Abstract
Biological membranes segregate into specialized functional domains of distinct composition, which can persist for the entire life of the cell. How separation of their lipid and (glyco)protein components is generated and maintained is not well understood, but the existence of diffusional barriers has been proposed. Remarkably, the physical nature of such barriers and the manner whereby they impede the free diffusion of molecules in the plane of the membrane has rarely been studied in depth. Moreover, alternative mechanisms capable of generating membrane inhomogeneity are often disregarded. Here we describe prototypical biological systems where membrane segregation has been amply documented and discuss the role of diffusional barriers and other processes in the generation and maintenance of their structural and functional compartmentalization.
Eukaryotic cells are highly compartmentalized. By confining specific functions within individual organelles, cells can provide optimal microenvironmental conditions for the operation of specialized metabolic pathways. Membranes delimit and maintain the individuality of organelles; they also serve to store and transduce energy as electrochemical gradients. While initially conceived as indistinct lipid bilayers studded with randomly distributed and freely mobile (glyco)proteins, membranes are increasingly understood to be inhomogeneous, and subdivided into domains. Segregation of organellar membranes into discrete domains is in all likelihood intended to isolate specific functional hubs, either by accumulation of defined, dedicated components and/or by exclusion of undesirable ones. Thus, a phosphorylation node would benefit from concentration of kinases and simultaneous exclusion of phosphatases, as reported to occur in immunological synapses (Batista and Dustin, 2013). The existence of specialized domains is most apparent in the plasma membrane, which has been studied extensively because of its size and accessibility. For these reasons, the plasmalemma will be the focus of this review; nevertheless, the principles discussed herein likely apply to other cellular membranes as well.
Maintenance of distinct, identifiable domains over extended periods of time (sometimes the entire life of a differentiated cell; Fig. 1 A) suggests that individual components do not move freely throughout the whole surface of the cell membrane. Indeed, estimates of lipid and protein diffusion rates in reconstituted artificial bilayers greatly exceed the rates observed in biological membranes of comparable lipid composition, often by more than an order of magnitude (Kusumi et al., 2005). Such observations imply that the free diffusion of membrane proteins and/or lipids in the plane of the bilayer is hindered by physical barriers; several such structures have been documented and at least partially characterized in several biological systems, and constitute the main subject of this article.
Figure 1.
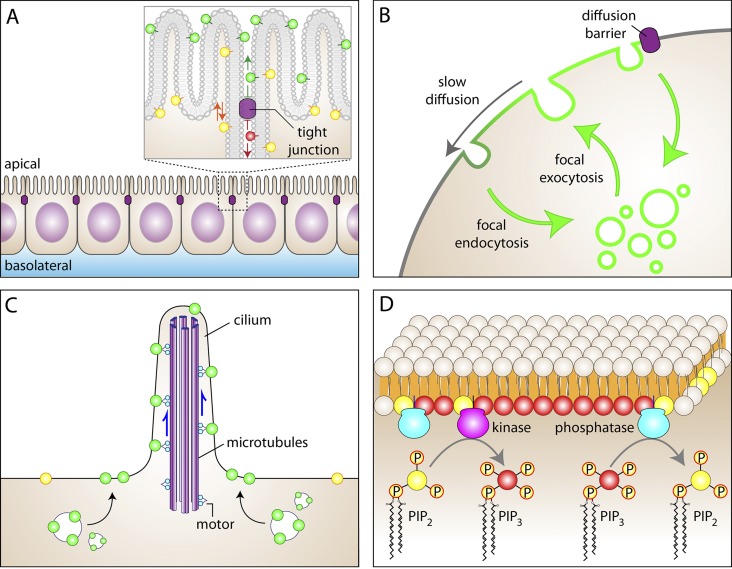
Mechanisms capable of generating and maintaining membrane inhomogeneity. (A) Epithelial tight junctions, which are bona fide diffusional barriers, occlude the passage of outer leaflet (exofacial) lipids from the apical membrane domain to the basolateral domain. For clarity, some exofacial apical lipids are highlighted in green while an exofacial basolateral lipid is shown in red. Note, however, that inner leaflet lipids (yellow) move freely between the apical and basolateral membranes. (B) Regions of the membrane with unique composition can be generated by targeting secretion focally and encircling this region with sites of active endocytosis. Also shown in this panel is the potential role of diffusional impediments in the maintenance of inhomogeneity. Slow diffusion along the plane of the membrane (e.g., mediated by hop diffusion) can reduce the rate of mixing of locally secreted material with the bulk plasmalemma, stabilizing standing gradients. More impenetrable barriers can effectively preclude the mixing of the two domains. (C) Active transport along microtubule-associated motors can also generate regions of differential accumulation of membrane proteins, as is the case in cilia. Components preferentially targeted and localized to cilia are shown in green; bulk (extraciliary) membrane components are shown in yellow. (D) The strategic distribution or activation of synthetic and degradative enzymes can generate standing gradients of the reaction substrates or products. In the example illustrated, an area of accumulation of phosphatidylinositol 3,4,5-trisphosphate (PIP3) can be created by local accumulation or activation of phosphatidylinositol 3-kinase, surrounded by membrane where a PIP3 phosphatase is active, regenerating PtdIns(4,5)P2 (designated as PIP2 in the figures for brevity). In this and all other figures the cytosol is colored beige.
Domain segregation in biological membranes can take multiple forms and span a range of sizes. Nanodomains can be generated upon passive association of lipids and/or proteins of compatible structure, hydrophobicity, and/or charge. The widely studied saturated lipid/cholesterol-rich “rafts” are a prime example of such nanodomains. However, much larger domains, beyond the 2–300 nm mesoscale defined by Kusumi et al. (2011), also exist and are of great importance in biology. These include the apical versus basolateral membranes of epithelial cells, the distinct buds of yeast, the somatodendritic versus axonal membranes of neurons, and the head, neck, and tail regions of sperm described in more detail later and illustrated elsewhere (see Fig. 6 of Kusumi et al., 2012). Active, energy-dependent processes are generally involved in generating these larger domains that are discernible by light microscopy. These processes include (but are not limited to):
(1) Spatially separated sites of exocytosis and endocytosis (Fig. 1 B). There is ample precedent for directed exocytosis: secretion is often polarized to an active region of the cell, whether in yeast, where vesicles are delivered to the growing bud, or in neurons, where neurotransmitter-containing vesicles are released exclusively at the synapse. A standing gradient in the composition of the membrane can be generated if continued secretion is accompanied by compensatory endocytosis elsewhere in the cell. Remarkably, regions of active endocytosis often surround the exocytic foci (Wahl et al., 2013). By preferentially internalizing the lipids and proteins that undergo exocytosis in their immediate vicinity, such rings of endocytic activity preclude their distribution throughout the rest of the cell. Thus, a steady-state gradient of plasmalemmal components can be generated that does not necessarily require diffusional barriers.
(2) Active displacement of membrane components by molecular motors (Fig. 1 C). Both actin- and tubulin-associated motors can operate in the immediate vicinity of the membrane. Indeed, some isoforms of myosin interact directly with the inner leaflet of the plasmalemma, and because microtubules sometimes run close to the membrane, dynein and/or kinesins can conceivably propel membrane-associated components, including transmembrane (glyco)proteins. As discussed in more detail later, such active displacement has been amply documented in cilia and flagella (Qin et al., 2004). Continued vectorial operation of such motors can generate inhomogeneities in the composition of the membrane.
(3) Strategic placement of enzymes with antagonistic activities (Fig. 1 D). The uneven distribution or activation of synthetic and degradative enzymes can generate standing gradients of the reaction substrates or products. A hypothetical case involves the local activation of a lipid kinase (e.g., class I phosphatidylinositol 3-kinase) at the leading edge of a migrating cell or at sites of contact with cognate ligands. The phosphatidylinositol 3,4,5-trisphosphate generated locally may not distribute equally throughout the cell if degradation occurs concomitantly. Although the formation of a gradient would be most noticeable if the degradative enzymes were themselves preferentially accumulated outside the site of synthesis, a gradient can also form even if hydrolysis occurs homogeneously throughout the cell surface. Over extended periods, the effect would be apparent only if the rate of hydrolysis is significant compared with that of synthesis.
The preceding examples illustrate various mechanisms whereby stationary gradients of membrane components can be generated without invoking diffusional impediments. While not essential for the separation of membrane domains, diffusional limitations can stabilize and accentuate them (Nicolson, 2014). Rapid isotropic (Brownian) diffusion of membrane components would tend to offset any accumulation generated by polarized membrane traffic, motors, or enzymatic reactions. Ultimately, the extent to which a gradient can be established and maintained is therefore a function of the relative rates of the polarizing and randomizing events. In this context, the formation of domains is aided by the unexpectedly slow diffusion of lipids and proteins in biological membranes referred to earlier. Thus, diffusional barriers may play a supporting role in the maintenance of inhomogeneities, without necessarily being the source of the molecular segregation process (Fig. 1 B).
It is clear from the preceding considerations that assessment of membrane appearance in the steady-state can document the existence of domains, but does not enlighten the underlying mechanism. To define the processes whereby domain segregation occurs, it is imperative to establish the mode and rate of molecular motion. This, in turn, requires highly sensitive techniques with exceptional temporal resolution. FRAP has been used extensively to assess the mobility of membrane components (Chen et al., 2006). Though technically simple, this method measures the average behavior of large ensembles of molecules and has limited temporal resolution (seconds). For these reasons, it has been superseded by approaches that can monitor the behavior of individual molecules. Fluorescence correlation spectroscopy and single-molecule tracking are increasingly the methods of choice (Kusumi et al., 2014). Because of its high spatial precision, single-molecule tracking can measure not only the diffusion coefficient of selected molecules but, empowered by the appropriate mathematical analysis, can determine whether the movement is random or directed, and can reveal the existence and properties of confinement zones. It is noteworthy that molecules can be confined transiently, and will therefore be categorized as subdiffusive by analysis of the mean squared displacement or of the moment scaling spectrum of the trajectories. Such transient events fail to be detected not only when molecular ensembles are analyzed, but even when detecting single molecules if the sampling rate is slower than the state transitions (i.e., the conversions from the confined state to the free state, and vice versa). It was only by implementation of ultrafast single-molecule tracking (with temporal resolution in the microsecond scale) that Kusumi and collaborators were able to reveal the “hop diffusion” of molecules between corrals where they are transiently confined (Fujiwara et al., 2002).
The molecular processes that account for the limited diffusion of lipids and proteins in membranes are not fully understood. The remainder of this review will consider mechanisms that can potentially curtail the diffusion of membrane components. Diffusional barriers are most readily conceived as stationary obstacles that preclude translocation of molecules from one compartment to another in a rather permanent and absolute fashion. This concept is restrictive and excludes a variety of possible mechanisms that can either limit diffusion transiently, or cause selective exclusion or retention of certain membrane components. We can envisage at least three different types of impediments that can restrict the free movement of lipids and proteins in biological membranes: physical obstacles, electrostatic attraction or repulsion nodes, and partitioning phenomena that preferentially retain or exclude certain membrane components. We will also present an overview of several biological systems where clear domain segregation has been documented. Whether diffusional barriers partake in the reported segregation will be discussed in each instance.
Physical obstacles
Arrays of (relatively) immobile molecules that are sufficiently crowded can obstruct the free passage of membrane proteins or lipids. Such physical barriers can form transiently or can be (semi)permanent fixtures of cells. Protein complexes are generally implicated, and both transmembrane and membrane-associated proteins can contribute to barrier formation. The size and degree of cross-linking of such complexes accounts for their poor mobility, which is often further reduced as a result of anchorage to extramembranous structures, such as intracellular components or the extracellular matrix. Four prototypical physical obstacles have been considered:
Cytoskeleton.
The inner aspect of the plasma membrane is lined by an intimately associated cortical skeleton. While present in virtually every eukaryotic cell, this actin-based mesh likely differs in composition between cells and even among regions of the same cell; in neurons, the cytoskeleton underneath the axonal membrane is distinctly different from that found in the soma and dendrites (Ludin and Matus, 1993). A myriad of proteins have been reported to constitute the cortical skeleton, but four of them (spectrin, ankyrin, band 4.1, and adducin) were proposed to be essential components of the framework that supports the actin-rich mesh (Baines, 2010). Others, like α-actinin, the ezrin-radixin-moesin group, septins, the talin–vinculin complex, and PDZ domain–containing proteins, may be more specialized, opportunistic constituents.
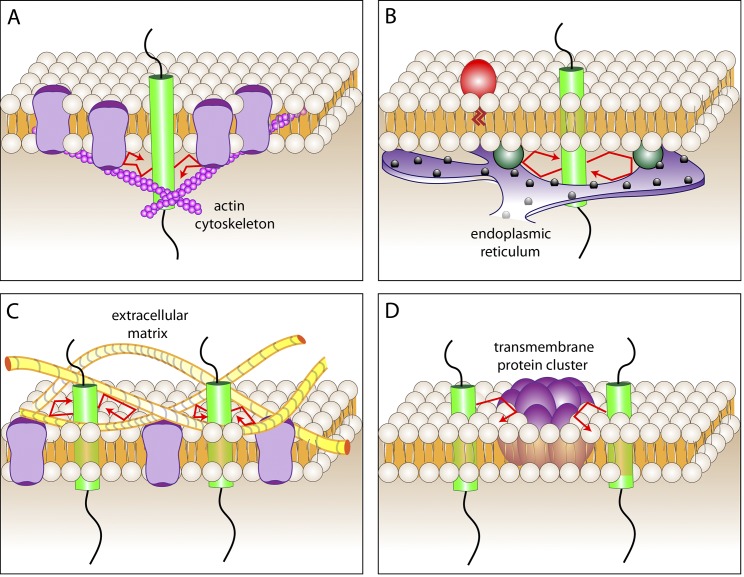
It is easy to visualize how a submembranous mesh would interfere with the lateral displacement of membrane-associated proteins bearing long cytosolic tails. However, the elegant measurements of Kusumi’s group revealed that the cortical skeleton also constrains the movement of outer leaflet (exofacial) lipid-anchored proteins and even of simple lipids (Fujiwara et al., 2002; Suzuki et al., 2007). This realization gave rise to the notion of a cytoskeleton-anchored picket fence (Fig. 2 A), where a fraction of the transmembrane proteins, which have been estimated to occupy nearly 50% of the membrane surface, are tethered to the underlying cytoskeletal mesh. These relatively immobile “pickets” obstruct the passage of unattached transmembrane proteins and also of lipids and lipid-anchored proteins. Thus, by stabilizing transmembrane components, the cortical skeleton not only impairs the free diffusion of proteins extending into the cytosol, but also of hydrophobic and even exofacial components.
Figure 2.
Physical barriers to the diffusion of membrane lipids or proteins. (A) The mesh formed by the cortical actin cytoskeleton serves to underpin a picket fence composed of transmembrane proteins and tightly associated lipids. (B) Regions of intimate contact between two organellar membranes can block the diffusion of molecules along the membrane of either organelle. A site of contact between the ER and the plasma membrane is illustrated as an example. Proteins that bridge the plasma membrane with the reticulum are shown as blue-green spheres. (C) Linkage of extracellular matrix components (yellow fibers) to membrane receptors can generate a mesh that impedes the diffusion of other membrane molecules. (D) Large, poorly mobile clusters of membrane proteins and lipids can block the passage of diffusing components. Freely mobile transmembrane proteins are illustrated by the green cylinder. Its trajectory is shown by the red arrows.
The fenestrated structure of the skeletal mesh that underpins the picket fence dictates the formation of confinement zones or “corrals” wherein diffusible molecules can move. It is important to note that confinement of mobile molecules within a given corral is not permanent. Instead, individual molecules are seen to “hop” intermittently between adjacent corrals. Two mechanisms can explain such hopping: first, the corrals themselves may be metastable, whether because of reversible detachment of the pickets from the underlying mesh or as a result of rearrangement of the mesh itself. That the actin cytoskeleton is a dynamic structure undergoing continuous remodeling is well established (Pollard et al., 2000). Second, the picket fence is unlikely to seal the confinement zones hermetically. Each corral may have incompletely sealed gates through which appropriately sized molecules can percolate. Either one or a combination of these mechanisms could account for the observed “hop diffusion” that, while allowing the eventual and seemingly random translocation of diffusible molecules along membranes, greatly curtails their diffusion coefficient.
The cortical cytoskeleton has also been implicated in the generation of nanoclusters of lipid-anchored exofacial proteins and glycolipids (Goswami et al., 2008). The microdomains formed by these molecules have been more often attributed to lipid-based partitioning processes (Lingwood and Simons, 2010), but Mayor and colleagues have argued that they can form via interactions with actin clusters or “asters,” which would explain their susceptibility to agents that disrupt actin assembly (Gowrishankar et al., 2012). It is not yet clear how glycolipids or lipid-anchored exofacial proteins would link to actin filaments; a transbilayer link could be provided by interdigitation with phosphoinositides on the inner leaflet, a process possibly assisted by cholesterol, or alternatively by unidentified membrane-spanning proteins that associate with both the exofacial components and with cytosolic actin filaments. Regardless of the mechanisms involved, such interactions would manifest themselves as limiting the free diffusion of the lipid-anchored proteins and glycolipids.
Membrane–membrane junctions.
Transmembrane pickets that obstruct free diffusion can be immobilized not only by the cortical cytoskeleton, but by association with other large and relatively immobile structures. Zones where two separate membranes interact intimately with one another can have this effect. Tight junctions (Fig. 1 A), adherens junctions, and other sites where the extracellular surfaces of two cells make stable contacts can concentrate and immobilize membrane proteins for extended periods of time, generating impenetrable barriers (van Meer and Simons, 1988; Gulino-Debrac, 2013). Similar effects can be expected at sites of contact between organellar membranes. The endoplasmic reticulum is increasingly appreciated to form intimate contacts with the plasma membrane (Carrasco and Meyer, 2011), and with the membranes of other organelles as well (Rowland and Voeltz, 2012; Hönscher and Ungermann, 2014). These would be predicted to deflect the free diffusion of unengaged membrane components (Fig. 2 B).
In the case of cell-to-cell contacts, the interacting (glyco)proteins need not span the membrane. This could result in the generation of barriers to the diffusion of components of the outer, but not the inner, leaflet, as discussed in more detail later in the context of epithelial junctions.
Membrane–matrix junctions.
Membrane components can also be immobilized by latching onto the extracellular matrix (Fig. 2 C). Integrins and other receptors bind tightly to matrix components and, if accumulated at sufficiently high densities, could impede the diffusion of other, more mobile molecules. The sealing zones that osteoclasts form on the surface of bones are presumably generated by this mechanism. The immobilization of the pickets in these cases is often buttressed by the development of sturdy, specialized cytoskeletal structures that assemble in response to the association of the matrix ligand with its transmembrane receptor.
A striking example of a diffusional barrier generated by membrane–matrix interaction was documented by Weaver and colleagues (Paszek et al., 2014), who showed that the close apposition of integrins with matrix proteins blocked the passage of membrane molecules whose extracellular dimensions (perpendicular to the plane of the membrane) exceeded the width of the receptor–ligand contact.
Intramembranous clusters.
In the preceding examples, the poor mobility of membrane proteins stems from their anchorage to other structures that are themselves relatively immobile. However, mobility can also decrease as a result of intrinsic clustering of membrane components. Thus, interestingly, the same forces that drive the formation of nano- or microdomains play a role in restricting the diffusion of other components. This applies to affinity-, partition-, and electrostatically driven associations, which will be discussed later.
The diffusion coefficient (D) of membrane-embedded particles decreases as a function of the radius of the cylinder (Rc) embedded in the bilayer. The dependence of D on the radius of the particle was predicted to be weak (D = (kT/4πµh)log[µh/µ′Rc – γ], where k is Boltzmann’s constant, h is the height of the cylinder, µ and µ′ are the viscosity of the membrane and aqueous media, respectively, and γ is Euler’s constant; Saffman and Delbrück, 1975). The validity of this prediction has been tested experimentally, with somewhat inconsistent results: although studies using penta-monododecylether bilayers initially failed to support the Saffman-Delbrück model (Gambin et al., 2006), more recent studies using phospholipid bilayers have generally validated its basic conclusions (Ramadurai et al., 2009; Domanov et al., 2011). More importantly, as their size increases, molecular aggregates are much more prone to become trapped in the corrals established by the picket fences, as described earlier (Iino et al., 2001). Such slow-moving islands will therefore constitute barriers to the rapid displacement of monomeric proteins or lipids (Fig. 2 D).
Electrostatic impediments
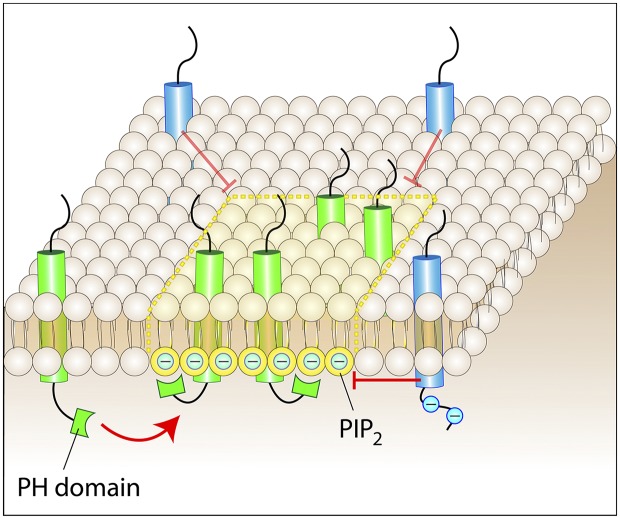
The free diffusion of membrane-associated molecules can be hindered by collisions with physical obstacles, but also by electrostatic interactions. Charged proteins or lipids can be repelled by like charges or attracted by opposite ones. Hence, the presence of poorly mobile, highly charged objects in or near the membrane can deflect or (temporarily) immobilize diffusible molecules (Fig. 3). Electrostatic mapping of the inner and outer surfaces of the plasma membrane is in its infancy, but there is compelling evidence that highly charged clusters of molecules exist, and that some of these are confined to specific regions of the cell, implying that they are somehow immobilized. McLaughlin and Murray (2005) noted that a striking number of proteins possess natively unfolded regions that contain both basic and hydrophobic residues that facilitate their partition into membranes and at the same time attract anionic lipids, particularly the tetravalent phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2), electrostatically. The strong electrostatic basin generated by the membrane-associated cationic residues can attract multiple PtdIns(4,5)P2 molecules, creating a negatively charged ring around the protein (it is noteworthy that the lipid is sequestered laterally near the protein, where its charge is concentrated, but not neutralized). These electrostatic islands can be comparatively small, such as those generated by the myristoylated MARCKS protein (Wang et al., 2001), but much larger ones have also been reported. Syntaxin-1A, a SNARE protein involved in exocytosis, contains an unstructured polycationic stretch; unlike the monomeric MARCKS, however, syntaxin-1A exists in clusters containing upwards of 60 monomers. These clusters sequester and thereby curtail the diffusion of a large number of PtdIns(4,5)P2 molecules (van den Bogaart et al., 2011). The resulting halo of anionic lipids can in turn alter the mobility of other charged species in the plane of the membrane. It is noteworthy that syntaxin-1A clusters are restricted to sites where synaptic vesicles undergo exocytosis, which implies that their mobility is limited. It can therefore be concluded that relatively stationary electrostatic barriers to the diffusion of charged molecules exist in some, or perhaps all, cell types.
Figure 3.
Attraction and repulsion of mobile proteins. Mobile membrane proteins or lipids can be immobilized within microdomains by stereospecific, hydrophobic, or electrostatic interactions. A protein bearing a PH domain is shown to be recruited to a patch rich in its ligand lipid, e.g., PtdIns(4,5)P2. The negative charge of PtdIns(4,5)P2 (designated as PIP2 in the figures for brevity; approximately −4 at physiological pH) is also shown as deflecting proteins with a negatively charged cytosolic domain.
Partition-induced sinks or barriers
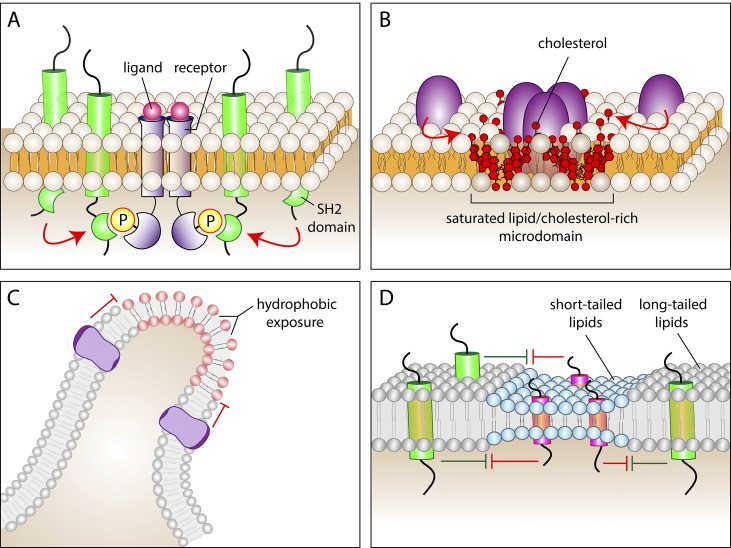
Other membrane properties can also interfere with the free displacement of molecules. Specific lipids or proteins can preferentially associate (partition) with defined regions or components of the membrane, resulting in atypical, subdiffusive behavior. In addition to the electrostatic interactions detailed in the previous section, proteins and lipids can associate with each other by several alternative means: (1) Proteins often recognize each other or specific lipids via stereospecific interactions that require precise tertiary folding. The association of protein PH domains with phosphoinositides (Lemmon, 2008; Fig. 3) or the recognition of phosphotyrosine moieties by SH2 domains (Pawson, 2004; Fig. 4 A) typify this kind of interaction. (2) Hydrophobic interactions can also induce the formation of metastable lipid and protein complexes of varying size (Fig. 4 B). The saturated lipid- and cholesterol-rich, detergent-resistant microdomains called “rafts” are perhaps the best known example of such hydrophobic partitioning (Lingwood and Simons, 2010). (3) Regions where the membrane curves sharply have distinct properties. Where the membrane bends, the headgroups of the lipids constituting the concave monolayer are uncharacteristically close together, whereas the opposite is the case for the lipids on the convex surface (Fig. 4 C). Lipid crowding on the concave aspect can alter diffusion physically or electrostatically. On the convex surface, the reduced packing tends to make the hydrophobic core of the membrane more accessible. This in turn can promote the binding and stabilization of proteins with exposed hydrophobic residues (Bigay and Antonny, 2012). Wedge-shaped intrinsic proteins such as the reticulons of the ER insert preferentially into curved membranes and may also contribute to the generation of curvature (Wong et al., 2014). They are localized preferentially in ER tubules and excluded from the flatter cisternae, except for the cisternal edges where curvature increases sharply. Curvature can also favor the partition of membrane extrinsic components: N- and F-BAR domain–containing proteins associate selectively with small tubules, and I-BAR proteins associate with outward membrane protrusions like the tips of filopodia. Multimeric assembly of such proteins could in principle generate barriers to diffusion. (4) The width of the bilayer can also affect the ability of molecules to traverse a region of the membrane (Bigay and Antonny, 2012). Bilayer height is dictated by the length of the lipid acyl chains and their degree of interdigitation. Once established, a particularly short bilayer (such as that thought to exist in the Golgi apparatus) will constitute an energetically unfavorable environment for long-tailed lipids or for proteins with tall transmembrane (hydrophobic) regions to partition into (Fig. 4 D), as the molecules try to minimize exposure of hydrophobic moieties to the aqueous space. The converse hydrophobic mismatch applies also to tall bilayers and short lipids and proteins. The reluctance of molecules to enter a membrane with a discordant height will effectively serve as a diffusional disincentive.
Figure 4.
Mechanisms that can impair the free mobility of membrane proteins or lipids. (A) Association of a protein bearing an SH2 domain with a receptor that became tyrosine-phosphorylated in response to ligand binding. (B) Partition of proteins into saturated lipid- and cholesterol-rich microdomains. Cholesterol is shown in red. (C) Sites where the membrane undergoes sharp curvature (e.g., the tip of filopodia) can force the exposure of hydrophobic regions on the outer leaflet and can also tighten the packaging of lipid headgroups of the inner leaflet, potentially altering the surface potential. These effects can alter the partition of other lipids or proteins into regions of extreme curvature. (D) Hydrophobic mismatch can restrict access of proteins or lipids into certain areas of the membrane. Regions of the bilayer where lipids with short acyl chains predominate (blue) tend to accumulate proteins with short transmembrane domains (pink), while excluding proteins with longer transmembrane domains (green). The opposite applies to regions with longer acyl chains (gray).
In summary, diffusion of molecules along membranes can in principle be altered by regions where poorly mobile lipids and/or proteins interact with each other stereospecifically or hydrophobically, and also where sharp curvature or bilayer height mismatches occur. Together with molecular crowding and electrostatic (and possible also van der Waals) interactions, these phenomena greatly expand the repertoire of impediments to the free diffusion of molecules in bilayers.
Membrane inhomogeneity: Diffusional barriers or other mechanisms?
Considering the number of mechanisms that could potentially limit the diffusion of membrane components, it is hardly surprising that the list of examples of inhomogeneous plasma membranes is growing steadily. Some of the best-studied examples are described here. As will become apparent from the accompanying discussion, the molecular basis of domain segregation is not always well understood, and it is imperative to bear in mind that inhomogeneity can arise from mechanisms other than diffusional barriers.
Tight junctions
Epithelia separate the inside of the body from the outside environment, whereas endothelia segregate the intravascular and extravascular compartments. The barrier properties of epithelial and endothelial cells are attributable to large multiprotein complexes, called tight junctions, which form intimate intercellular contacts that limit paracellular leak. The tight junction also defines the polarity of the membrane, segregating the apical and basolateral surfaces. This polarity is essential for the directional transport of fluids, ions, and macromolecules across the cell layer.
The polarity of epithelial cells is characterized by the distinct lipid and protein compositions of their apical and basolateral membranes. The apical membrane is usually enriched several-fold in glycolipids and cholesterol, compared with the basal and lateral membranes. More strikingly, many proteins have been demonstrated to be enriched or even localized exclusively at either the apical or basolateral surfaces. Not surprisingly, transporters and ion channels are frequently limited to one surface or the other, allowing the cells to create gradients that drive net unidirectional transport of substrates between the compartments they separate (Simons and Fuller, 1985). The preferential appearance of lipids and proteins in apical versus basolateral membranes likely reflects their targeted secretion, directed to the relevant surface (Stoops and Caplan, 2014), but the maintenance of the resulting enrichment requires the existence of a barrier to lateral diffusion, a function associated with the tight junction.
Experimental evidence for a diffusion barrier was obtained by monitoring the movement of fluorescent lipids. When inserted into the extracellular leaflet of the apical membrane, the labeled lipids were unable to diffuse past the tight junction to reach the basolateral surface. Notably, when the lipids were capable of flipping, or of reaching the inner leaflet by other means, they were able to cross the junction (Dragsten et al., 1981; van Meer et al., 1986), which suggests that the exofacial barrier does not extend to the inner leaflet. There has been speculation that transmembrane proteins would also be restricted from diffusion by this barrier, although direct evidence is lacking.
Although the precise nature of the diffusion barrier is still under investigation, clues of its structure have come from analysis of the proteins found at the tight junction and correlated structures observed by electron microscopy. The extracellular domains of transmembrane proteins such as the claudins and occludin are thought to mediate cell–cell contact at the tight junction; their cytoplasmic domains are bound to multi-PDZ-containing proteins such as ZO-1 that attach, via linker proteins, to the filamentous actin and microtubule cytoskeleton. Freeze fracture of the lipid bilayer revealed the presence of anastomosing transjunctional strands, thought to be comprised of polymers of claudin proteins (Furuse, 2010). These detergent-resistant structures (Stevenson and Goodenough, 1984) are believed to form a continuous fence within the lipid bilayer. However, occludin also plays a role in establishing the diffusion barrier. Expression of truncated occludin lacking the C-terminal PDZ-binding motif did not affect tight junction appearance, but caused a size-specific increase in paracellular ion permeability and resulted in diffusion of lipids from the apical to the lateral membrane (Balda et al., 1996). Remarkably, tight junctions are not static structures; their component proteins undergo rapid turnover by endocytosis and recycling back to the plasma membrane, providing a means to regulate paracellular leakage.
The protein-centric model of the tight junction diffusion barrier suggests that transmembrane proteins form a fence that physically restricts the mobility of proteins and lipids. However, if the diffusion barrier is achieved by a protein-based fence of transmembrane molecules locked in by homotypic interactions between neighboring cells, it is unclear why diffusion of lipids of the extracellular leaflet is restricted, while that of inner leaflet lipids is not. An alternative explanation is that the extracellular leaflet may exist in a hemi-fused state between cells (Kachar and Reese, 1982). Accordingly, several lipid probes have been shown to diffuse from cell to cell. For example, C6-NBD-phosphatidylcholine can diffuse along the apical surfaces of polarized MDCK cells apparently without partitioning into the aqueous phase (Grebenkämper and Galla, 1994), and endogenous GM1 alone, or with cholera toxin B bound, can also cross from cell to cell (Laffafian and Hallett, 2007), which supports the hemi-fusion concept. However, the Forssman antigen, an extracellular leaflet glycolipid, does not cross from cell to cell (van Meer et al., 1986), an observation not easily reconciled with the hemi-fusion model. Because of these uncertainties, hybrid models combining protein- and lipid-based barriers have also been proposed (Lee et al., 2008). Clearly, additional work is required to fully define the mechanisms controlling lateral diffusion at the tight junction.
Axon initial segment (AIS)
Neuronal communication is the basis of sensory perception, motor control, reasoning, and memory, and results from the conversion of stimulatory and inhibitory chemical stimuli into electrical signals at synapses. These inputs are integrated at the AIS, the boundary between the neuronal body and the axon. This site is rich in voltage-gated ion channels that respond to depolarization; when a threshold is reached, activation of these channels generates an action potential that propagates down the axon. The localization of these ion channels at the AIS reflects their limited mobility within the membrane.
The composition of the membranes lining the axon and soma differs considerably; NgCAM, for instance, is found primarily in the axon (Silverman et al., 2001), whereas the K+/Cl− cotransporter KCC2 is excluded from the axon and found only in the somatodendritic compartment (Hedstrom et al., 2008). The observation that certain membrane proteins are restricted to axonal or somatodendritic compartments led to speculation that a barrier to free diffusion may exist at the AIS. Intriguingly, studies showed that viral glycoproteins that localize to the apical surface when expressed in epithelial cells are targeted primarily to axons of polarized neurons. Conversely, proteins targeted basolaterally in epithelia are expressed preferentially in the soma/dendrites (Dotti and Simons, 1990). This raised the possibility that partitioning of proteins in neurons may be akin to that of epithelial cells, where the junctional complex restricts diffusion.
In search of a diffusion barrier, Kobayashi et al. (1992) introduced fluorescent phospholipids into neurons by labeling enveloped viruses that selectively fuse with axons. Remarkably, the incorporated lipids were limited to the axonal membrane, and unable to cross the AIS barrier regardless of whether they resided in the inner or outer leaflet. However, the ability of lipids to traverse the barrier has remained controversial, as the amphiphilic fluorescent label DiI, which incorporates readily into the outer leaflet of biological membranes, manages to traverse the AIS (Winckler and Poo, 1996). However, the existence of a diffusional barrier was supported by experiments measuring the mobility of membrane proteins. Using laser tweezers, Winckler et al. (1999) found that beads attached to Thy1 (a glycophosphatidylinositol-anchored protein) or to L1 (a transmembrane protein) could be moved easily along the axon, but could not be dragged past the AIS. The reduced mobility at the AIS could be alleviated by disrupting F-actin with latrunculin, which caused the loss of polarity of membrane proteins normally restricted to the axon.
Single-particle tracking of gold-labeled lipids revealed that the barrier to diffusion developed 7–10 d after neurons were plated, and correlated with the accumulation of ankyrin G and actin at the AIS. In addition, accumulation at the AIS of transmembrane proteins, such as NrCAM, neurofascin, and the voltage-gated sodium channel, all of which are anchored to the cytoskeleton (Nakada et al., 2003), occurs at this time. Ankyrin, a protein that bridges the cytoskeleton with membrane proteins, directly binds to an ankyrin-binding motif in Nav1 channels (Garrido et al., 2003; Gasser et al., 2012), thus providing a potential basis for the formation of a picket fence at the AIS. Of note, ankyrin G and sodium channels begin to accumulate before the diffusional barrier is established (Brachet et al., 2010), which suggests that a tight barrier capable of blocking lipid diffusion is achieved only when the local protein concentration is sufficiently high to assemble a dense picket fence. Moreover, depletion of ankyrin G results in the leakage of somatodendritic proteins, such as KCC2, into the axon, and loss of neuronal polarity (Hedstrom et al., 2008). Unlike the epithelial tight junctions, these pickets seem to traverse the membrane, interfering with the movement of components on both leaflets, explaining why membrane proteins and even some, but not all, lipids might be affected. In fact, the AIS even creates a filter for cytoplasmic molecules, as large cytosolic solutes (such as 70 kD dextran) are unable to traverse the narrowing between the soma and axon (Song et al., 2009), whereas smaller solutes like GFP or 10 kD dextran can diffuse across. Perhaps the dense meshwork of actin filaments forms a sieve-like structure that excludes large objects. If so, this mesh must be dynamically controlled to allow the passage to the axon of even larger objects such as mitochondria and synaptic vesicle precursors.
Spermatozoa
For fertilization to occur, sperm cells must swim up a gradient of chemoattractants and attach to a jelly coat surrounding the egg. This is followed by the release of enzymes that allow the sperm to burrow toward the egg and ultimately fuse with it. Achieving this goal requires a variety of adhesion molecules, receptors, and enzymes to be appropriately localized on the sperm surface. During maturation, the surface membrane of the sperm cell develops lateral inhomogeneity, with specific proteins being restricted to one of four major compartments: the anterior head (or acrosomal region), posterior head (postacrosomal region), anterior tail (midpiece), and posterior tail (primary piece; Cowan et al., 1997). Notable changes in the distribution of the membrane proteins are seen as sperm progress from the testes to the epididymis, and also after capacitation, which renders the sperm competent for fertilization. These observations suggest that barriers that preclude the free diffusion of membrane proteins may exist and that they are dynamically regulated.
The anterior and posterior regions of the sperm head are separated by the subacrosomal ring. The anterior region is enriched in GM1 (Selvaraj et al., 2006) and sterols (Selvaraj et al., 2009) compared with the posterior region. However, the means whereby this segregation is established and maintained are not clear. Individual sterol molecules can traverse the subacrosomal ring. Moreover, the anterior GM1 and sterol enrichment is lost in dead or fixed sperm. Thus, rather than separation by a diffusion barrier, it seems likely that proteins with affinity for GM1 and sterols are concentrated in the anterior section by an energy-dependent process. Consistent with this conclusion, freeze-fracture analysis of the plasma membrane did not reveal any transmembrane structures that could limit the diffusion of lipids between the two segments of the sperm head (De Leeuw et al., 1990), yet the mobility of lipids was found to be different in the two regions (Bruckbauer et al., 2007).
A second ring-like structure separates the head from the tail. This so-called posterior ring is visible as an indentation by scanning electron microscopy and as an electron-dense element by transmission electron microscopy. The composition of this poorly characterized structure is not known. Fluorescent lipid probes are able to traverse this ring (Christova et al., 2004), but the permeation of membrane proteins has not been directly tested. It therefore remains unclear whether the posterior ring plays a role as a diffusional barrier.
Between the principal piece and the midpiece of the tail there is a third ring-like electron-dense structure called the annulus. The annulus is thought to be composed of septins, a family of proteins that form heteromultimeric complexes that assemble into filamentous structures. Accordingly, deletion of one of the septin components of the complex (SEPT4) resulted in loss of the annulus and incomplete tail maturation (Ihara et al., 2005; Kissel et al., 2005). Septins have a largely cationic surface and can thereby bind directly to negatively charged lipids such as PtdIns(4,5)P2 (Zhang et al., 1999). They have been speculated to function as molecular fences by forming long polymers that bind avidly to the inner leaflet of the plasma membrane, which is particularly enriched in PtdIns(4,5)P2 (Golebiewska et al., 2011).
Lipid probes were found to diffuse freely along the tail, indicating that the annulus is not a barrier to lipid diffusion (Christova et al., 2004). However, protein segregation across the annulus has been well documented. Initially, both CE9 (also called Basigin or CD147) and PT-1 are localized to the principal piece. During epididymal maturation, CE9 translocates to the midpiece, whereas PT-1 relocalizes there during capacitation (Bartles, 1995). The role of septins in this segregation has been addressed using gene knockout strategies. In SEPT4−/− mice, CE9 was found throughout the plasma membrane, which suggests loss of a diffusion barrier that maintains protein segregation (Kwitny et al., 2010); however, diffusion was not directly measured. It remains possible that the homogeneous distribution of proteins along the tail of SEPT4−/− sperm may reflect a defect in targeting, rather than loss of a diffusion barrier.
It is worth noting that, in addition to the morphologically apparent rings that separate the individual regions, sperm have unique features that could affect the migration of their membrane proteins. First, sperm cells have an extraordinarily small amount of cytoplasm; as a consequence, membrane proteins with intracellular domains may collide not only with the cortical cytoskeleton, but also with underlying cellular structures such as the nucleus or mitochondria. In addition, extracellular interactions may limit the mobility of glycoproteins. This is illustrated by PH20, an extracellular glycosylphosphatidylinositol (GPI)-anchored protein that was shown to diffuse >1,000-fold slower than lipids. The protein ectodomain of PH20 therefore appears to dictate its immobility (Phelps et al., 1988).
In summary, although the segregation of the sperm plasmalemma into distinct domains is striking, the mechanisms underlying the generation of the domains remain largely obscure. In particular, the putative existence of diffusional barriers and their molecular nature remain uncertain.
Yeast bud neck
Budding yeast undergo asymmetric divisions. New membrane is secreted in a polarized fashion, giving rise to the nascent bud. Remarkably, many plasma membrane proteins are uniquely concentrated at the bud, raising the possibility that a diffusion barrier prevents their dispersal back into the mother cell. As in the case of sperm, septins have been implicated in limiting the diffusion of polarized plasma membrane proteins in yeast. During budding, mRNA encoding Ist2, an integral membrane protein, is transported into the bud, where it is translated. The Ist2 protein accumulates in the bud, and this asymmetrical distribution is maintained by the presence of a septin ring at the bud–mother junction (Takizawa et al., 2000). FRAP studies in yeast expressing GFP-Ist2p revealed that although the protein was restricted to the bud in Cdc12-6 septin mutants at the permissive temperature, it diffused back into the mother at the restrictive temperature. This result has been widely interpreted as evidence for a barrier that curtails the diffusion of plasma membrane proteins. However, more recent studies have revealed that although Ist2p appears to be plasmalemmal, it is in fact present exclusively in the cortical ER, tethering the reticulum to the plasma membrane (Manford et al., 2012; Wolf et al., 2012). Therefore, rather than demonstrating the existence of a barrier to the diffusion of plasmalemmal proteins, the Ist2p data in fact argue in favor of a septin-based barrier to the movement of ER membrane–associated components across the mother–bud junction. Indeed, this notion is supported by much additional evidence. For instance, although GFP targeted to the lumen of the ER diffuses readily through the bud neck, integral membrane proteins such as Sec61p do not (Luedeke et al., 2005). In the absence of functional septins, however, Sec61p can readily diffuse across the neck. It is interesting to note that the ER localized within the neck is predominantly smooth ER, and that the diffusion barrier is dependent on the presence of the sphinganine C4-hydroxylase Sur2 (Clay et al., 2014), which produces sphingosine. Moreover septins genetically interact with Sur2 (Costanzo et al., 2010) and appear to act upstream of sphingosine production. It is therefore tempting to speculate that the diffusion barrier may be generated by a membrane domain enriched in long chain lipids, which may segregate into a specialized microdomain that excludes certain membrane proteins, obstructing their passage. In fact, the role of septins may be to function as a scaffold to control the local production and/or retention of such lipids in the ER, rather than to directly inhibit the movement of proteins.
Although reinterpretation of the Ist2p data provides support for the existence of impaired diffusion of ER proteins across the yeast bud neck, comparable evidence for a barrier to lateral diffusion of plasma membrane components is currently lacking. Instead, the marked accumulation of certain plasmalemmal proteins at the bud can be explained, at least in some cases, by polarized exocytosis coupled to localized endocytosis (Fig. 1 B), which is particularly active at the bud neck. For instance, the vSNARE protein Snc1 is preferentially secreted at the bud, where it accumulates. Remarkably, this accumulation is abrogated in endocytosis-deficient mutants. In contrast, the tSNARE protein Sso1p, which normally lacks polarization, becomes polarized when an endocytosis motif is introduced to its cytosolic tail (Valdez-Taubas and Pelham, 2003).
Primary cilia
The primary cilium is a projection found in most nonmitotic cells of vertebrates. The high concentration of signaling molecules found in primary cilia has led to their designation as the “antenna” of the cell (Singla and Reiter, 2006). The importance of primary cilia is highlighted by the number and severity of diseases associated with their loss, which are collectively known as ciliopathies (Bettencourt-Dias et al., 2011).
The membrane covering the cilium is topologically continuous with the plasma membrane, yet has a unique composition of proteins and lipids. This segregation is caused, at least in part, by a mechanism whereby membrane proteins enter the cilium after exocytosis near its base and are transported to the tip by microtubule motors in a process called intraflagellar transport (Qin et al., 2004; Fig. 1 C). Once released from the motors, however, the membrane proteins move within the cilium membrane primarily by diffusion (Ye et al., 2013), which would be expected to counteract the concentrative effect of motor-driven transport. Because the membrane of the cilium nevertheless maintains a distinct composition of proteins and lipids, it has been speculated that a diffusion barrier exists at the base of the cilium. Morphological findings are consistent with this concept. Freeze-fracture EM studies revealed the presence of the “ciliary necklace,” an array of transmembrane particles at the base of the cilium (Gilula and Satir, 1972), raising the possibility that this structure may act like a fence to limit membrane diffusion. Recently, complexes of proteins including microtubule-binding proteins, cytoplasmic proteins, transmembrane proteins, and extracellular proteins have been identified and shown to localize to an area at the base of the cilium called the transition zone. Microtubules appear tethered to the membrane at the transition zone (Chih et al., 2012; Garcia-Gonzalo et al., 2011), where they may function as gatekeepers for entry into the cilia. Consistent with this, it has been suggested that a sieve-like barrier regulates entry into the cilium of soluble proteins in a size-dependent manner (Gulino-Debrac, 2013), and a similar restriction could exist for membrane proteins.
The first suggestive evidence for a ciliary diffusion barrier came from studies of GPI-anchored GFP, which could diffuse freely in the apical membrane of MDCK cells, but was excluded from a zone of membrane around the cilium in fixed cells. However, in live cells, GPI-GFP freely diffused into the cilium, implying that there was no barrier on the extracellular leaflet and suggesting that the exclusion observed earlier may be an artifact of fixation (Francis et al., 2011).
More compelling evidence for a diffusion barrier for membrane proteins came from studies by Hu et al. (2010), who used FRAP to show that several membrane proteins were mobile within the cilium, but could neither exit nor enter the ciliary membrane. They also found that septins localized in a ring-like structure at the base of cilia, and that in the absence of septins the shortened cilia still contained specific membrane proteins, yet these were now able to freely diffuse across the base into the cilium (Hu et al., 2010). It is unclear, however, how these proteins remained enriched in the cilia of cells lacking septins, as they should have been able to diffuse away. Moreover, septins are not seen at the base of all cilia (for examples see Ghossoub et al., 2013; Fliegauf et al., 2014), which suggests that, if barriers exist, they must be selective and may involve other proteins.
Exclusion of certain membrane proteins from the cilium has also been cited as evidence for the existence of a diffusion barrier. Podocalyxin is one such protein. However, exclusion of podocalyxin from cilia depends on its retention in the apical membrane by interaction with the PDZ-domain–containing protein NEHRF1, which is in turn anchored to the membrane and the actin matrix via ERM domain–containing proteins. When its anchorage to the apical membrane is released by deletion of the ERM domain, podocalyxin diffuses freely into the cilium (Francis et al., 2011), disproving the notion that a diffusional barrier prevents the entry of membrane proteins into the cilium.
It thus appears that, despite the demonstrable segregation of proteins between the cilium and the surrounding (bulk) membrane, the evidence for a diffusional barrier at the base of the cilium is scant. Instead, membrane protein distribution in cilia may be influenced by lipid order and partitioning. Laurdan staining revealed a highly condensed bilayer domain at the base of the cilium (Vieira et al., 2006), which implies lipid ordered structures. The cilia protein fibrocystin has a carboxy-terminal targeting motif that is sufficient to target GFP to cilia, but in nonciliated cells is associated with rafts (Follit et al., 2010). GM1, GM3, and the cholesterol-binding protein prominin, which localize to lipid rafts, also all overlap within the cilia (Janich and Corbeil, 2007). Alternatively, and perhaps more importantly, proteins that accumulate in the cilium as a result of active transport by microtubule-associated motors may be selectively removed when they reach the transition zone by active endocytosis. Endocytic recycling is critically important for ciliogenesis (Kim et al., 2010), and the preciliary membrane at the base of the cilium is enriched in clathrin-coated pits (Molla-Herman et al., 2010). These observations suggest that, as in yeast, coupling of targeted exocytosis, in tandem with active transport mechanisms and focal endocytosis, could contribute significantly to retention of specific membrane proteins in cilia.
Cytokinesis
During cell division a contractile actomyosin ring forms in anaphase to divide the cytoplasm of the two daughter cells. In late telophase, the cleavage furrow formed by this structure is constricted tightly around the spindle microtubules, and the cell remains in this state, connected by a thin midbody bridge, until abscission (breakage of the bridge) occurs. By performing FRAP on cells expressing GFP-tagged membrane proteins, Schmidt and Nichols (2004) found that while soluble GFP could move from one cell to another, membrane proteins were restricted, unable to move between cells. The passage of transmembrane proteins and proteins anchored in the intracellular leaflet was impeded, whereas a GPI-anchored GFP protein and DiI lipids were free to diffuse. The molecular basis for this barrier is not currently known, and although the septins were speculatively implicated (Schmidt and Nichols, 2004; Caudron and Barral, 2009), other protein filamentous structures, such as the ESCRT complex, are also localized to this bridge and tightly associated with the membrane (Guizetti et al., 2011). In addition, lipidomics have revealed that a unique subset of lipids are concentrated at the midbody, including long-chain ceramides and triglycerides (Atilla-Gokcumen et al., 2014), which may alter the phase properties of the membrane at this site. Future studies will be needed to identify the molecular basis of the barrier that separates membrane components during cytokinesis.
Concluding remarks
In closing, we would like to emphasize three concepts that we regard as important. First, while membranes are unquestionably segregated into subcompartments, detection of persistent inhomogeneity (e.g., by visualizing the distribution of membrane components at steady-state) is an insufficient criterion to establish the presence of diffusional barriers. Other mechanisms can yield an equivalent outcome. In fact, diffusional limitations are more likely to aid in the preservation of dynamic gradients established by other means. Second, when present, diffusion barriers need not be permanent. On the contrary, metastable barriers that undergo continuous remodeling are likely the norm. Even long-lasting structures such as tight junctions and the AIS must undergo restructuring to allow for turnover of their components or to allow for the passage of axonal cargo, respectively. Detection of short-lived transitions in the tightness of a diffusional barrier requires comparably fast acquisition of information regarding the mobility of the diffusing components. Lastly, it is worth remembering that diffusion of proteins or lipids in the plane of the membrane can be limited not only by direct collisions with structures where immobile proteins are crowded, but also by electrostatic deflection, hydrophobic mismatches, and other mechanisms. We hope that bearing these considerations in mind will aid future studies of this fascinating topic.
Acknowledgments
Research in the authors’ laboratory is supported by the Canadian Institutes for Health Research grants MOP7075 and MOP4665 to S. Grinstein, MOP123405 and MOP133660 to W.S. Trimble, and MOP102474 and MOP126069 to S. Grinstein and W.S. Trimble.
The authors declare no competing financial interests.
Footnotes
Abbreviations used it this paper:
- AIS
- axon initial segment
- GPI
- glycosylphosphatidylinositol
- PtdIns(45)P2
- phosphatidylinositol 4,5-bisphosphate
References
- Atilla-Gokcumen G.E., Muro E., Relat-Goberna J., Sasse S., Bedigian A., Coughlin M.L., Garcia-Manyes S., and Eggert U.S.. 2014. Dividing cells regulate their lipid composition and localization. Cell. 156:428–439 10.1016/j.cell.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines A.J.2010. The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma. 244:99–131 10.1007/s00709-010-0181-1 [DOI] [PubMed] [Google Scholar]
- Balda M.S., Whitney J.A., Flores C., González S., Cereijido M., and Matter K.. 1996. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134:1031–1049 10.1083/jcb.134.4.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J.R.1995. The spermatid plasma membrane comes of age. Trends Cell Biol. 5:400–404 10.1016/S0962-8924(00)89089-3 [DOI] [PubMed] [Google Scholar]
- Batista F.D., and Dustin M.L.. 2013. Cell:cell interactions in the immune system. Immunol. Rev. 251:7–12 10.1111/imr.12025 [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M., Hildebrandt F., Pellman D., Woods G., and Godinho S.A.. 2011. Centrosomes and cilia in human disease. Trends Genet. 27:307–315 10.1016/j.tig.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J., and Antonny B.. 2012. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev. Cell. 23:886–895 10.1016/j.devcel.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Brachet A., Leterrier C., Irondelle M., Fache M.P., Racine V., Sibarita J.B., Choquet D., and Dargent B.. 2010. Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier. J. Cell Biol. 191:383–395 10.1083/jcb.201003042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckbauer A., James P., Zhou D., Yoon J.W., Excell D., Korchev Y., Jones R., and Klenerman D.. 2007. Nanopipette delivery of individual molecules to cellular compartments for single-molecule fluorescence tracking. Biophys. J. 93:3120–3131 10.1529/biophysj.107.104737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco S., and Meyer T.. 2011. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu. Rev. Biochem. 80:973–1000 10.1146/annurev-biochem-061609-165311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron F., and Barral Y.. 2009. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell. 16:493–506 10.1016/j.devcel.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Chen Y., Lagerholm B.C., Yang B., and Jacobson K.. 2006. Methods to measure the lateral diffusion of membrane lipids and proteins. Methods. 39:147–153 10.1016/j.ymeth.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Chih B., Liu P., Chinn Y., Chalouni C., Komuves L.G., Hass P.E., Sandoval W., and Peterson A.S.. 2012. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 14:61–72 10.1038/ncb2410 [DOI] [PubMed] [Google Scholar]
- Christova Y., James P., Mackie A., Cooper T.G., and Jones R.. 2004. Molecular diffusion in sperm plasma membranes during epididymal maturation. Mol. Cell. Endocrinol. 216:41–46 10.1016/j.mce.2003.10.075 [DOI] [PubMed] [Google Scholar]
- Clay L., Caudron F., Denoth-Lippuner A., Boettcher B., Buvelot Frei S., Snapp E.L., and Barral Y.. 2014. A sphingolipid-dependent diffusion barrier confines ER stress to the yeast mother cell. eLife. 3:e01883 10.7554/eLife.01883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., Baryshnikova A., Bellay J., Kim Y., Spear E.D., Sevier C.S., Ding H., Koh J.L., Toufighi K., Mostafavi S., et al. . 2010. The genetic landscape of a cell. Science. 327:425–431 10.1126/science.1180823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A.E., Nakhimovsky L., Myles D.G., and Koppel D.E.. 1997. Barriers to diffusion of plasma membrane proteins form early during guinea pig spermiogenesis. Biophys. J. 73:507–516 10.1016/S0006-3495(97)78089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leeuw F.E., Chen H.C., Colenbrander B., and Verkleij A.J.. 1990. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology. 27:171–183 10.1016/0011-2240(90)90009-S [DOI] [PubMed] [Google Scholar]
- Domanov Y.A., Aimon S., Toombes G.E., Renner M., Quemeneur F., Triller A., Turner M.S., and Bassereau P.. 2011. Mobility in geometrically confined membranes. Proc. Natl. Acad. Sci. USA. 108:12605–12610 10.1073/pnas.1102646108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C.G., and Simons K.. 1990. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 62:63–72 10.1016/0092-8674(90)90240-F [DOI] [PubMed] [Google Scholar]
- Dragsten P.R., Blumenthal R., and Handler J.S.. 1981. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane? Nature. 294:718–722 10.1038/294718a0 [DOI] [PubMed] [Google Scholar]
- Fliegauf M., Kahle A., Häffner K., and Zieger B.. 2014. Distinct localization of septin proteins to ciliary sub-compartments in airway epithelial cells. Biol. Chem. 395:151–156 10.1515/hsz-2013-0252 [DOI] [PubMed] [Google Scholar]
- Follit J.A., Li L., Vucica Y., and Pazour G.J.. 2010. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J. Cell Biol. 188:21–28 10.1083/jcb.200910096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S.S., Sfakianos J., Lo B., and Mellman I.. 2011. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J. Cell Biol. 193:219–233 10.1083/jcb.201009001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Ritchie K., Murakoshi H., Jacobson K., and Kusumi A.. 2002. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 157:1071–1082 10.1083/jcb.200202050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M.2010. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2:a002907 10.1101/cshperspect.a002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambin Y., Lopez-Esparza R., Reffay M., Sierecki E., Gov N.S., Genest M., Hodges R.S., and Urbach W.. 2006. Lateral mobility of proteins in liquid membranes revisited. Proc. Natl. Acad. Sci. USA. 103:2098–2102 10.1073/pnas.0511026103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo F.R., Corbit K.C., Sirerol-Piquer M.S., Ramaswami G., Otto E.A., Noriega T.R., Seol A.D., Robinson J.F., Bennett C.L., Josifova D.J., et al. . 2011. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43:776–784 10.1038/ng.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido J.J., Giraud P., Carlier E., Fernandes F., Moussif A., Fache M.P., Debanne D., and Dargent B.. 2003. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 300:2091–2094 10.1126/science.1085167 [DOI] [PubMed] [Google Scholar]
- Gasser A., Ho T.S., Cheng X., Chang K.J., Waxman S.G., Rasband M.N., and Dib-Hajj S.D.. 2012. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. J. Neurosci. 32:7232–7243 10.1523/JNEUROSCI.5434-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghossoub R., Hu Q., Failler M., Rouyez M.C., Spitzbarth B., Mostowy S., Wolfrum U., Saunier S., Cossart P., Jamesnelson W., and Benmerah A.. 2013. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J. Cell Sci. 126:2583–2594 10.1242/jcs.111377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula N.B., and Satir P.. 1972. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 53:494–509 10.1083/jcb.53.2.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewska U., Kay J.G., Masters T., Grinstein S., Im W., Pastor R.W., Scarlata S., and McLaughlin S.. 2011. Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol. Biol. Cell. 22:3498–3507 10.1091/mbc.E11-02-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami D., Gowrishankar K., Bilgrami S., Ghosh S., Raghupathy R., Chadda R., Vishwakarma R., Rao M., and Mayor S.. 2008. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 135:1085–1097 10.1016/j.cell.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar K., Ghosh S., Saha S., C R., Mayor S., and Rao M.. 2012. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell. 149:1353–1367 10.1016/j.cell.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Grebenkämper K., and Galla H.J.. 1994. Translational diffusion measurements of a fluorescent phospholipid between MDCK-I cells support the lipid model of the tight junctions. Chem. Phys. Lipids. 71:133–143 10.1016/0009-3084(94)90066-3 [DOI] [PubMed] [Google Scholar]
- Guizetti J., Schermelleh L., Mäntler J., Maar S., Poser I., Leonhardt H., Müller-Reichert T., and Gerlich D.W.. 2011. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 331:1616–1620 10.1126/science.1201847 [DOI] [PubMed] [Google Scholar]
- Gulino-Debrac D.2013. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers. 1:e24180 10.4161/tisb.24180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom K.L., Ogawa Y., and Rasband M.N.. 2008. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 183:635–640 10.1083/jcb.200806112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönscher C., and Ungermann C.. 2014. A close-up view of membrane contact sites between the endoplasmic reticulum and the endolysosomal system: from yeast to man. Crit. Rev. Biochem. Mol. Biol. 49:262–268 10.3109/10409238.2013.875512 [DOI] [PubMed] [Google Scholar]
- Hu Q., Milenkovic L., Jin H., Scott M.P., Nachury M.V., Spiliotis E.T., and Nelson W.J.. 2010. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 329:436–439 10.1126/science.1191054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M., Kinoshita A., Yamada S., Tanaka H., Tanigaki A., Kitano A., Goto M., Okubo K., Nishiyama H., Ogawa O., et al. . 2005. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell. 8:343–352 10.1016/j.devcel.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Iino R., Koyama I., and Kusumi A.. 2001. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys. J. 80:2667–2677 10.1016/S0006-3495(01)76236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janich P., and Corbeil D.. 2007. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 581:1783–1787 10.1016/j.febslet.2007.03.065 [DOI] [PubMed] [Google Scholar]
- Kachar B., and Reese T.S.. 1982. Evidence for the lipidic nature of tight junction strands. Nature. 296:464–466 10.1038/296464a0 [DOI] [PubMed] [Google Scholar]
- Kim J., Lee J.E., Heynen-Genel S., Suyama E., Ono K., Lee K., Ideker T., Aza-Blanc P., and Gleeson J.G.. 2010. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 464:1048–1051 10.1038/nature08895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel H., Georgescu M.M., Larisch S., Manova K., Hunnicutt G.R., and Steller H.. 2005. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell. 8:353–364 10.1016/j.devcel.2005.01.021 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Storrie B., Simons K., and Dotti C.G.. 1992. A functional barrier to movement of lipids in polarized neurons. Nature. 359:647–650 10.1038/359647a0 [DOI] [PubMed] [Google Scholar]
- Kusumi A., Nakada C., Ritchie K., Murase K., Suzuki K., Murakoshi H., Kasai R.S., Kondo J., and Fujiwara T.. 2005. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 34:351–378 10.1146/annurev.biophys.34.040204.144637 [DOI] [PubMed] [Google Scholar]
- Kusumi A., Suzuki K.G., Kasai R.S., Ritchie K., and Fujiwara T.K.. 2011. Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem. Sci. 36:604–615 10.1016/j.tibs.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Kusumi A., Fujiwara T.K., Morone N., Yoshida K.J., Chadda R., Xie M., Kasai R.S., and Suzuki K.G.. 2012. Membrane mechanisms for signal transduction: the coupling of the meso-scale raft domains to membrane-skeleton-induced compartments and dynamic protein complexes. Semin. Cell Dev. Biol. 23:126–144 10.1016/j.semcdb.2012.01.018 [DOI] [PubMed] [Google Scholar]
- Kusumi A., Tsunoyama T.A., Hirosawa K.M., Kasai R.S., and Fujiwara T.K.. 2014. Tracking single molecules at work in living cells. Nat. Chem. Biol. 10:524–532 10.1038/nchembio.1558 [DOI] [PubMed] [Google Scholar]
- Kwitny S., Klaus A.V., and Hunnicutt G.R.. 2010. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol. Reprod. 82:669–678 10.1095/biolreprod.109.079566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffafian I., and Hallett M.B.. 2007. Lipid-protein cargo transfer: a mode of direct cell-to-cell communication for lipids and their associated proteins. J. Cell. Physiol. 210:336–342 10.1002/jcp.20851 [DOI] [PubMed] [Google Scholar]
- Lee D.B., Jamgotchian N., Allen S.G., Abeles M.B., and Ward H.J.. 2008. A lipid-protein hybrid model for tight junction. Am. J. Physiol. Renal Physiol. 295:F1601–F1612 10.1152/ajprenal.00097.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M.A.2008. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 9:99–111 10.1038/nrm2328 [DOI] [PubMed] [Google Scholar]
- Lingwood D., and Simons K.. 2010. Lipid rafts as a membrane-organizing principle. Science. 327:46–50 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- Ludin B., and Matus A.. 1993. The neuronal cytoskeleton and its role in axonal and dendritic plasticity. Hippocampus. 3:61–71. [PubMed] [Google Scholar]
- Luedeke C., Frei S.B., Sbalzarini I., Schwarz H., Spang A., and Barral Y.. 2005. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J. Cell Biol. 169:897–908 10.1083/jcb.200412143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manford A.G., Stefan C.J., Yuan H.L., Macgurn J.A., and Emr S.D.. 2012. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev. Cell. 23:1129–1140 10.1016/j.devcel.2012.11.004 [DOI] [PubMed] [Google Scholar]
- McLaughlin S., and Murray D.. 2005. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 438:605–611 10.1038/nature04398 [DOI] [PubMed] [Google Scholar]
- Molla-Herman A., Ghossoub R., Blisnick T., Meunier A., Serres C., Silbermann F., Emmerson C., Romeo K., Bourdoncle P., Schmitt A., et al. . 2010. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 123:1785–1795 10.1242/jcs.059519 [DOI] [PubMed] [Google Scholar]
- Nakada C., Ritchie K., Oba Y., Nakamura M., Hotta Y., Iino R., Kasai R.S., Yamaguchi K., Fujiwara T., and Kusumi A.. 2003. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 5:626–632 10.1038/ncb1009 [DOI] [PubMed] [Google Scholar]
- Nicolson G.L.2014. The Fluid-Mosaic Model of Membrane Structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta. 1838:1451–1466 10.1016/j.bbamem.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Paszek M.J., DuFort C.C., Rossier O., Bainer R., Mouw J.K., Godula K., Hudak J.E., Lakins J.N., Wijekoon A.C., Cassereau L., et al. . 2014. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 511:319–325 10.1038/nature13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T.2004. Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell. 116:191–203 10.1016/S0092-8674(03)01077-8 [DOI] [PubMed] [Google Scholar]
- Phelps B.M., Primakoff P., Koppel D.E., Low M.G., and Myles D.G.. 1988. Restricted lateral diffusion of PH-20, a PI-anchored sperm membrane protein. Science. 240:1780–1782 10.1126/science.3381102 [DOI] [PubMed] [Google Scholar]
- Pollard T.D., Blanchoin L., and Mullins R.D.. 2000. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29:545–576 10.1146/annurev.biophys.29.1.545 [DOI] [PubMed] [Google Scholar]
- Qin H., Diener D.R., Geimer S., Cole D.G., and Rosenbaum J.L.. 2004. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164:255–266 10.1083/jcb.200308132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadurai S., Holt A., Krasnikov V., van den Bogaart G., Killian J.A., and Poolman B.. 2009. Lateral diffusion of membrane proteins. J. Am. Chem. Soc. 131:12650–12656 10.1021/ja902853g [DOI] [PubMed] [Google Scholar]
- Rowland A.A., and Voeltz G.K.. 2012. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 13:607–625 10.1038/nrm3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman P.G., and Delbrück M.. 1975. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. USA. 72:3111–3113 10.1073/pnas.72.8.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K., and Nichols B.J.. 2004. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr. Biol. 14:1002–1006 10.1016/j.cub.2004.05.044 [DOI] [PubMed] [Google Scholar]
- Selvaraj V., Asano A., Buttke D.E., McElwee J.L., Nelson J.L., Wolff C.A., Merdiushev T., Fornés M.W., Cohen A.W., Lisanti M.P., et al. . 2006. Segregation of micron-scale membrane sub-domains in live murine sperm. J. Cell. Physiol. 206:636–646 10.1002/jcp.20504 [DOI] [PubMed] [Google Scholar]
- Selvaraj V., Asano A., Buttke D.E., Sengupta P., Weiss R.S., and Travis A.J.. 2009. Mechanisms underlying the micron-scale segregation of sterols and GM1 in live mammalian sperm. J. Cell. Physiol. 218:522–536 10.1002/jcp.21624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M.A., Kaech S., Jareb M., Burack M.A., Vogt L., Sonderegger P., and Banker G.. 2001. Sorting and directed transport of membrane proteins during development of hippocampal neurons in culture. Proc. Natl. Acad. Sci. USA. 98:7051–7057 10.1073/pnas.111146198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., and Fuller S.D.. 1985. Cell surface polarity in epithelia. Annu. Rev. Cell Biol. 1:243–288 10.1146/annurev.cb.01.110185.001331 [DOI] [PubMed] [Google Scholar]
- Singla V., and Reiter J.F.. 2006. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 313:629–633 10.1126/science.1124534 [DOI] [PubMed] [Google Scholar]
- Song A.H., Wang D., Chen G., Li Y., Luo J., Duan S., and Poo M.M.. 2009. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 136:1148–1160 10.1016/j.cell.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Stevenson B.R., and Goodenough D.A.. 1984. Zonulae occludentes in junctional complex-enriched fractions from mouse liver: preliminary morphological and biochemical characterization. J. Cell Biol. 98:1209–1221 10.1083/jcb.98.4.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops E.H., and Caplan M.J.. 2014. Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 25:1375–1386 10.1681/ASN.2013080883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K.G., Fujiwara T.K., Sanematsu F., Iino R., Edidin M., and Kusumi A.. 2007. GPI-anchored receptor clusters transiently recruit Lyn and Gα for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177:717–730 10.1083/jcb.200609174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa P.A., DeRisi J.L., Wilhelm J.E., and Vale R.D.. 2000. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 290:341–344 10.1126/science.290.5490.341 [DOI] [PubMed] [Google Scholar]
- Valdez-Taubas J., and Pelham H.R.. 2003. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr. Biol. 13:1636–1640 10.1016/j.cub.2003.09.001 [DOI] [PubMed] [Google Scholar]
- van den Bogaart G., Meyenberg K., Risselada H.J., Amin H., Willig K.I., Hubrich B.E., Dier M., Hell S.W., Grubmüller H., Diederichsen U., and Jahn R.. 2011. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 479:552–555 10.1038/nature10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., and Simons K.. 1988. Lipid polarity and sorting in epithelial cells. J. Cell. Biochem. 36:51–58 10.1002/jcb.240360106 [DOI] [PubMed] [Google Scholar]
- van Meer G., Gumbiner B., and Simons K.. 1986. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 322:639–641 10.1038/322639a0 [DOI] [PubMed] [Google Scholar]
- Vieira O.V., Gaus K., Verkade P., Fullekrug J., Vaz W.L., and Simons K.. 2006. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. USA. 103:18556–18561 10.1073/pnas.0608291103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S., Katiyar R., and Schmitz F.. 2013. A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons. J. Neurosci. 33:10278–10300 10.1523/JNEUROSCI.5048-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Arbuzova A., Hangyás-Mihályné G., and McLaughlin S.. 2001. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 276:5012–5019 10.1074/jbc.M008355200 [DOI] [PubMed] [Google Scholar]
- Winckler B., and Poo M.M.. 1996. No diffusion barrier at axon hillock. Nature. 379:213 10.1038/379213a0 [DOI] [PubMed] [Google Scholar]
- Winckler B., Forscher P., and Mellman I.. 1999. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 397:698–701 10.1038/17806 [DOI] [PubMed] [Google Scholar]
- Wolf W., Kilic A., Schrul B., Lorenz H., Schwappach B., and Seedorf M.. 2012. Yeast Ist2 recruits the endoplasmic reticulum to the plasma membrane and creates a ribosome-free membrane microcompartment. PLoS ONE. 7:e39703 10.1371/journal.pone.0039703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.K., Chao J.T., and Loewen C.J.. 2014. Barriers to uniformity within the endoplasmic reticulum. Curr. Opin. Cell Biol. 29:31–38 10.1016/j.ceb.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Ye F., Breslow D.K., Koslover E.F., Spakowitz A.J., Nelson W.J., and Nachury M.V.. 2013. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife. 2:e00654 10.7554/eLife.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kong C., Xie H., McPherson P.S., Grinstein S., and Trimble W.S.. 1999. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 9:1458–1467 10.1016/S0960-9822(00)80115-3 [DOI] [PubMed] [Google Scholar]