Abstract
Two lines of evidence point to a hairpin DNA intermediate in V(D)J joining (V, variable; D, diversity; J, joining) [Lieber, M.R. (1991) FASEB J. 4, 2934-2944]. One is the presence of P nucleotide insertions (short inverted-repeat sequence) in V(D)J junctions [Lafaille, J. J., DeCloux, A., Bonneville, M., Takagaki, Y. & Tonegawa, S. (1989) Cell 59, 859-870]; a second is the detection of site-specifically broken DNA molecules with covalently closed (hairpin) termini in thymus DNA [Roth, D. B., Menetski, J. P., Nakajima, P., Bosma, M. J. & Gellert, M. (1989) Cell 70, 983-991]. However, P nucleotide insertions could be generated in ways not involving a hairpin structure, and because physical evidence for hairpin-ended DNA fragments has been obtained only with mutant mice, there is some uncertainty regarding the role of hairpin molecules in the normal V(D)J joining pathway. To determine whether mammalian cells are capable of metabolizing this odd type of DNA terminus and whether, in doing so, junctions with P insertions are in fact created, a linear DNA molecule with a hairpin closure at each end was transfected into several murine cell lines. The hairpin-ended molecules were recircularized, and the junctions exhibited P insertions at a high frequency. This result directly links the presence of P insertions to a hairpin precursor, providing strong evidence for the notion that a hairpin DNA intermediate exists in V(D)J recombination. A comparison of hairpin end joining in various cells, including those derived from mice with the severe combined immunodeficiency (scid) mutation, is presented.
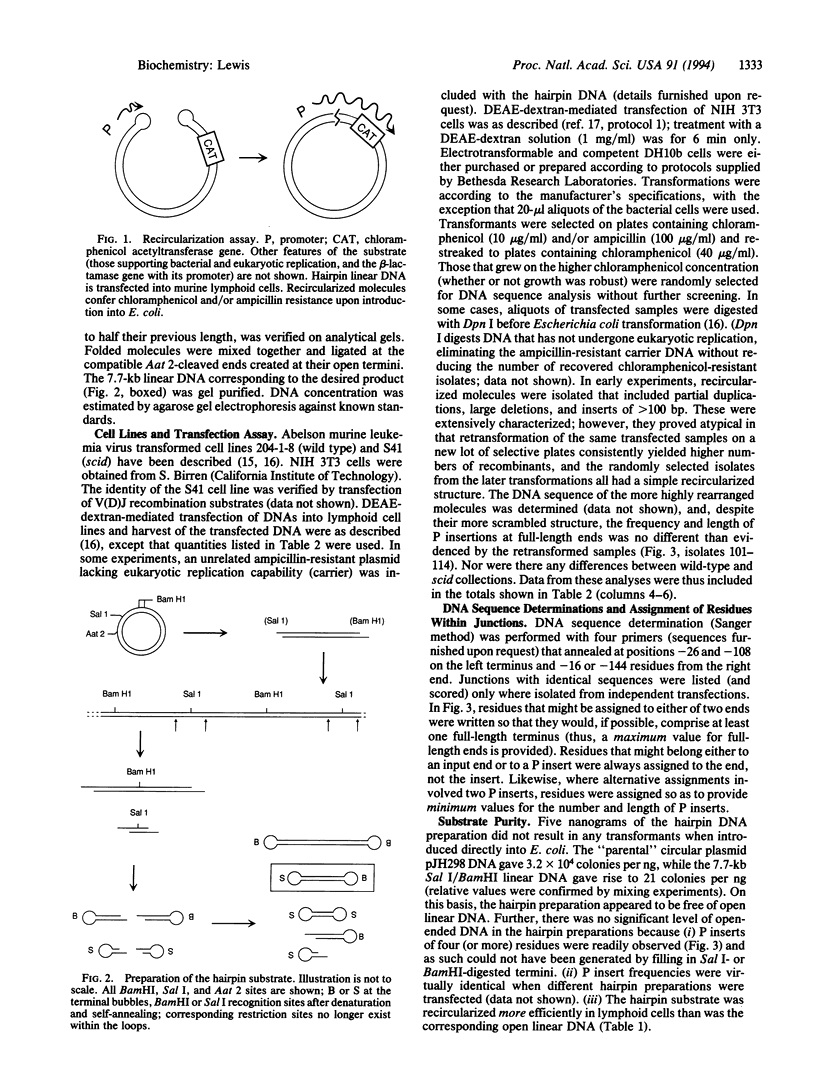
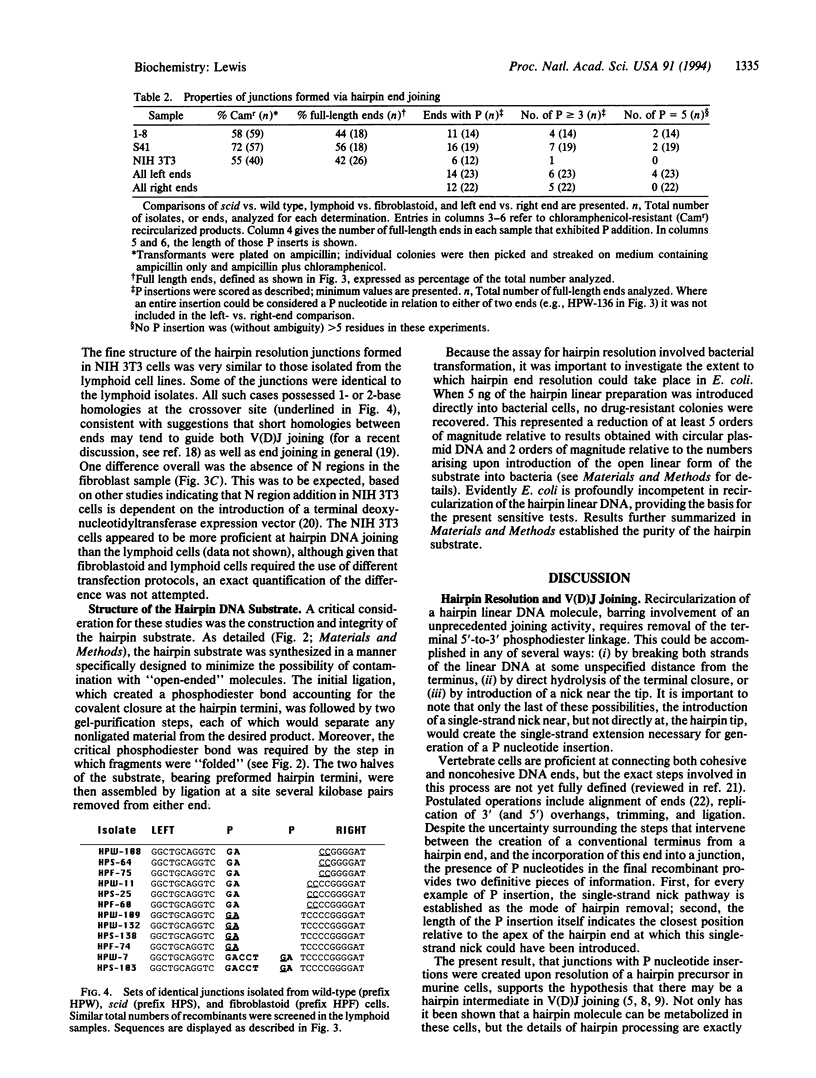
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Biedermann K. A., Mezzina M., Brown J. M. Characterization of the DNA double strand break repair defect in scid mice. Cancer Res. 1993 Mar 15;53(6):1244–1248. [PubMed] [Google Scholar]
- Coen E. S., Carpenter R., Martin C. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell. 1986 Oct 24;47(2):285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Gerstein R. M., Lieber M. R. Extent to which homology can constrain coding exon junctional diversity in V(D)J recombination. Nature. 1993 Jun 17;363(6430):625–627. doi: 10.1038/363625a0. [DOI] [PubMed] [Google Scholar]
- Gilfillan S., Dierich A., Lemeur M., Benoist C., Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993 Aug 27;261(5125):1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- Harrington J., Hsieh C. L., Gerton J., Bosma G., Lieber M. R. Analysis of the defect in DNA end joining in the murine scid mutation. Mol Cell Biol. 1992 Oct;12(10):4758–4768. doi: 10.1128/mcb.12.10.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Kallenbach S., Doyen N., Fanton d'Andon M., Rougeon F. Three lymphoid-specific factors account for all junctional diversity characteristic of somatic assembly of T-cell receptor and immunoglobulin genes. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2799–2803. doi: 10.1073/pnas.89.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Okada A., Stewart V., Alt F. W. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993 Aug 27;261(5125):1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Lieber M. R. Site-specific recombination in the immune system. FASEB J. 1991 Nov;5(14):2934–2944. doi: 10.1096/fasebj.5.14.1752360. [DOI] [PubMed] [Google Scholar]
- Meier J. T., Lewis S. M. P nucleotides in V(D)J recombination: a fine-structure analysis. Mol Cell Biol. 1993 Feb;13(2):1078–1092. doi: 10.1128/mcb.13.2.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Nevers P. Transposition in plants: a molecular model. EMBO J. 1985 Mar;4(3):585–590. doi: 10.1002/j.1460-2075.1985.tb03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schuler W., Schuler A., Bosma M. J. Defective V-to-J recombination of T cell receptor gamma chain genes in scid mice. Eur J Immunol. 1990 Mar;20(3):545–550. doi: 10.1002/eji.1830200313. [DOI] [PubMed] [Google Scholar]
- Thode S., Schäfer A., Pfeiffer P., Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990 Mar 23;60(6):921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]



