Abstract
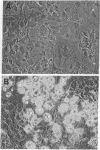
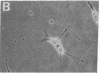
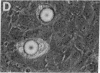
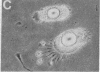
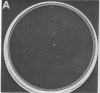
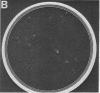
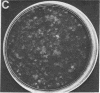
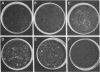
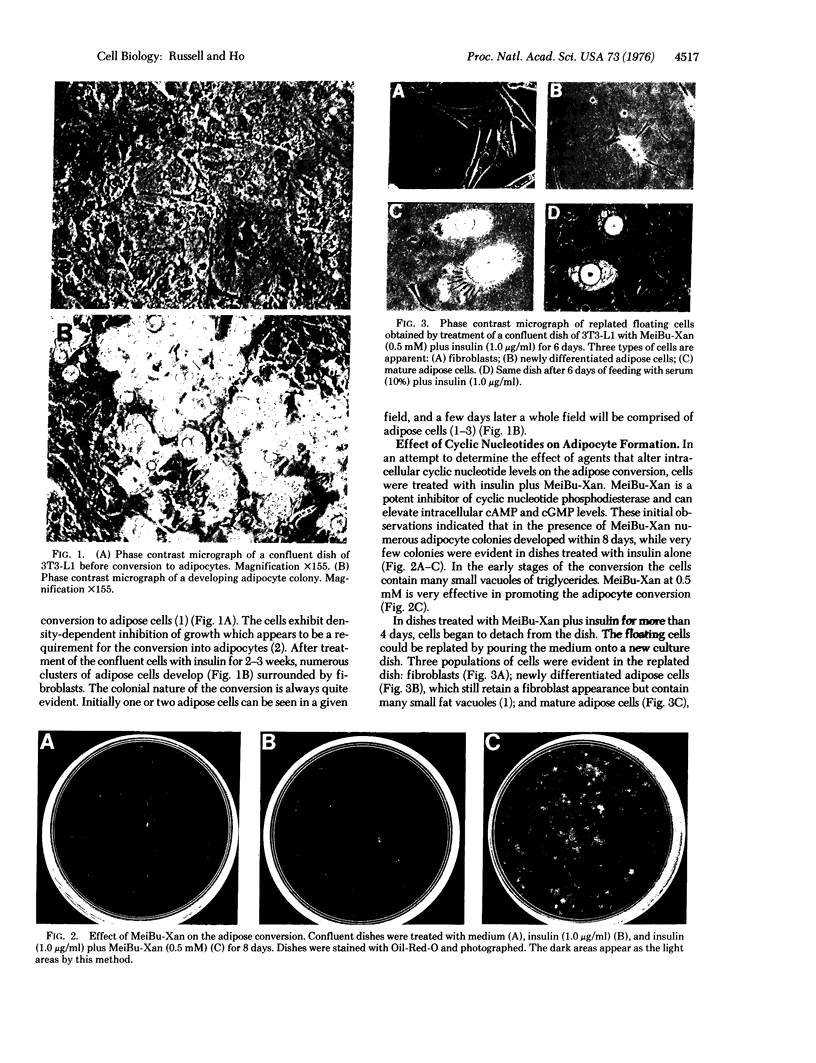
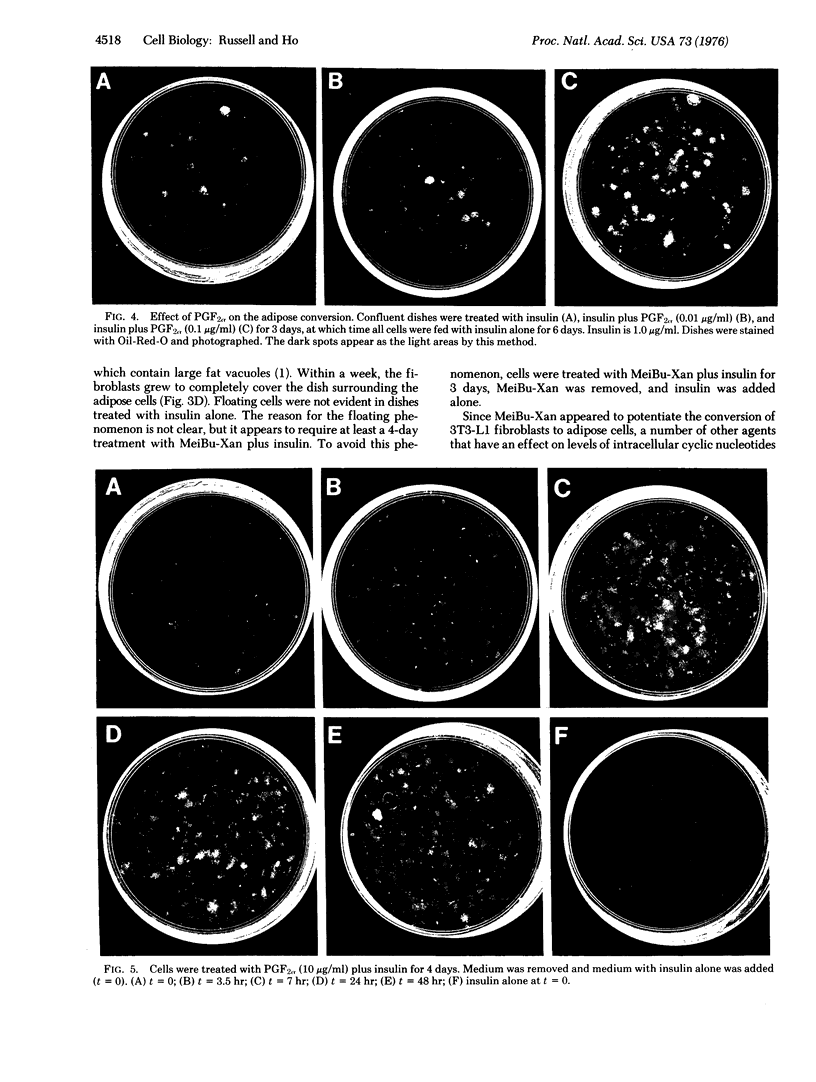
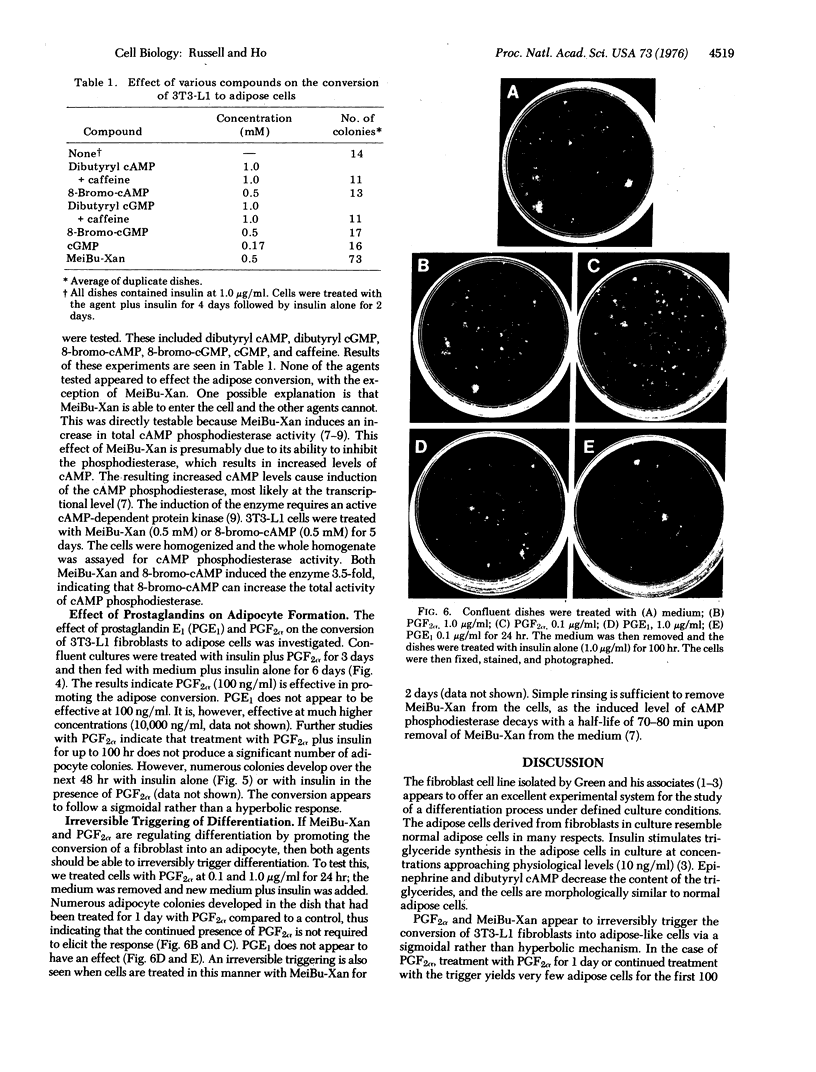
Green and Kehinde [(1974) cell 1, 113-116] have isolated clones of Swiss 3T3 fibriblasts that are able to convert to adipose cells. In this paper we report on two chemicals (prostaglandin F2alpha, 0.1 mug ml, and 1-methyl-3-isobutyl xanthine, 0.5 mM) that are able to rapidly and irreversibly program the fibroblasts to differentiate into adipose cells. Confluent cultures treated with prostaglandin F2alpha and insulin for 3-5 days, followed by insulin alone for 7-48 hr, yield numerous adipocyte colonies compared to control dishes and dishes treated with insulin alone. Cells treated with prostaglandin F2alpha or 1-methyl-3-isobutyl xanthine alone, rinsed, and then exposed to insulin gave similar results, indicating that the continuous presence of the triggering agent is not required to elicit the conversion of the fibroblasts to adipocytes. Agents that raise intracellular levels of 3':5'-cyclic AMP. 1.0 mM; 8-bromo-cyclic AMP,0.5 mM; and prostaglandin E1, 0.1 mug/ml) do not trigger the conversion process, suggesting that cyclic AMP may not be the mediator of differentiation in these cells. 8-Bromo-cyclic AMP, however, does induce thase; 3':5'-nucleotidohydrolase; EC 3.1.4.17) in these cells; the induction appears to be mediated by increases in intracellular cyclic AMP levels. These results indicate that this cell line might offer a system for studying the regulation of a type of cellular differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Asua L. J., Clingan D., Rudland P. S. Initiation of cell proliferation in cultured mouse fibroblasts by prostaglandin F2alpha. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2724–2728. doi: 10.1073/pnas.72.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science. 1975 Nov 28;190(4217):896–898. doi: 10.1126/science.171770. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Russell T. R., Pastan I. H. Cyclic adenosine 3':5'-monophosphate and cyclic guanosine 3':5'-monophosphate phosphodiesterase activities are under separate genetic control. J Biol Chem. 1974 Dec 25;249(24):7764–7769. [PubMed] [Google Scholar]
- Samuelsson B., Granström E., Green K., Hamberg M., Hammarström S. Prostaglandins. Annu Rev Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Baird C. E., Hardman Y. J., Wu J. G. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975 Apr 19;384(2):430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]