levels (Figure 4G–H, Suppl. Figure 1), but instead, the Wwoxhep−/− mouse model harbors a strong gender-specific effect on circulating TG levels.
Impaired HDL biogenesis in Wwox null mice
Having observed that Wwoxhep−/− mice show an incomplete penetrance of the HDL phenotype observed in human families with WWOX variants, we next extended our analyses to Wwox null mice. We first examined lipid levels of Wwox−/− mice, previously described as having impaired expression of key steroidogenic enzymes10,11,20. HPLC serum characterization revealed a marked reduction in HDL-C (Figure 5A), concomitant with a significant decrease in circulating ApoA-I levels (***P=0.0002, Figure 5B–C). Additionally, 2D-PAGGE analysis of HDL sub-species in Wwox null mice showed significantly reduced amounts of larger α-ApoA-I-containing particles (LpAI), as well as pre-β migrating subpopulations (Figure 5D). Separation of serum lipoproteins by 2D-PAGGE in heterozygous Wwox+/− mice revealed no difference in lipid profiles as compared to WT mice (data not shown), as expected.11,13
Figure 5.
Impaired expression of key nascent HDL players in Wwox null mice. (A) Serum (80 µl) from Wwox wild-type (WT) and knock-out (KO) mice was separated using HPLC, and total cholesterol was measured enzymatically as described in Methods. Results are representative of four independent experiments. (B) ApoA-I from duplicate serum samples (5ul) from a Wwox WT and a Wwox KO mouse were analysed by western blot using antibodies against apoA-I and alpha-2-macroglobulin (loading control). (C) Band intensities were quantified and relative apoA-I levels were determined. Average values shown are means ± SEM of four independent experiments. (D) Serum (25 µl) from individual Wwox null and WT mice was separated by 2D-PAGGE and apoA-I-containing particles were detected by immunopurified polyclonal anti-apoA-I antibody labeled with 125I. Molecular size markers are indicated on the right side of the gel. Results are representative of three independent experiments. (E) Total RNA was isolated from WT and Wwox total KO mice livers as described in Methods. Analysis of mRNA expression of Wwox, ApoA-I, and AbcA1 was carried by qRT-PCR from 200 ng RNA/sample. Values shown are means ± SEM of three independent experiments, each performed in triplicates. Expression of each gene was normalized to GAPDH expression, and mRNA fold changes relative to controls were determined. ***P < 0.00058 by Student's t -test. (F) Analysis of mRNA expression was performed as in (E), but in a pure FVB total Wwox KO mouse model. **P =0.0013 and *P=0.0145 by Student's t-test. (G-J) Total protein extracts were isolated from liver samples from WT and Wwox total KO mice as described in Methods. Equivalent quantities of lysate were separated by SDS-PAGE and analyzed by western blot, using antibodies against ABCA1, WWOX, GAPDH (loading control) in (G) and ApoA-I, WWOX and α-tubulin (loading control) in (I). Band intensities of ABCA1, ApoA-I and loading controls were quantified, and relative levels of ABCA1 (H) and ApoA-I (J) were graphed. Graphs represent the average values of 4 (H) and 3 (J) different mice samples, with error bars representing the SEM. Statistical analysis was performed as described (**P=0.0015 and ***P=0.007 by Student's t-test). Blots shown are representative of four independent experiments.
We further assessed the effect of Wwox deletion in hepatic tissues from two days old Wwox−/− mice. Expression of key regulators of HDL biogenesis, ApoA-I and ABCA1, were examined. Levels of both AbcA1 and ApoA-I mRNA were decreased in Wwox−/− compared to WT, as assessed by RT-PCR (AbcA1 ***P<0.00058, Figure 5E). These results obtained from a 129/SvjxC57BL/6J mixed background mouse model were subsequently confirmed in hepatocytes from a FVB pure background model: mRNA levels of both Abca1 and ApoA-I were significantly decreased by 45% and 35%, respectively (Abca1 **P=0.0013, ApoA-I *P=0.0145, Figure 5F). Hepatic ABCA1 and ApoA-I protein levels were also significantly reduced, by 40% and 80%, respectively (Abca1 **P=0.015, ApoA-I ***P=0.0007, Figure 5G–J). This suggests that Wwox ablation may alter endogenous production of ApoA-I and subsequent lipidation through the ABCA1 transporter.
Together, these data are consistent with the observed decrease in HDL-C serum levels and nascent HDL subspecies, as well as human genetic findings. Wwox−/− mice exhibit not only a reduction in hepatic HDL production, but also systemic impairment in HDL metabolism. The HDL phenotype observed in these Wwox null mice is thus consistent with the low HDL trait in families sharing the WWOX variants allele haplotype.
Discussion
In this study, we show that WWOX is involved in HDL, TG and overall lipoprotein metabolism, using a combination of next-generation resequencing in HDL-deficient families, in vivo functional studies using liver-specific Wwoxhep−/− and total Wwox−/− mouse models, and conventional and modern-WGCNA gene microarray analyses.
First, we report a strong co-segregation of WWOX with the low HDL trait in French Canadian families with HDL-C≤5th age-sex percentile (Figure 1, Table 2). We have previously identified a variant in the WWOX gene region to be associated with low serum HDL-C levels in a study sample comprising 9,798 subjects3. The same region has also been implicated in multiple linkage studies for HDL-C31,45–47. Here, we determined that the minor allele haplotype ‘ATT’ (rs72790052, rs4462603 and rs5818121) perfectly co-segregated with the low HDL-C in families (P=0.0013-0.0005; beta=-0.3544-−0.3333), suggesting intron 5 of WWOX as the location of the functional variant. Additionally, there was a borderline significant gender-effect (P=0.059) between segregating WWOX variants and the low HDL-C trait (given a limited number of individuals in our families). These findings validate however the effect of WWOX in sex-specific interactions.
Using two Wwox-deficient mouse models, we determined that in the absence of Wwox, mRNA and protein levels of key regulators of HDL metabolism are altered. Both Wwox null and Wwoxhep−/− mice demonstrated reductions in ApoA-I and ABCA1 levels, critical components in reverse cholesterol transport and generation of nascent HDL particles48 (Figures 4, 5). Interestingly, the small decrease in AbcA1 mRNA levels in Wwoxhep−/− males (FC= −1.14) observed by microarray analyses translated to a significant reduction in ABCA1 protein levels (50% reduction, *P=0.035, Figure 4A, D). Conversely, Wwoxhep−/− females displayed unchanged mRNA and protein ABCA1 levels (FC= −1.04, Figure 4B, E). This might be due to gender specific translational regulation. ApoA-I protein levels were however observed to be reduced in both Wwoxhep−/− genders (males 55% reduction, **P=0.0068, Figure 4A, D and females 50% reduction, ***P=0.00073, Figure 4B, E) in line with decreases in total KO mice findings (Figure 5). These results in both Wwox null and Wwoxhep−/− models, coupled with serum analyses, not only suggest a key role for Wwox in HDL biogenesis, but most importantly, that ApoA-I and ABCA1 might be among Wwox targets for regulating HDL-C serum concentrations. To date however, limited information has been found on Wwox interacting partners in lipid metabolism, though it has been shown to alter the activity of transcription factors through binding to PPxY rich motifs 49,50.
Despite consistent results in liver tissues of both mice models, differences in serum lipid levels between total and liver-specific Wwox KO models were observed. Wwox null mice have significantly lower HDL-C and serum ApoA-I levels (Figure 5A–C), confirming previous observations10,11,20 and correlating with decreased α1-LpAI and pre-β migrating particles (Figure 5D), consistent with human disorders of HDL biogenesis51. In contrast, liver-specific Wwox deletion did not result in a substantial change in serum cholesterol or HDL levels (Figure 4G–H, Suppl. Figure 1A–D). It was determined that circulating HDL-C were subject to small variations between individual Wwoxhep−/− mice (Suppl. Figure 1). Instead, a significant increase in overall TG levels and VLDL-TG content in serum lipoproteins was observed in older Wwoxhep−/− female mice, by both plasma lipid measurements and HPLC lipoprotein profiling (Figure 4I–J, Suppl. Figure 1F). These results are in agreement with previous human genetic studies documenting an association between Wwox and TG6.
Specific disruption of Wwox in the liver revealed important roles in lipid metabolism. In addition to identifying the lipid metabolism function as the most significantly deregulated function in female Wwoxhep−/− mice, and also prominently affected in males, microarray analyses identified several lipid-related canonical pathways differentially expressed between WT and Wwoxhep−/− female and male mice (Figure 2). Furthermore, when specifically examining the 64 female and 56 male genes (P<0.05, |FC|>1.5) associated with the lipid metabolism (Figure 3A, B and Suppl. Tables 2A, B), we observed multiple genes involved in cholesterol homeostasis, hydrolysis and biosynthesis of TG and FA biosynthesis. As demonstrated through network analyses (Suppl. Figure 3A, B), upregulation of these genes, such as Angptl4, Fasn, Pltp, Gpam, Lipg and downregulation of ApoA-I, Lpl, Insig2, suggest global effects on several pathways in lipid metabolism, modulated by Wwox ablation in livers of both genders (Suppl. Tables 3A, B). Importantly, these networks point towards a decrease in HDL metabolism in both males and females (Suppl. Figure 3A, B). Complementing this, it was also observed through both conventional and WGCNA studies that male canonical pathways associated with acute phase signaling and immune response genes were greatly affected by Wwox liver disruption (Figure 2, Suppl. Figure 3B– Figure 4, and Suppl. Figure 4). The functional WGCNA analyses further demonstrated a novel gene network co-expressed with Wwox and enriched in lipid and acute phase functions. This is in concert with previous findings where WWOX plays a central role in multiple signal transduction pathways14.
Microarray studies led us to pursue several genomic targets involved in lipoprotein metabolism. In addition to downregulated mRNA levels of ApoA-I and Abca1, which findings were subsequently validated through protein analyses (Figure 4A–B, D–E), we also investigated Angptl4, an inhibitor of LPL43. In line with the array data, depletion of Wwox in liver tissues caused a 60% and 35% increase in Angptl4 protein expression in males and females, respectively (Figure 4C, F). In combination with other TG-regulating genes, such as LPL which was reduced 2.08 fold, Angptl4 upregulation might be responsible for the observed increase in circulating TG levels and VLDL-TG content in Wwoxhep−/− female mice. Supporting these findings, Lichtenstein et al showed that Angptl4 overexpression increases plasma TG by decreasing LPL activity, converting LPL from a catalytically active dimer to an inactive monomer, and thus increasing VLDL-associated TG levels44. Angptl4 overexpression thus impairs LPL-dependent plasma TG and cholesteryl ester clearance and subsequent uptake of FAs and cholesterol into tissues, explaining the identified upregulation of FA biosynthesis pathways and TG-related genes in Wwoxhep−/− mice. Additionally, while ANGPTL4 primarily affects plasma levels of TG, the gene was recently identified in GWAS studies to also affect other related metabolic parameters, such as HDL metabolism52.
Gender was an important factor in determining biological roles for Wwox. Unlike the considerable difference in hepatic ABCA1 protein identified in males (Figure 4A, D), the ABCA1 levels in Wwoxhep−/− females were comparable to WT mice (Figure 4B, E). In contrast, the increased production of hepatic Angptl4 in Wwoxhep−/− females (Suppl. Table 2A, Figure 4C, F) was concomitant with the observed elevated serum TG levels (Figure 4J, Suppl. Figure 1F), whereas Angptl4 in Wwoxhep−/− males had a modest influence on TG concentrations (Figure 4I, Suppl. Figure 1F). This gender-specific effect was also maintained by differentially expressed pathways in conventional microarray and WGCNA analyses (Figure 2 vs Suppl. Figure 4). These data support the observation that Wwox seems to play a more prominent role in female HDL, TG and FA metabolisms. This is in concordance with previous studies where WWOX was found to regulate steroid hormone pathways as it is highly expressed in steroidogenic tissues, such as testis and ovaries, both in humans and mice20. Altogether, our observations thus suggest a Wwox-dependent hormonal effect on the lipid metabolism.
Interestingly, and in line with these investigations, are our findings that Wwoxhep−/− mice maintain normal circulating HDL-C and ApoA-I levels, despite a significant reduction in Abca1 and/or ApoA-I hepatic production. Although Timmins et al. reported that hepatic Abca1 KO mice show a ~80% decrease in plasma HDL and ApoA-I levels, indicative of the decisive role of hepatic Abca1 in HDL biogenesis53, our Wwoxhep−/− model did not demonstrate these significant plasma changes. As such, in the male Wwoxhep−/− mice, despite reductions of 50% and 55% in hepatic ABCA1 and ApoA-I proteins respectively, HDL serum levels were subject to small variations between individual Wwoxhep−/− mice and, on average, remained unchanged (Figure 4A, D, G; Suppl. Figures 1C–D). These findings could be due to several reasons. First, Wwox levels were only reduced by 2.85 fold in female and 2.34 fold in male Wwoxhep−/− mice, which might be insufficient to decrease whole-body circulating HDL-C levels. Second, although our knockout mouse model was hepatocyte-specific, livers contain other cells including stellate, sinusoidal endothelial and Kupffer cells, which may express Wwox in the livers that were isolated. The effect of hepatic Wwox disruption may thus be diluted by other Wwox-expressing cells. Third, our study comprised a limited number of mice with individual variations in HDL-C plasma concentrations. Despite this number, we were able to show that WWOX is significantly involved in modulating HDL and lipid metabolism through a combination of techniques (next-generation resequencing in HDL-deficient families, in vivo animal studies and gene microarray analyses), which results we believe, validate one another. Finally, but most notably however, these findings also strongly suggest that other HDL-producing tissues, biochemical and genetic regulators of HDL may compensate for HDL production and contribute to the circulating pool of HDL-C in the absence of hepatic Wwox. The intestine may thus compensate for plasma HDL biosynthesis in this mouse model, warranting additional investigations. Nonetheless, this supports the notion that the effects of WWOX extend beyond the liver, as evidenced by its involvement in multiple genetic pathways (Figure 2, Suppl. Figure 3, Suppl. Table 3A, B).
Importantly, despite the plasma HDL-C levels in Wwoxhep−/− mice, our microarray findings report both through mRNA, subsequent ABCA1 and ApoA-I protein levels, as well as genomic pathway analyses, an overall decrease in HDL production (Suppl. Figure 3). These observations led us to extend our analyses to a total Wwox−/− mouse model, where WWOX was ablated in all tissues. Although it is known that whole body Wwox deletion leads to growth retardation, resulting in early death by four weeks of age, it was identified that homozygous Wwox-null pups were healthy and indistinguishable from their WT littermates up to 4 days postpartum, with no histological lesions in the liver or other organs13. In our studies, we thus assessed the effect of Wwox deletion in hepatic tissues from two days old Wwox KO mice. Our results demonstrate a considerable decrease in ApoA-I and ABCA1 expression levels, and subsequent HDL-C concentrations (Figure 5), underlining a critical role for Wwox in lipid metabolism. Several HDL-producing sources might therefore be affected by Wwox ablation and thus be responsible for the decrease in HDL.
Using the Wwox null model, we were also able to complement and translate the human genetic phenotype observed in the French Canadian families to a physiological effect of WWOX on HDL metabolism. As such, the markedly low levels of circulating HDL and ApoA-I in total Wwox KO mice, strongly supported by impaired key regulators of HDL biogenesis, are consistent with the human observations of a region-wide association between WWOX variants and decreased plasma HDL-C. Specifically, the WWOX variants may account for the low-HDL phenotype in humans, as they were found to perfectly segregate in multigenerational HDL-deficient families, correlating with a whole-body genetic association between low HDL-C and human WWOX variants, similar to the decreased HDL-C concentrations in whole-body Wwox disruption. These further extend the observation that hepatic Wwox is sufficient to alter TG metabolism in a gender-specific way, but that whole-body Wwox might be required to modulate circulating HDL levels. Therefore, this underlines the importance of selecting specific mouse models for future analyses and substantiates that, in addition to the liver, other HDL-producing sources might be responsible for circulating HDL-C levels.
Collectively, our data has established a physiological significant role for WWOX in lipid and lipoprotein metabolism in mouse models and human genetic studies. This may be, in part, mediated through the ABCA1/ApoA-I pathway, raising the possibility that WWOX may be involved in the complex network of cellular cholesterol homeostasis. While WWOX had been previously linked to HDL-C in genetic association studies3–6, this is the first study that combines human genetics, animal models and biochemical methods to demonstrate WWOX involvement in HDL and lipid metabolism. This report is therefore a first line of functional evidence and comprehensive examination of WWOX in lipoprotein metabolism, implicating it as a novel, important and influential modulator of HDL-C and TG levels, both in mice and humans. These findings thus emphasize the need to further elucidate the mechanisms of action of WWOX in the HDL metabolism, which may have important implications in preventing and treating atherosclerotic cardiovascular disease.
Supplementary Material
Acknowledgments
We thank the family members who participated in the study, as well as Francois Lefebvre for his great help with the microarray analyses. The technical assistance of Anouar Hafiane MSc is also gratefully acknowledged.
Funding Sources: This research was supported by the Canadian Institutes of Health Research (CIHR) grant MOP 97752 and CIHR MOP 15042, Heart and Stroke Foundation of Canada and grants HL095056 and HL-28481 from the National Institutes of Health (PP). FP7 Marie Curie Reintegration Grant to RIA, National Institutes of Health/National Cancer Institute (USA) grant R01 CA102444-7 to CMA. Iulia Iatan was supported by the CIHR’s Frederick Banting and Charles Best Canada Graduate Doctoral award, and MV Prasad Linga Reddy by the American Heart Association grant 11POST7380028.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Heller DA, de FU, Pedersen NL, Dahlen G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–1156. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Weissglas-Volkov D, Kyttala M, Dastani Z, Cantor RM, Sobel EM, et al. WW-domain-containing oxidoreductase is associated with low plasma HDL-C levels. Am J Hum Genet. 2008;83:180–192. doi: 10.1016/j.ajhg.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leduc MS, Lyons M, Darvishi K, Walsh K, Sheehan S, Amend S, et al. The mouse QTL map helps interpret human genome-wide association studies for HDL cholesterol. J Lipid Res. 2011;52:1139–1149. doi: 10.1194/jlr.M009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saez ME, Gonzalez-Perez A, Martinez-Larrad MT, Gayan J, Real LM, Serrano-Rios M, et al. WWOX gene is associated with HDL cholesterol and triglyceride levels. BMC Med Genet. 2010;11:148. doi: 10.1186/1471-2350-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasan RS, Glazer NL, Felix JF, Lieb W, Wild PS, Felix SB, et al. Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA. 2009;302:168–178. doi: 10.1001/jama.2009.978-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60:2140–2145. [PubMed] [Google Scholar]
- 9.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, et al. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet. 2000;9:1651–1663. doi: 10.1093/hmg/9.11.1651. [DOI] [PubMed] [Google Scholar]
- 10.Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, et al. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci U S A. 2007;104:3949–3954. doi: 10.1073/pnas.0609783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One. 2009;4:e7775. doi: 10.1371/journal.pone.0007775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del MS, Kurek KC, Stein GS, Lian JB, Aqeilan RI. Role of the WWOX tumor suppressor gene in bone homeostasis and the pathogenesis of osteosarcoma. Am J Cancer Res. 2011;1:585–594. [PMC free article] [PubMed] [Google Scholar]
- 13.Aqeilan RI, Hassan MQ, de BA, Hagan JP, Volinia S, Palumbo T, et al. The WWOX tumor suppressor is essential for postnatal survival and normal bone metabolism. J Biol Chem. 2008;283:21629–21639. doi: 10.1074/jbc.M800855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del MS, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem. 2009;108:737–745. doi: 10.1002/jcb.22298. [DOI] [PubMed] [Google Scholar]
- 15.Sudol M. Structure and function of the WW domain. Prog Biophys Mol Biol. 1996;65:113–132. doi: 10.1016/s0079-6107(96)00008-9. [DOI] [PubMed] [Google Scholar]
- 16.Salah Z, Alian A, Aqeilan RI. WW domain-containing proteins: retrospectives and the future. Front Biosci. 2012;17:331–348. doi: 10.2741/3930. [DOI] [PubMed] [Google Scholar]
- 17.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci U S A. 2004;101:4401–4406. doi: 10.1073/pnas.0400805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludes-Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT, Aldaz CM. WWOX binds the specific proline-rich ligand PPXY: identification of candidate interacting proteins. Oncogene. 2004;23:5049–5055. doi: 10.1038/sj.onc.1207680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salah Z, Aqeilan R, Huebner K. WWOX gene and gene product: tumor suppression through specific protein interactions. Future Oncol. 2010;6:249–259. doi: 10.2217/fon.09.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aqeilan RI, Hagan JP, de BA, Rawahneh M, Salah Z, Gaudio E, et al. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology. 2009;150:1530–1535. doi: 10.1210/en.2008-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 22.Krimbou L, Hajj HH, Blain S, Rashid S, Denis M, Marcil M, et al. Biogenesis and speciation of nascent apoA-I-containing particles in various cell lines. J Lipid Res. 2005;46:1668–1677. doi: 10.1194/jlr.M500038-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- 25.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 26.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaisier CL, Horvath S, Huertas-Vazquez A, Cruz-Bautista I, Herrera MF, Tusie-Luna T, et al. A systems genetics approach implicates USF1, FADS3, and other causal candidate genes for familial combined hyperlipidemia. PLoS Genet. 2009;5:e1000642. doi: 10.1371/journal.pgen.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics. 2008;24:719–720. doi: 10.1093/bioinformatics/btm563. [DOI] [PubMed] [Google Scholar]
- 29.Plaisier CL, Horvath S, Huertas-Vazquez A, Cruz-Bautista I, Herrera MF, Tusie-Luna T, et al. A systems genetics approach implicates USF1, FADS3, and other causal candidate genes for familial combined hyperlipidemia. PLoS Genet. 2009;5:e1000642. doi: 10.1371/journal.pgen.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman BT, Huang dW, Tan Q, Guo Y, Bour S, Liu D, et al. DAVID Knowledgebase: a gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysis. BMC Bioinformatics. 2007;8:426. doi: 10.1186/1471-2105-8-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dastani Z, Quiogue L, Plaisier C, Engert JC, Marcil M, Genest J, et al. Evidence for a gene influencing high-density lipoprotein cholesterol on chromosome 4q31.21. Arterioscler Thromb Vasc Biol. 2006;26:392–397. doi: 10.1161/01.ATV.0000198243.83781.a3. [DOI] [PubMed] [Google Scholar]
- 32.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van DM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 33.Dastani Z, Pajukanta P, Marcil M, Rudzicz N, Ruel I, Bailey SD, et al. Fine mapping and association studies of a high-density lipoprotein cholesterol linkage region on chromosome 16 in French-Canadian subjects. Eur J Hum Genet. 2010;18:342–347. doi: 10.1038/ejhg.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange K, Sinsheimer JS, Sobel E. Association testing with Mendel. Genet Epidemiol. 2005;29:36–50. doi: 10.1002/gepi.20073. [DOI] [PubMed] [Google Scholar]
- 40.Dastani Z, Pajukanta P, Marcil M, Rudzicz N, Ruel I, Bailey SD, et al. Fine mapping and association studies of a high-density lipoprotein cholesterol linkage region on chromosome 16 in French-Canadian subjects. Eur J Hum Genet. 2010;18:342–347. doi: 10.1038/ejhg.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JC, Weissglas-Volkov D, Kyttala M, Dastani Z, Cantor RM, Sobel EM, et al. WW-domain-containing oxidoreductase is associated with low plasma HDL-C levels. Am J Hum Genet. 2008;83:180–192. doi: 10.1016/j.ajhg.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolle M, Huang T, Schuchardt M, Jankowski V, Prufer N, Jankowski J, et al. High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc Res. 2012;94:154–162. doi: 10.1093/cvr/cvs089. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenstein L, Kersten S. Modulation of plasma TG lipolysis by Angiopoietin-like proteins and GPIHBP1. Biochim Biophys Acta. 2010;1801:415–420. doi: 10.1016/j.bbalip.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenstein L, Berbee JF, van Dijk SJ, van Dijk KW, Bensadoun A, Kema IP, et al. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arterioscler Thromb Vasc Biol. 2007;27:2420–2427. doi: 10.1161/ATVBAHA.107.151894. [DOI] [PubMed] [Google Scholar]
- 45.Mahaney MC, Almasy L, Rainwater DL, VandeBerg JL, Cole SA, Hixson JE, et al. A quantitative trait locus on chromosome 16q influences variation in plasma HDL-C levels in Mexican Americans. Arterioscler Thromb Vasc Biol. 2003;23:339–345. doi: 10.1161/01.atv.0000051406.14162.6a. [DOI] [PubMed] [Google Scholar]
- 46.Shearman AM, Ordovas JM, Cupples LA, Schaefer EJ, Harmon MD, Shao Y, et al. Evidence for a gene influencing the TG/HDL-C ratio on chromosome 7q32.3-qter: a genome-wide scan in the Framingham study. Hum Mol Genet. 2000;9:1315–1320. doi: 10.1093/hmg/9.9.1315. [DOI] [PubMed] [Google Scholar]
- 47.Soro A, Pajukanta P, Lilja HE, Ylitalo K, Hiekkalinna T, Perola M, et al. Genome scans provide evidence for low-HDL-C loci on chromosomes 8q23, 16q24.1-24.2, and 20q13.11 in Finnish families. Am J Hum Genet. 2002;70:1333–1340. doi: 10.1086/339988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iatan I, Bailey D, Ruel I, Hafiane A, Campbell S, Krimbou L, et al. Membrane microdomains modulate oligomeric ABCA1 function: impact on apoAI-mediated lipid removal and phosphatidylcholine biosynthesis. J Lipid Res. 2011;52:2043–2055. doi: 10.1194/jlr.M016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, et al. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005;65:6764–6772. doi: 10.1158/0008-5472.CAN-05-1150. [DOI] [PubMed] [Google Scholar]
- 50.Aqeilan RI, Croce CM. WWOX in biological control and tumorigenesis. J Cell Physiol. 2007;212:307–310. doi: 10.1002/jcp.21099. [DOI] [PubMed] [Google Scholar]
- 51.Marcil M, Bissonnette R, Vincent J, Krimbou L, Genest J. Cellular phospholipid and cholesterol efflux in high-density lipoprotein deficiency. Circulation. 2003;107:1366–1371. doi: 10.1161/01.cir.0000056764.53152.f9. [DOI] [PubMed] [Google Scholar]
- 52.Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.