Abstract
Background
The clinical presentation of sarcoidosis can be varied. Prior investigations have shown that diagnosis is often delayed over six months, particularly in patients with pulmonary symptoms. Delays may lead to high healthcare use prior to diagnosis.
Objective
To investigate healthcare use prior to diagnosis of sarcoidosis for a cohort of insured patients.
Methods
We conducted a case-control study using a de-identified limited dataset of private health insurance claims. Cases were identified as persons with sarcoidosis from 2003-2009. Controls with other respiratory-related diagnoses (asthma, chronic obstructive pulmonary disease, pneumonia) were matched by age, gender, and diagnosis date. We compared frequencies of doctor visits, prescriptions, and imaging in the year prior to established diagnosis.
Results
We identified 206 cases and 2060 controls and compared healthcare use patterns in the year prior to diagnosis. Among those receiving prescriptions, a larger proportion of cases received two or more antibiotic courses (69% vs. 55%, p=0.0020) or two or more corticosteroid prescriptions (63% vs. 50%, p=0.0137). On average, cases had more doctor visits (14.7 vs. 7.8, p<0.0001), saw more specialties (3.9 vs. 2.1, p<0.0001), and underwent more chest x-rays (2.0 vs. 1.5, p<0.0001). A larger proportion of cases underwent two or more chest x-rays (54% vs. 24%, p<0.0001).
Conclusions
Patients with sarcoidosis undergo a large amount of healthcare prior to diagnosis, some of which may not be necessary, compared to controls with respiratory-related disease. These results highlight the need for improved diagnostic algorithms to identify patients with sarcoidosis and avoid potentially excessive delays in diagnosis.
Keywords: Sarcoidosis, Healthcare Utilization, Prescriptions
Introduction
Sarcoidosis is a multi-system disease of unclear etiology affecting the lungs in over 90% of cases.(1) Sarcoidosis has been referred to as the ‘Great Pretender’ in the radiology literature, as CT patterns can mimic a large number of other pulmonary diseases.(2) The clinical presentation of sarcoidosis is also varied.(1) Patients present with vague, nonspecific symptoms such as fatigue, shortness of breath, fevers, or cough, and the diagnosis may initially be mistaken for other pulmonary diseases.(3)
Prior work has shown that the diagnosis of sarcoidosis is often delayed over six months in patients presenting with respiratory symptoms and decreased lung function.(3) Recent data also suggest that mortality and hospitalizations in people with this disease appear to be increasing over time.(4, 5) Sarcoidosis patients on higher doses of corticosteroids also seek more medical care related to infection, and have increased emergency department visits.(6) Thus, the diagnostic costs and resource utilization may be becoming increasingly significant.
Although there are reports of increased healthcare use of patients already diagnosed with sarcoidosis, few reports focus on the use of resources prior to diagnosis. The purpose of this study was to describe healthcare utilization patterns one year prior to diagnosis of sarcoidosis for a cohort of insured patients and compare their healthcare use to a cohort of patients with other respiratory diagnoses.
Methods
Dataset
We conducted a retrospective case-control study using 7 years (2003-2009) of a de-identified, limited dataset of employer-based health insurance claims data, housed at the University of Iowa College of Public Health, to identify persons with sarcoidosis. The Data Repository contains longitudinal data of claims for members and their covered family members who are fully insured through policies underwritten by the insurer. This study was approved by the University of Iowa Institutional Review Board.
We examined insurance claims for outpatient and emergency department visits and outpatient pharmacy healthcare services provided to members with health and prescription drug coverage. These data included enrolment information, insurance coverage, provider information, patient demographic information, diagnosis codes, procedure codes, dates of service, and outpatient pharmacy data, including fill dates and drug-days supplied.
Identification of Sarcoidosis Cases
We identified cases as persons with a primary or secondary diagnosis of ICD-9 code 135 for ‘sarcoidosis’ listed on an outpatient insurance claim. Case subjects were required to have a minimum of 12 months of continuous health and pharmacy insurance coverage before their diagnosis, and six months of coverage after diagnosis. The diagnosis date was defined as the date on which the ICD-9 code for sarcoidosis first appeared on a claim, with no prior claim for sarcoidosis in the prior 12 months. Each case in our series also must have had a second claim for sarcoidosis in the six months after index date to further validate the diagnosis.
Identification of Control Group
The control group was selected from the same outpatient claims database. Control subjects were selected based on having another respiratory-related diagnosis (other than sarcoidosis) including chronic obstructive pulmonary disease (ICD-9 codes 490.xx, 491.xx, 492.xx, and 496.xx), asthma (ICD-9 code 493.xx), or pneumonia (ICD-9 codes 480.xx, 481.xx, 482.xx, 483.xx, 485.xx, and 486.xx). Control subjects were required to have at least 12 months of insurance coverage prior to the diagnosis date and at least six months of coverage after the diagnosis date. Ten controls per case were matched by age strata (age below 30, 31-40 years, 41-50 years, 51-60 years, over 60 years), year of diagnosis, and gender. Similar to cases, the diagnosis date was defined as the date on which the ICD-9 code first appeared on the claims records, with no prior claim for the diagnosis within the prior 12 months.
Assessment of Antibiotic and Corticosteroid Prescriptions
We examined outpatient prescription use of antimicrobials and corticosteroids among sarcoidosis cases and matched controls in the 365 days before the date of established diagnosis. Prescription medications were identified through National Drug Codes on outpatient prescription drug claims. We examined exposure to antimicrobial classes or agents (aminoglycosides, beta-lactam/beta-lactamase inhibitors, cephalosporins, clindamycin, fluoroquinolones, macrolides, penicillins, sulfonamides, tetracyclines, and intravenous vancomycin), the total number of different antimicrobial agents received, and the timing of antimicrobial use in relation to the diagnosis date. We did not examine use of topical or ophthalmic antimicrobials. In a similar fashion, we examined all systemic corticosteroid prescriptions, including those for oral formulations, injections, or intravenous prescriptions. We did not include topical or ophthalmic steroid prescriptions.
Assessment of Imaging and Provider Visits
We investigated the frequency and timing of imaging exams by identifying medical claims for the test or procedure by current procedural terminology (CPT) codes. For chest x-rays, we used CPT codes 71010, 71015, 71020-71022, 71030, 71035. For computed tomography (CT) imaging, we used CPT codes 71250 (CT thorax without dye), 71260 (CT thorax with dye), 71270 (CT thorax without and with dye), and 71275 (CT angiography, chest). We also examined the frequency of provider visits by identifying the total number of provider visits, the number of different individual providers seen, and the number of unique specialties seen by each patient. Specialists included primary care providers, internal medicine specialities, general surgery, surgical subspecialties, psychiatry, obstetrics/gynecology, dermatology, neurology, sleep medicine, emergency department, and ophthalmology.
Statistical Analyses
We calculated summary statistics for demographic characteristics, medications, imaging, and frequency of provider visits among sarcoidosis patients. Comparisons between cases and controls were performed using t-tests for continuous variables, and chi-square tests for binary responses. SAS version 9.3 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Demographics
During the study period, we identified 206 cases of sarcoidosis that met our inclusion criteria. The mean age was 50.1 years, median age of 51.5 years, with a range of 15-84 years. Fifty-six percent of the cohort was female. Race information was not available in the data set. Cases were compared to 2060 matched control subjects on age and gender, and thus did not differ from cases.
Prescription Utilization: Antibiotics and Corticosteroids
One hundred and thirty-seven cases with sarcoidosis (67%) filled antibiotic prescriptions in the year prior to diagnosis, while 1557 control subjects (76%) filled antibiotic prescriptions. For cases receiving antibiotics, the average number of antibiotic prescriptions was 3.1 (SD=2.7) ,compared to 2.5 prescriptions (SD=2.5) in the control group (p=0.011). Subjects with sarcoidosis more frequently filled two or more antibiotic prescriptions in the year prior to diagnosis than the control subjects (69% vs. 55%, p=0.002).
One hundred and five patients with sarcoidosis (51%) received at least one corticosteroid prescription, while 600 control subjects (29%) received corticosteroids. For those receiving corticosteroids, the average number of prescriptions was 3.2 (SD=3.3) compared to 2.7 (SD=2.8) in the control group (p=0.106). However, in patients receiving corticosteroids, cases with sarcoidosis more often filled two or more prescriptions compared to those in the control group (63% vs. 50%, p=0.014).
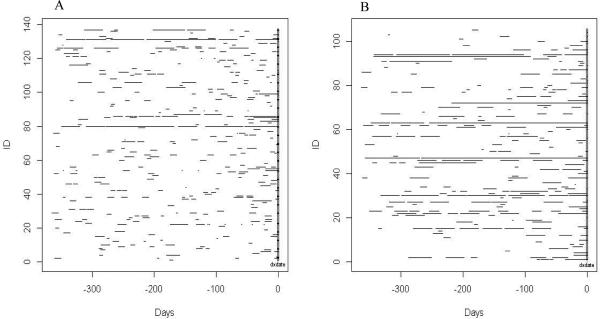
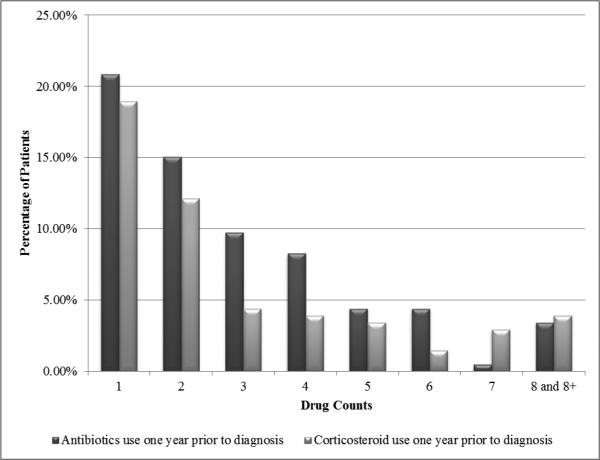
The frequency, timing, and duration of antibiotic and corticosteroid use in sarcoidosis cases are plotted in Figures 1A and 1B. The distribution of number of prescriptions per patient with sarcoidosis is displayed in Figure 2.
Figure 1.
Frequency and timing of antibiotic (1A) and corticosteroid (1B) prescriptions in the year prior to diagnosis by individual sarcoidosis patients.
Figure 2.
Distribution of the number of antibiotic and corticosteroid prescriptions per person in the year prior to diagnosis of sarcoidosis.
Utilization of Imaging
One hundred and sixty-nine patients (82%) of cases with sarcoidosis underwent at least one chest x-ray in the year prior to diagnosis, while only 1054 (51%) of control patients did so. Among those who received chest x-rays, patients with sarcoidosis averaged 2.0 x-rays (SD 1.4), while control subjects averaged 1.5 x-rays (SD 1.6) per patient (p<0.0001). Sarcoidosis cases had a markedly higher frequency of receiving two or more chest x-rays compared to those in the control group (54% vs. 24%, p<0.0001).
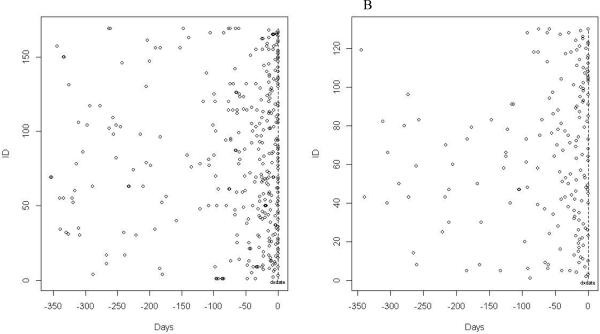
One hundred and thirty cases with sarcoidosis (63%) had at least one chest CT imaging procedure, while only 83 (6.3%) of patients in the control group had a chest CT. Of the patients receiving chest CTs, there were similar rates of receiving two or more chest CT imaging in both groups (28% of the sarcoidosis patients vs. 27% of the control patients, p=0.920) The frequency, timing, and duration of chest x-rays and CT scans for patients with sarcoidosis are plotted in Figures 3A and 3B.
Figure 3.
Frequency and timing of chest x-rays (3A) and CT Chest imaging (3B) in the year prior to diagnosis by individual sarcoidosis patient.
Health Provider Visits
The average number of doctor visits of cases with sarcoidosis was 14.7 (SD=11.3, median=12.0) in the year prior to diagnosis. Compared to the case group, control patients had significantly fewer doctor visits of 7.8 (SD=8.4, median 5), p<0.0001). Cases saw, on average, 6.1 different individual providers (SD=3.4, median=5.0), and 3.9 different types of specialists (SD=1.9, median=4.0). Control patients saw an average of 3.4 different individual providers (SD=2.6, median 3) and 2.1 specialists (SD=1.4, median 2), both significantly fewer than case patients (p<0.0001).
Discussion
We found that patients with sarcoidosis frequently see healthcare providers and receive a large number of prescriptions and imaging exams in the year prior to diagnosis. Compared to patients with alternative respiratory diagnoses (asthma, COPD, and pneumonia), patients with sarcoidosis had a significantly higher frequency of receiving two or more courses of antibiotics, corticosteroids, and chest x-rays, and saw a markedly greater number of doctors and specialists in the year prior to diagnosis. Our study includes patients from rural, urban, academic, and community practices, and offers a view of the patterns of care of insured sarcoidosis patients.
Although it is evident that patients with sarcoidosis undergo a substantial amount of medical care prior to diagnosis, it is the repeated testing and prescriptions that are distinctive compared to patients with other respiratory diagnoses. Identifying unique indicators (or patterns of care) could indicate to providers that a patient may need further workup of a rare disease such as sarcoidosis or specialized coordination of care. For example, identifying patients with multiple courses of antibiotics, steroids, and chest x-rays, may prompt a provider or payer to reassess a patient's underlying diagnosis. In this study, patients with sarcoidosis were much more likely to receive more than one course of antibiotics, steroids, or chest x-rays than a population with general respiratory complaints. Using criteria for high frequency use of these three variables may be considered for future automated algorithms that flag a patient with a potential missed diagnosis. Further evaluation of the appropriate ‘cut-off’ values or combinations of variables to obtain an acceptable rate of sensitivity and specificity for identifying missed or delayed diagnosis is warranted. For example, search engines that utilize automated algorithms specific to rare diseases have recently been developed to help search and find rare conditions on the internet, with improved diagnostic potential compared to traditional search engines.(7)
Although, our claims data does not allow us to evaluate indirect costs of sarcoidosis on society, such a high number of doctor visits in one year (an average of 14.7 visits) would imply an impact on work productivity (or sick days), disability, and costs in this population. Other chronic diseases with respiratory presentations, such as COPD, have been shown to be associated with a significant loss of wages due to lost time from work.(8) Similarly, rheumatoid arthritis patients have monthly health care costs two-three times the general population in the year of diagnosis.(9) Future investigations should consider the costs related to delayed diagnosis of sarcoidosis.
Rare diseases frequently are associated with delay in diagnosis, often for years.(10-12) Reasons for delayed diagnosis can be due to patient-related, disease-related, and healthcare-related factors. Even common diseases, such as COPD, have been found to have a high rate of under-diagnosis in patients who do not exhibit classic features such as active smoking and chronic cough.(13) Characteristics that could explain some reasons for difficulty in the diagnosis include atypical, nonspecific presentations, rarity of the disease, or lack of provider awareness of the disease.(14) Further research and understanding of how to support and educate clinicians in the diagnostic process of sarcoidosis are warranted.
Our study has several limitations. First, we do not have patient-reported or provider-reported data that would indicate exactly when symptoms began. Second, in our dataset, we were not able to analyze all potential healthcare use such as laboratory testing, pulmonary function, or cardiac testing that often is obtained in patients with sarcoidosis, and could not do a full cost analysis. These would be important next steps in estimating the impact of sarcoidosis on the healthcare system. Third, we do not have the ability to assess specific organ involvement. Fourth, we cannot be entirely certain that these are all incident cases. Our case definition attempts to identify incident cases by having no prior claims for sarcoidosis in the year prior. Some patients may be seen as a general re-evaluation. However, this bias would likely result in an underestimation of healthcare use since the diagnosis had already been made. Fifth, despite documentation of high healthcare use in the year prior to diagnosis, our study data do not allow us to assess whether there are true delays in diagnosis, or if this is the expected process of diagnosis. Furthermore, it is unknown whether delays in diagnosis equate to adverse outcomes in this population. More encompassing datasets following prospective outcomes related to diagnostic times are needed to assess the consequences of long diagnostic periods. Last, we chose a diseased control group with respiratory diagnoses to best represent pulmonary complaints that may be seen in a primary care clinic. Although it is possible that some sarcoidosis patients could be misdiagnosed with other respiratory disease, we feel that the results are not likely to be greatly impacted given the large number of controls and the rarity of sarcoidosis. Comparisons with other control groups may also be warranted. A majority of these limitations are inherent to a claims database; establishing a more comprehensive factor analysis of reasons for high healthcare use would require a combination of patient medical charts, claims databases, and clinical care registries; combining overlapping datasets may be helpful to improve sensitivity of identifying rare outcomes.(15)
Despite these limitations, our results clearly show that sarcoidosis patients are subjected to a variety of tests and therapies, many of which may not be necessary. These results are of interest to clinicians, patients, and healthcare payers seeking to improve care to patients with sarcoidosis. Further research is needed to determine how healthcare use patterns compare to other populations and to the non-diseased, or to determine how resources are utilized in patients without insurance and whether there are other socioeconomic, racial, or geographic disparities affecting patterns of care. The results of this study show why more comprehensive datasets are needed that specifically include patient symptoms, outcomes, and clinical management. Finally, our results highlight the need for improved diagnostic algorithms to identify patients with sarcoidosis.
Acknowledgements
The authors appreciate the collaborative relationship between Wellmark, Inc. (dba as Wellmark Blue Cross and Blue Shield of Iowa and Wellmark Blue Cross and Blue Shield of South Dakota) and the University of Iowa, which has created this secured, IRB-approved Data Repository, accessible without charge or censorship rights to the University of Iowa faculty researchers.
Funding Sources: This work was supported in part by the National Institutes of Health Grant: 1K23HL114640-01A1 (AKG). The funding sources did not have involvement in the study design, data analysis, writing, or submission of the manuscript.
Footnotes
Competing Interests: The authors have no conflicts of interest.
Authors’ Contributions:
Dr. Alicia Gerke, the guarantor of the manuscript, contributed to the design of the study, was responsible for original design, data collection and analyses, and contributed to the writing and revising all drafts of the manuscript.
Fan Tang was responsible for data analyses, and contributed to writing and revising the manuscript.
Dr. Jane Pendergast contributed to the design of the study and data analyses.
Dr. Joseph Cavanaugh contributed to the design of the study, data analyses, and to the writing and revising of the manuscript.
Dr. Philip Polgreen contributed to the original idea and design of the study, data collection and analyses, and to the writing and revising of the first draft and subsequent drafts of the manuscript.
All authors contributed to final approval of the manuscript.
References
- 1.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 2.Hawtin KE, Roddie ME, Mauri FA, Copley SJ. Pulmonary sarcoidosis: the ‘Great Pretender’. Clin Radiol. 2010;65(8):642–50. doi: 10.1016/j.crad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123(2):406–12. doi: 10.1378/chest.123.2.406. [DOI] [PubMed] [Google Scholar]
- 4.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–30. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerke AK, Yang M, Tang F, Cavanaugh JE, Polgreen PM. Increased hospitalizations among sarcoidosis patients from 1998 to 2008: a population-based cohort study. BMC Pulm Med. 2012;12:19. doi: 10.1186/1471-2466-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ligon CB, Judson MA. Impact of systemic corticosteroids on healthcare utilization in patients with sarcoidosis. Am J Med Sci. 2011;341(3):196–201. doi: 10.1097/maj.0b013e3181fe3eb2. [DOI] [PubMed] [Google Scholar]
- 7.Dragusin R, Petcu P, Lioma C, et al. FindZebra: a search engine for rare diseases. Int J Med Inform. 2013;82(6):528–38. doi: 10.1016/j.ijmedinf.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher MJ, Upton J, Taylor-Fishwick J, et al. COPD uncovered: an international survey on the impact of chronic obstructive pulmonary disease [COPD] on a working age population. BMC Public Health. 2011;11:612. doi: 10.1186/1471-2458-11-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson JK, Johansson K, Askling J, Neovius M. Costs for hospital care, drugs and lost work days in incident and prevalent rheumatoid arthritis: how large, and how are they distributed? Ann Rheum Dis. 2013 Dec 9; doi: 10.1136/annrheumdis-2013-204080. doi: 10.1136/annrheumdis-2013-204080. [DOI] [PubMed] [Google Scholar]
- 10.Patterson MC, Mengel E, Wijburg FA, et al. Disease and patient characteristics in NP-C patients: findings from an international disease registry. Orphanet J Rare Dis. 2013;8:12. doi: 10.1186/1750-1172-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson M, Elliott EJ, Zurynski YA. Australian families living with rare disease: experiences of diagnosis, health services use and needs for psychosocial support. Orphanet J Rare Dis. 2013;8(1):22. doi: 10.1186/1750-1172-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierucci P, Lenato GM, Suppressa P, et al. A long diagnostic delay in patients with Hereditary Haemorrhagic Telangiectasia: a questionnaire-based retrospective study. Orphanet J Rare Dis. 2012;7:33. doi: 10.1186/1750-1172-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourbeau J, O'Donnell D, Maltais F, et al. What are the factors related to misdiagnosis of COPD? Eur Resp J. 2011;38(sup55):261. [Google Scholar]
- 14.Kostopoulou O, Delaney BC, Munro CW. Diagnostic difficulty and error in primary care-a systematic review. Fam Pract. 2008;25(6):400–13. doi: 10.1093/fampra/cmn071. [DOI] [PubMed] [Google Scholar]
- 15.Zampi JD, Donohue JE, Charpie JR, Yu S, Hanauer DA, Hirsch JC. Retrospective database research in pediatric cardiology and congenital heart surgery: an illustrative example of limitations and possible solutions. World J Pediatr Congenit Heart Surg. 2012;3(3):283–7. doi: 10.1177/2150135112440462. [DOI] [PubMed] [Google Scholar]