Abstract
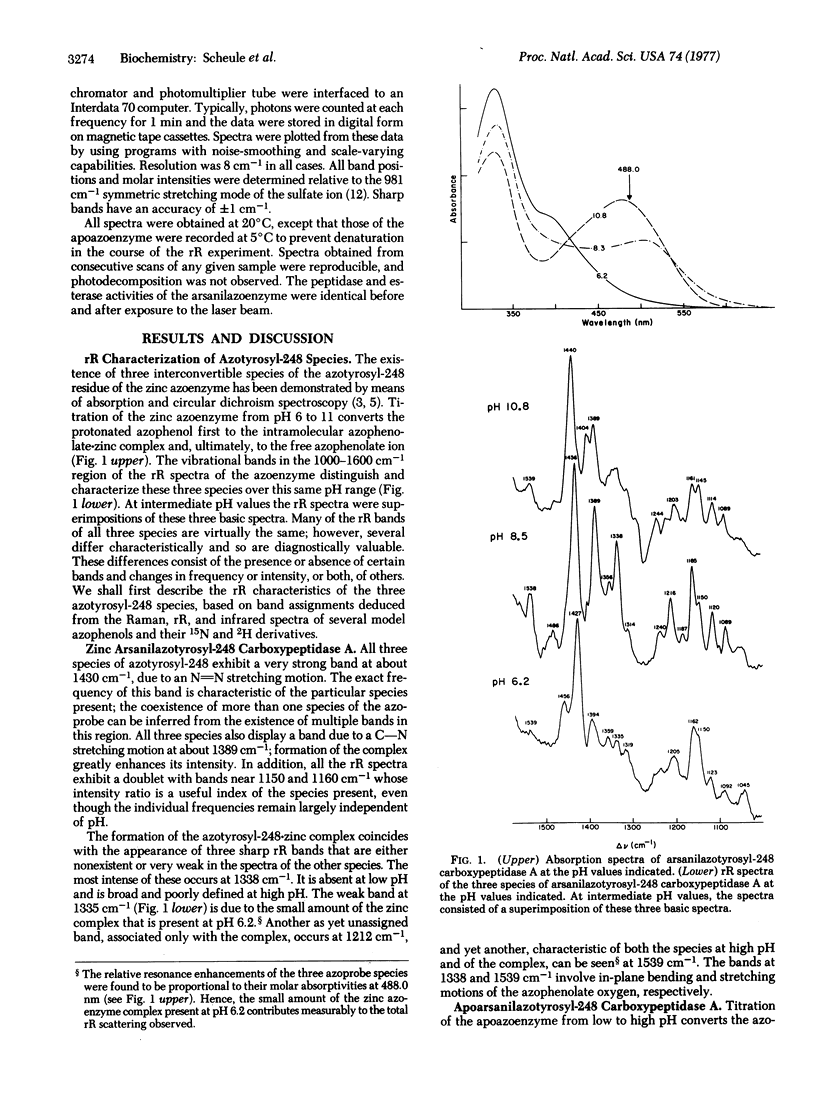
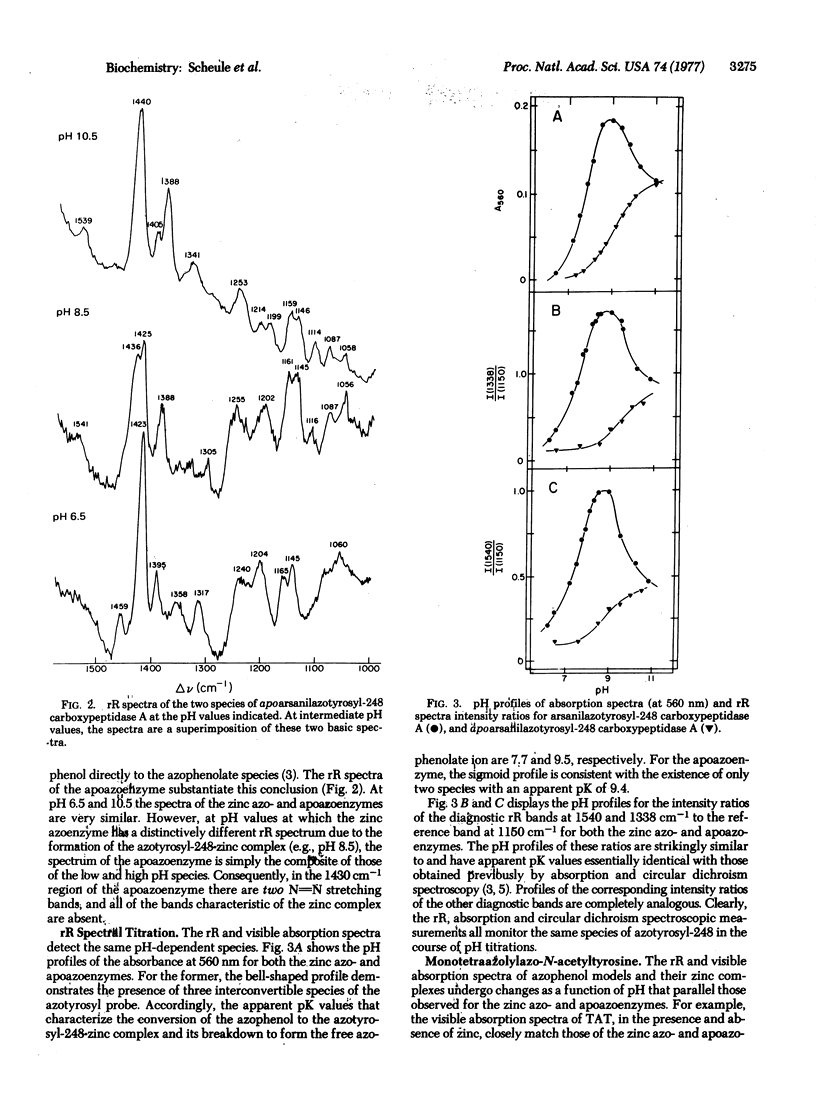
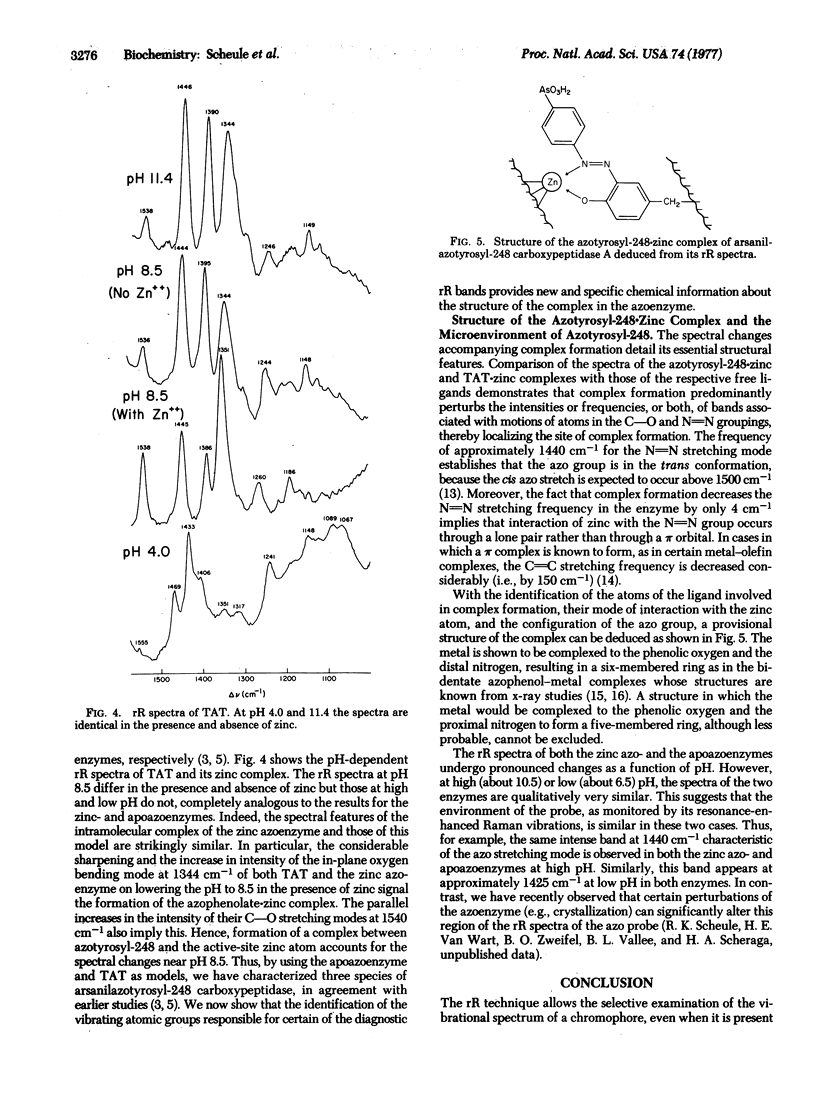
Resonance Raman spectra of arsanilazotryosyl-248 carboxypeptidase A (peptidyl-L-amino-acid hydrolase, EC 3.4.12.2) exhibit only the vibrational bands of its chromophoric azotyrosyl-248 residue uncomplicated by background interference from either water or other components of the protein. The resonance Raman spectra contain multiple, discrete bands which change as a function of pH, thereby demonstrating the existence of interconvertible species of the azotyrosine probe in solution. Spectra of model azophenols and of the apoazoenzyme establish the identity of these species. All conclusions about the azoenzyme based on the resonance Raman spectra, including the apparent pK values for the interconversion of these species, are in complete agreement with those drawn earlier from studies by absorption spectroscopy. In addition, the properties of resonance Raman bands that have been identified with the motions of specific atoms of azotyrosyl-248 provide details of the interactions of specific atoms of this chromophore with the catalytic zinc atom at the active site. In particular, this has allowed elucidation of the structure of the azotyrosyl-248-zinc coordination complex. Such experiments are also providing information on the effects of crystallization on the enzyme and on its interaction with inhibitors. The important potential of resonance Raman spectroscopy for the study of the structure of chromophoric components of active enzymatic sites and of metal complex ions is discussed.
Keywords: active site, conformation, intramolecular coordination
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. II. Inhibitors of the hydrolysis of oligopeptides. Biochemistry. 1970 Feb 3;9(3):602–609. doi: 10.1021/bi00805a022. [DOI] [PubMed] [Google Scholar]
- COX D. J., BOVARD F. C., BARGETZI J. P., WALSH K. A., NEURATH H. PROCEDURES FOR THE ISOLATION OF CRYSTALLINE BOVINE PANCREATIC CARBOXYPEPTIDASE A. II. ISOLATION OF CARBOXYPEPTIDASE A-ALPHA FROM PROCARBOXYPEPTIDASE A. Biochemistry. 1964 Jan;3:44–47. doi: 10.1021/bi00889a008. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Klyosov A. A., Vallee B. L. Circular dichroism-inhibitor titrations of arsanilazotyrosine-248 carboxypeptidase A. Biochemistry. 1976 Jan 27;15(2):296–303. doi: 10.1021/bi00647a009. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Livingston D. M., Vallee B. L. Chemical modification of carboxypeptidase A crystals. Azo coupling with tyrosine-248. Biochemistry. 1972 Jul 4;11(14):2584–2588. doi: 10.1021/bi00764a005. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Conformations of arsanilazotyrosine-248 carboxypeptidase A alpha, beta, gamma, comparison of crystals and solution. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2006–2010. doi: 10.1073/pnas.70.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Differences between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2532–2535. doi: 10.1073/pnas.68.10.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Environment and conformation dependent sensitivity of the arsanilazotyrosine-248 carboxypeptidase A chromophore. Biochemistry. 1975 Feb 25;14(4):649–660. doi: 10.1021/bi00675a001. [DOI] [PubMed] [Google Scholar]
- Peterson L. M., Sokolovsky M., Vallee B. L. Purification and crystallization of human carboxypeptidase A. Biochemistry. 1976 Jun 15;15(12):2501–2508. doi: 10.1021/bi00657a001. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Vallee B. L. The reaction of diazonium-1H-tetrazole with proteins. Determination of tyrosine and histidine content. Biochemistry. 1966 Nov;5(11):3574–3581. doi: 10.1021/bi00875a028. [DOI] [PubMed] [Google Scholar]


