Abstract
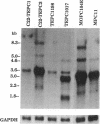
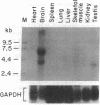
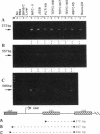
Plasmacytomagenesis provides a murine model to decipher progressive genetic events culminating in a B-cell neoplasia. Activation of the c-myc protooncogene by chromosomal translocation is considered an initiating event. Intracisternal A-type particles (IAPs) are defective retroviral-like structures present in the endoplasmic reticulum of plasmacytomas (PCTs). IAP proviral insertions have been documented to engender negative or positive effects on the expression of nearby cellular genes. We have isolated a gene, PANG (plasmacytoma-associated neuronal glycoprotein), that is ectopically transcribed in a number of PCTs due to IAP long terminal repeat (LTR) activation. A full-length PANG cDNA was isolated from an MPC-11 plasma cell tumor cDNA library and encodes a polypeptide of about 113 kDa with six immunoglobulin C2-like and four type III fibronectin-like domains. PANG bears a striking resemblance to axonal glycoproteins TAG-1 and F11 known to function in neuronal outgrowth. An extensive survey revealed a predominant 3.6-kb PANG transcript in 60% (30 of 50) of PCTs as well as unique smaller and larger species. All other normal and transformed lymphoid and nonlymphoid cell lines and normal tissues were negative for PANG expression except for the brain, wherein unique 4.0- and 6.1-kb transcripts were detected. Reverse transcriptase PCR analysis revealed IAP LTR fusion to PANG mRNAs in five PCTs and in a neuroblastoma line. The 5' end of a mouse brain PANG cDNA was identical to the MPC-11 PANG transcript except for the precise replacement of its 5' LTR sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arquint M., Roder J., Chia L. S., Down J., Wilkinson D., Bayley H., Braun P., Dunn R. Molecular cloning and primary structure of myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1987 Jan;84(2):600–604. doi: 10.1073/pnas.84.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Bieber A. J., Snow P. M., Hortsch M., Patel N. H., Jacobs J. R., Traquina Z. R., Schilling J., Goodman C. S. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989 Nov 3;59(3):447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Wolff J. M., Frank R., Rathjen F. G. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989 Apr;2(4):1351–1361. doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Chang-Yeh A., Mold D. E., Brilliant M. H., Huang R. C. The mouse intracisternal A particle-promoted placental gene retrotransposition is mouse-strain-specific. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):292–296. doi: 10.1073/pnas.90.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. B., Unger T., Rechavi G., Canaani E., Givol D. Rearrangement of the oncogene c-mos in mouse myeloma NSI and hybridomas. Nature. 1983 Dec 22;306(5945):797–799. doi: 10.1038/306797a0. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Intracisternal A-particle genes: structure of adjacent genes and mapping of the boundaries of the transcriptional unit. J Virol. 1982 Apr;42(1):123–130. doi: 10.1128/jvi.42.1.123-130.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M. A., Grady R. C., Mushinski J. F., Marcu K. B. PCS, a gene related to the immunoglobulin super family of axonal glycoproteins is expressed in murine plasma cell tumors. Curr Top Microbiol Immunol. 1992;182:229–236. doi: 10.1007/978-3-642-77633-5_28. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Furley A. J., Morton S. B., Manalo D., Karagogeos D., Dodd J., Jessell T. M. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990 Apr 6;61(1):157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Gennarini G., Cibelli G., Rougon G., Mattei M. G., Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989 Aug;109(2):775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann O., Heidmann T. Retrotransposition of a mouse IAP sequence tagged with an indicator gene. Cell. 1991 Jan 11;64(1):159–170. doi: 10.1016/0092-8674(91)90217-m. [DOI] [PubMed] [Google Scholar]
- Jessell T. M. Adhesion molecules and the hierarchy of neural development. Neuron. 1988 Mar;1(1):3–13. doi: 10.1016/0896-6273(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Julius M. A., Street A. J., Fahrlander P. D., Yang J. Q., Eisenman R. N., Marcu K. B. Translocated c-myc genes produce chimeric transcripts containing antisense sequences of the immunoglobulin heavy chain locus in mouse plasmacytomas. Oncogene. 1988 May;2(5):469–476. [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Moos M., Tacke R., Scherer H., Teplow D., Früh K., Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988 Aug 25;334(6184):701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Cole M. D. Differing populations of intracisternal A-particle genes in myeloma tumors and mouse subspecies. J Virol. 1982 May;42(2):411–421. doi: 10.1128/jvi.42.2.411-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Campbell H. D., Young I. G. Nucleotide sequence of the intracisternal A-particle genome inserted 5' to the interleukin-3 gene of the leukemia cell line WEHI-3B. Nucleic Acids Res. 1986 Jul 25;14(14):5901–5918. doi: 10.1093/nar/14.14.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]