Abstract
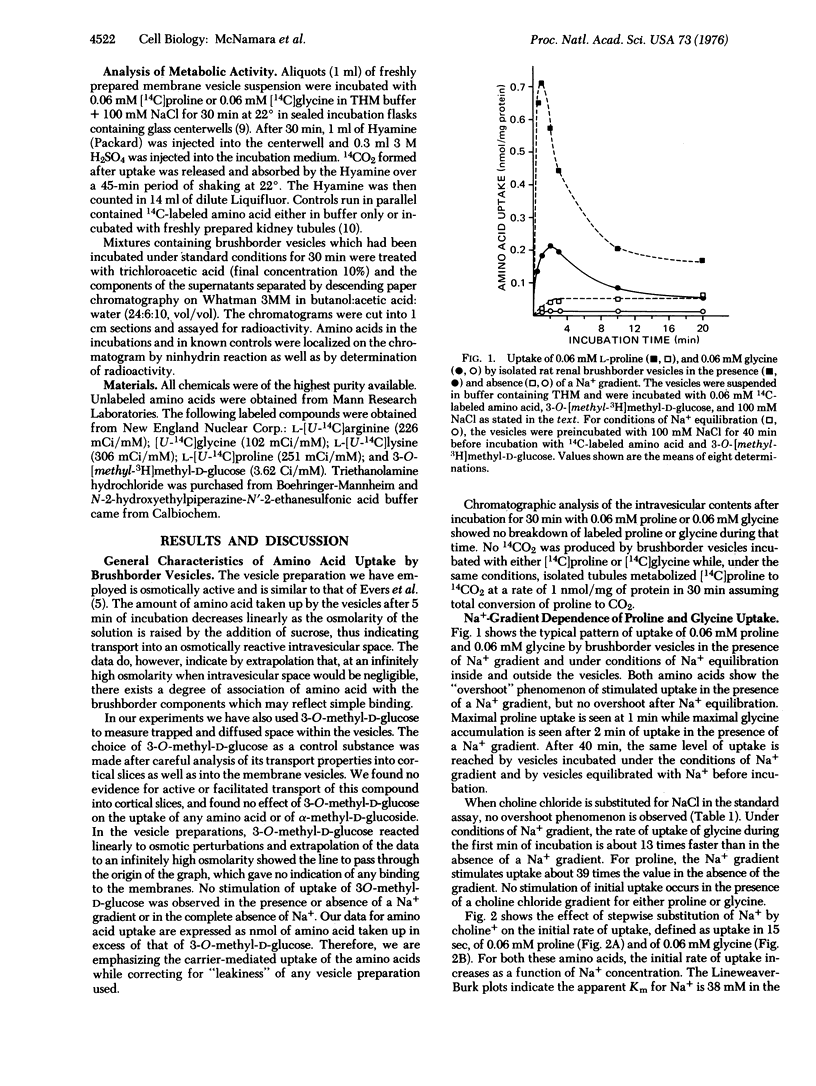
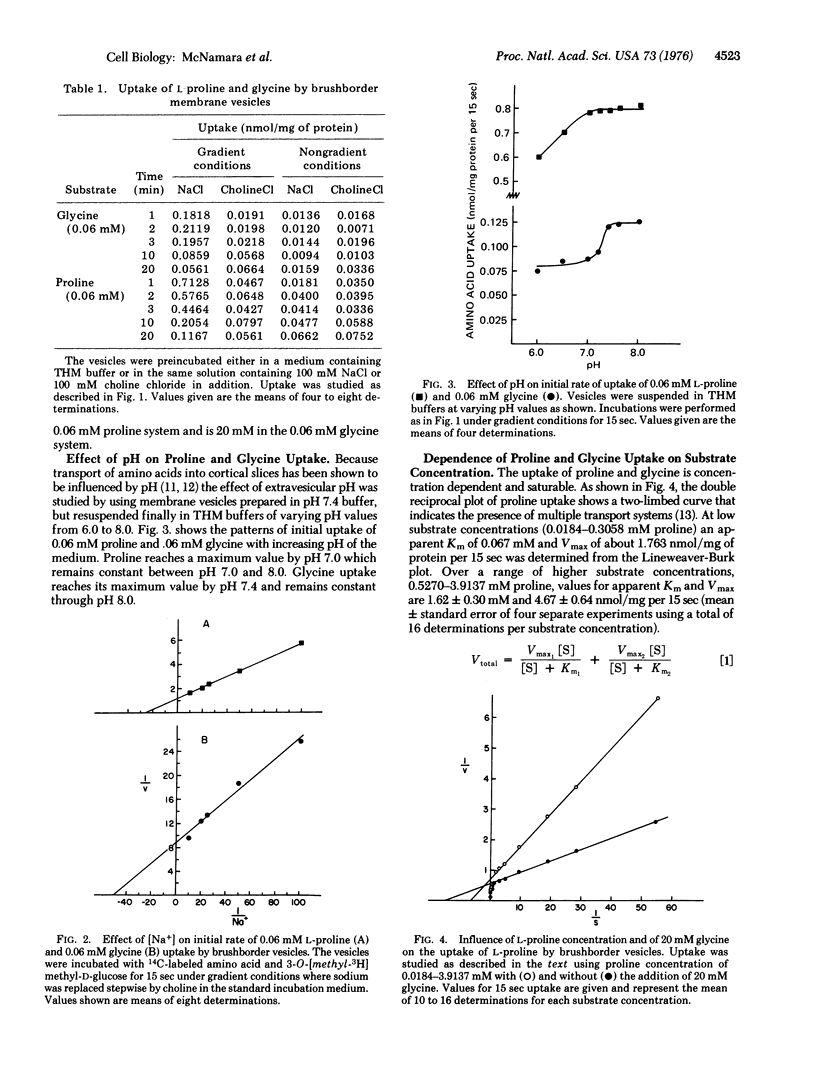
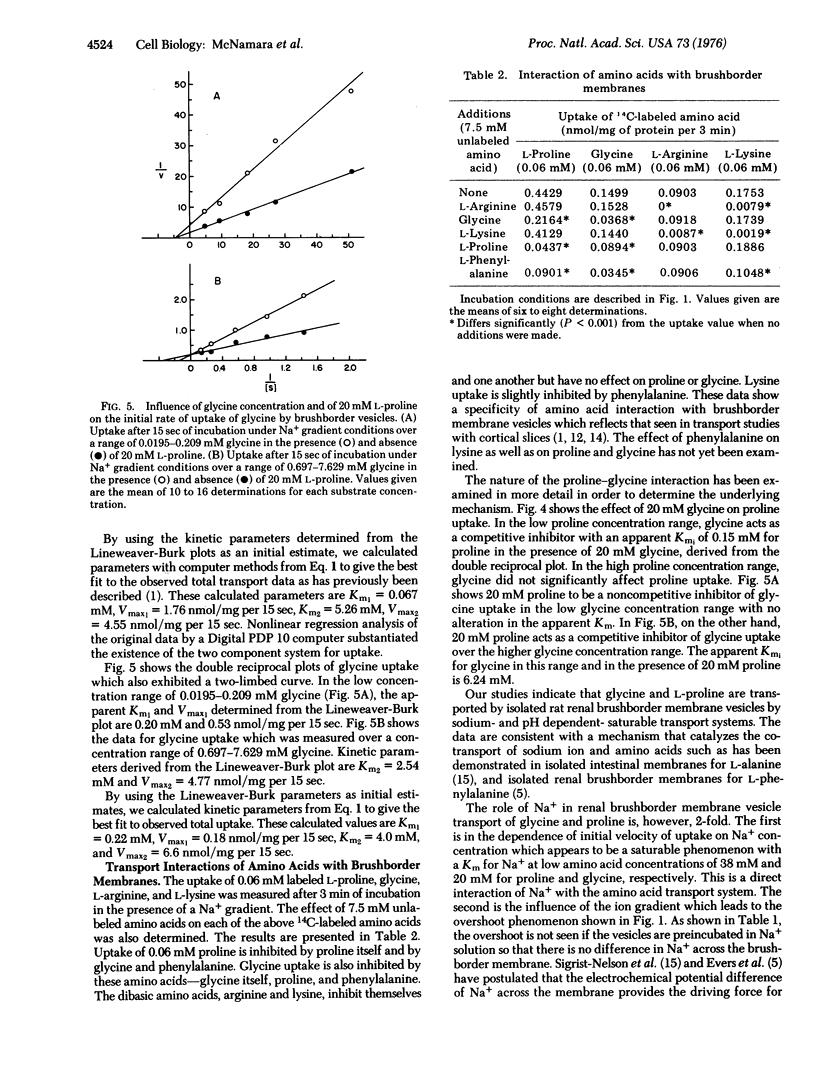
Uptake of L-proline and glycine by rat renal brushborder membrane vesicles was seen to be osmotically sensitive, pH dependent,and occurred in the absence of proline and glycine metabolism. The uptake system for proline was Na+ gradient dependent, and exhibited a dual system for entry, Km1 = 0.067 mM and Km2 = 5.26 mM. The uptake of glycine was also Na+ gradient dependent, and exhibited a two Km system, Km1 = 0.22 mM and Km2 = 4.00 mM. Studies of proline and glycine interactions indicate a shared site which has a lower affinity and higher capacity for glycine than for proline. The high affinity glycine site and low affinity proline site do not appear to be shared.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baerlocher K. E., Scriver C. R., Mohyuddin F. The ontogeny of amino acid transport in rat kidney. I. Effect on distribution ratios and intracellular metabolism of proline and glycine. Biochim Biophys Acta. 1971 Dec 3;249(2):353–363. doi: 10.1016/0005-2736(71)90114-3. [DOI] [PubMed] [Google Scholar]
- Evers J., Murer H., Kinne R. Phenylalanine uptake in isolated renal brush border vesicles. Biochim Biophys Acta. 1976 Apr 5;426(4):598–615. doi: 10.1016/0005-2736(76)90124-3. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Albrecht I., Rosenberg L. E. Identification and analysis of multiple glycine transport systems in isolated mammalian renal tubules. J Biol Chem. 1968 Nov 10;243(21):5566–5571. [PubMed] [Google Scholar]
- Hillman R. E., Rosenberg L. E. Amino acid transport by isolated mammalian renal tubules. II. Transport systems for L-proline. J Biol Chem. 1969 Aug 25;244(16):4494–4498. [PubMed] [Google Scholar]
- Holtzapple P., Genel M., Rea C., Segal S. Metabolism and uptake of l-proline by human kidney cortex. Pediatr Res. 1973 Oct;7(10):818–825. doi: 10.1203/00006450-197310000-00005. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lingard J., Rumrich G., Young J. A. Kinetics of L-histidine transport in the proximal convolution of the rat nephron studied using the stationary microperfusion technique. Pflugers Arch. 1973 Jul 25;342(1):13–28. doi: 10.1007/BF00593247. [DOI] [PubMed] [Google Scholar]
- Mohyuddin F., Scriver C. R. Amino acid transport in mammalian kidney: Multiple systems for imino acids and glycine in rat kidney. Am J Physiol. 1970 Jul;219(1):1–8. doi: 10.1152/ajplegacy.1970.219.1.1. [DOI] [PubMed] [Google Scholar]
- Pockrandt-Hemstedt H., Schmitz J. E., Kinne-Saffran E., Kinne R. Morphologische und biochemische Untersuchungen über die Oberflächenstruktur der Bürstensaummenbran der Rattenniere. Pflugers Arch. 1972;333(4):297–313. doi: 10.1007/BF00586210. [DOI] [PubMed] [Google Scholar]
- Roth K. S., Hwang S. M., Segal S. Effect of maleic acid on the kinetics of alpha-methyl-D-glucoside uptake by isolated rat renal tubules. Biochim Biophys Acta. 1976 Apr 5;426(4):675–687. doi: 10.1016/0005-2736(76)90132-2. [DOI] [PubMed] [Google Scholar]
- Scriver C. R. Renal tubular transport of proline, hydroxyproline, and glycine. 3. Genetic basis for more than one mode of transport in human kidney. J Clin Invest. 1968 Apr;47(4):823–835. doi: 10.1172/JCI105776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Rea C., Smith I. Separate transport systems for sugars and amino acids in developing rat kidney cortex. Proc Natl Acad Sci U S A. 1971 Feb;68(2):372–376. doi: 10.1073/pnas.68.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Schwartzman L., Blair A., Bertoli D. Dibasic amino acid transport in rat-kidney cortex slices. Biochim Biophys Acta. 1967 Feb 1;135(1):127–135. doi: 10.1016/0005-2736(67)90015-6. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Murer H., Hopfer U. Active alanine transport in isolated brush border membranes. J Biol Chem. 1975 Jul 25;250(14):5674–5680. [PubMed] [Google Scholar]
- Weinstein A. N., Segal S. The metabolic fate of [I-14C]galactitol in mammalian tissue. Biochim Biophys Acta. 1968 Feb 1;156(1):9–16. doi: 10.1016/0304-4165(68)90098-6. [DOI] [PubMed] [Google Scholar]
- Wilson O. H., Scriver C. R. Specificity of transport of neutral and basic amino acids in rat kidney. Am J Physiol. 1967 Jul;213(1):185–190. doi: 10.1152/ajplegacy.1967.213.1.185. [DOI] [PubMed] [Google Scholar]


