Abstract
Background
While hemodynamic monitoring is often performed following coronary artery bypass grafting (CABG), the relationship between postoperative central venous pressure (CVP) measurement and clinical outcomes is unknown.
Methods
Detailed clinical data were analyzed from 2,390 randomly selected patients undergoing high risk CABG or CABG/valve at 55 hospitals participating in the Society of Thoracic Surgeons' National Cardiac Surgery Database from 2004 to 2005. Eligible patients underwent elective/urgent isolated CABG with an ejection fraction < 40%, or elective/urgent CABG at age ≥65 years with diabetes or a glomerular filtration rate 60 mL/min per 1.73 m2. Correlation between post-operative CVP and in-hospital / 30-day mortality and renal failure was assessed as a continuous variable, both unadjusted and after adjusting for important clinical factors using logistic regression modeling.
Results
Mean age was 72 years, 54% of patients had diabetes mellitus, 49% were urgent procedures, and mean cardiopulmonary bypass time was 105 minutes. Patients’ CVP 6 hours post-operation was strongly associated with in-hospital and 30 day mortality: odds ratio (OR) 1.5 (95% confidence interval [CI] 1.23, 1.87) for every 5 mmHg increase in CVP, p<0.0001. This association remained significant after risk-adjustment for cardiac index: adjusted OR 1.44 (95% CI 1.10, 1.89), p<0.01. A model adjusting for cardiac index also revealed increased incidence of mortality or renal failure: adjusted OR 1.5 (95% CI 1.28, 1.86) for every 5 mmHg increase in CVP, p<0.0001.
Conclusion
Patients’ central venous pressure at 6 hours following CABG surgery was highly predictive of operative mortality or renal failure, independent of cardiac index and other important clinical variables. Future studies will need to assess whether post-operative CVP can be used to guide intervention and improve outcomes.
Many higher risk coronary artery bypass graft (CABG) surgery patients have a pulmonary artery catheter placed pre-operatively to help guide clinical management [1, 2]. Much attention is often paid to assuring patients’ have adequate cardiac output and index in the perioperative period. However, central venous pressure (CVP) is another quick and simple hemodynamic parameter in the acute recovery period [1]. Despite its frequent measurement, the importance and role of CVP as a prognostic indicator has not received wide-spread study or attention. As such, we sought to investigate the prognostic value of CVP measurement among a multi-institutional cohort of high-risk CABG patients in the post-surgical intensive care unit setting. Our specific questions were whether CVP correlates with death and/or renal failure, whether the relationship, if any, is continuous or stepped, and whether there exists any association between CVP and outcome independent of baseline preoperative factors and/or cardiac index.
METHODS
The CAPS-Care Registry
The Contemporary Analysis of Perioperative Cardiovascular Surgical Care (CAPS-Care) registry is a sample of 2,390 high risk patients from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS ACSD) in whom retrospective chart reviews were performed to supplement existing registry data. Clinical data for the STS ACSD were collected using methods described fully elsewhere [3]. Data definitions are standardized, and site data coordinators receive initial and ongoing training in these definitions. Sites voluntarily submit data to the data coordinating center and receive site-specific feedback, benchmarked against regional and national results. Overall completeness of procedure reporting and mortality event reporting in patients age ≥65 has been validated against national Medicare claims files [4]. Accuracy of individual data elements has been validated in regional analyses with agreement rate >95% [3]. The STS also conducts annual on-site data audits for randomly selected database participants.
From among the greater than 1,000 STS ACSD participant centers, 50 sites were chosen for inclusion in CAPS-Care based on records of high quality data submission. Forty-eight of these selected sites, collecting data for 55 hospitals, secured institutional review board approval for study participation. Sites were trained to abstract charts using the standardized CAPS-Care case report form. Detailed pre-operative, operative, and post-operative information was collected.
Patient Population
Patients undergoing CABG between January 2004 and June 2005 were screened for inclusion in CAPS-Care. For eligibility, EF< 40% or age ≥ 65 years with either diabetes mellitus or a glomerular filtration rate (GFR) <60 mL/min per 1.73 m2 was required. Patients ≤18 years old and those undergoing emergent surgery or with preoperative cardiogenic shock were excluded. Among eligible cases, 50 were randomly selected at each of the 48 participating sites (n=2390 cases). Of these 2390 CABG cases, 263 also underwent concomitant tricuspid or mitral valve repair or replacement and those patients were excluded from the present analysis.
CVP measurements
To create the CAPS-Care registry, abstracted hemodynamic data were directly linked to the STS ACSD using unique patient identifiers. The Duke University School of Medicine Institutional Review Board granted a waiver of informed consent and authorization for this study. CVP measurements were successfully abstracted at 2 time points as part of the CAPS-Care study: immediately upon ICU arrival and 6 hours postoperatively. The change in CVP from ICU arrival to 6 hours postoperatively was also calculated for study.
Clinical Outcomes
The primary endpoints were operative mortality defined as in-hospital or 30-day mortality and renal failure defined as postoperative in-hospital renal failure, new-onset dialysis, or readmission for renal failure, was identified as a secondary outcome of interest as a composite endpoint with mortality [5].
Statistical Analysis
Patient and operative characteristics were summarized for the study cohort, with categorical variables presented as percentages and continuous variables presented as medians with 25th75th percentiles unless otherwise stated. CVP was selected a priori as our exposure of interest. CVP was normally distributed across the study population, allowing the use of parametric modeling techniques.
We studied the correlation between mortality and CVP at ICU arrival and 6 hours, as well as the magnitude of the change between these two time points, in order to determine at which point CVP was most closely correlated with clinical outcomes. Patients with missing CVP at arrival and/or at 6 hours were excluded from the analysis. Primary analysis of CVP measurement was selected at the time point which correlated most strongly with outcome.
Logistic regression modeling was used to determine the relationship between CVP and mortality. Unadjusted and risk-adjusted models were constructed. Variables included in the risk-adjusted model were those known to be most predictive of mortality following CABG (age, perfusion time, diabetes, lung disease) [6]. The relationship between CVP and endpoints was assessed for linearity and CVP was transformed when needed.
In an additional sensitivity analysis among those patients for whom cardiac index was also measured at 6 hours postoperatively, we assessed the relationship between CVP and mortality stratified by cardiac index ≥ or < 2.0 L/min/m2.
All analyses were performed using SAS System version 9.2.
RESULTS
Patient Population
CVP was not captured in 126 patients at either ICU arrival or at 6 hours. In-hospital/30-day mortality rate in patients excluded from and included in the analysis were similar (5.0% vs. 4.8%). Table 1 displays the baseline clinical and operative characteristics of the 2001 CABG patients with CVP captured in the CAPS-Care registry. Mean age was 72 with 54% of patients having diabetes mellitus and a mean cardiopulmonary bypass time of 105 minutes [25th75th percentile = 82, 135]. Excluded from risk-adjusted analysis were 337 patients with a missing value for age, perfusion time, diabetes mellitus, or lung disease and were not included in the risk-adjusted model. The subsets of patients included in the unadjusted (n=1834) and risk-adjusted (n=1497) models for mortality at 30 days were not clinically different from the overall study cohort (Table 1).
Table 1.
Patient baseline clinical and operative characteristics. Values are presented as mean or median [25th, 75th percentile].
| Characteristic | Overall (n=2001) |
Unadjusted model (n=1834) |
Risk-adjusted model (n=1497) |
|---|---|---|---|
| Age | 72 [66,77] | 72 [66,77] | 72 [66,77] |
| Female | 33.1 | 33.0 | 32.7 |
| Diabetes mellitus | 53.4 | 53.5 | 54.4 |
| Morbid obesity | 13.6 | 13.7 | 12.9 |
| Dyslipidemia | 77.2 | 76.8 | 77.4 |
| Current smoker | 180 | 17.5 | 17.1 |
| Severe chronic lung disease | 5.4 | 5.3 | 4.9 |
| Preoperative creatinine | 1.1 [1.0,1.4] | 1.1 [1.0,1.4] | 1.1 [1.0,1.4] |
| Hypertension | 84.7 | 84.6 | 84.9 |
| Arrhythmia | 15.3 | 15.2 | 15.0 |
| PVD | 20.3 | 20.4 | 19.4 |
| History of stroke | 11.5 | 11.3 | 11.4 |
| Previous myocardial infarction | 46.9 | 46.5 | 44.7 |
| Prior CV surgery | 29.0 | 29.4 | 28.1 |
| Urgent procedure | 49.6 | 48.2 | 48.7 |
| CABG only | 86.0 | 85.9 | 84.4 |
| Preoperative IABP | 6.4 | 6.6 | 6.9 |
| Postoperative blood product transfusion | 51.0 | 50.9 | 52.1 |
| CPB time (minutes) | 104 [81,135] | 103 [80,135] | 103 [80,135] |
| Cross-clamp time (minutes) | 71 [53,97] | 71 [53,97] | 71 [53,97] |
Abbreviations: CAPS-Care= contemporary analysis of perioperative cardiovascular surgical care; PVD=peripheral vascular disease; CV=cardiovascular; CABG=coronary artery bypass grafting; IABP=intra-aortic balloon pump; CPB=cardiopulmonary bypass
Unadjusted Models
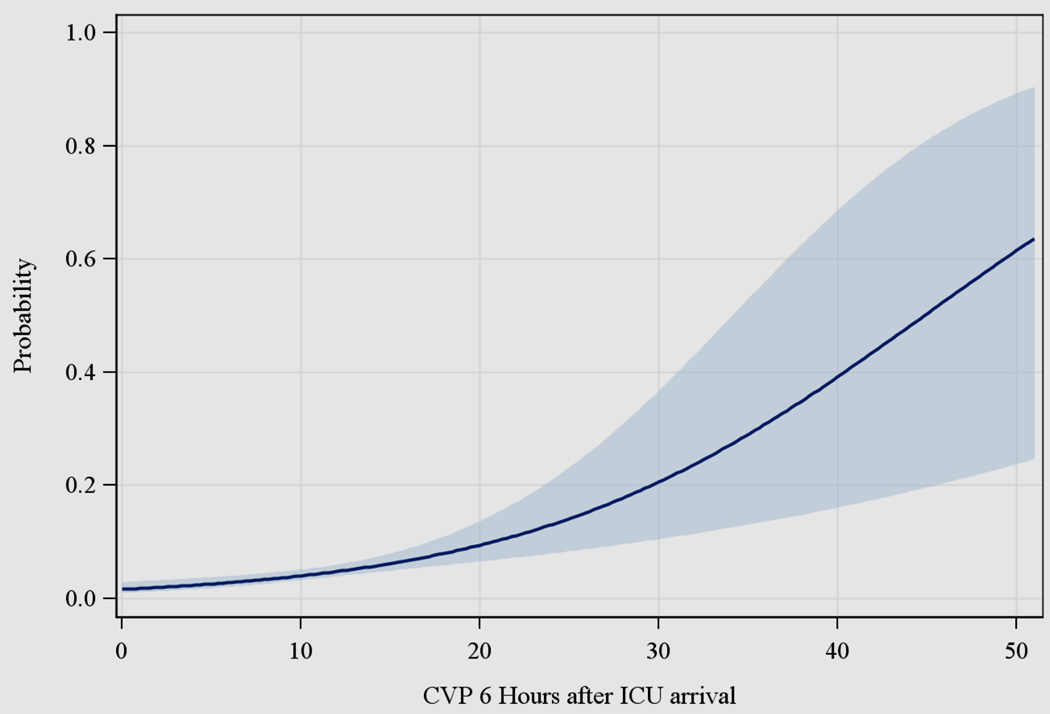
Figure 1 displays the association between central venous pressure (CVP) at 6 hours and predicted probability of operative death. Increasing CVP correlated with increasing risk of operative mortality across the entire range of recorded CVP values. CVP at 6 hours postoperatively was associated most strongly with mortality. The correlation between the outcome and both CVP at ICU arrival and the change in CVP between ICU arrival and 6 hours postoperatively were similar to the results displayed in Figure 1. CVP at 6 hours was selected as the CVP measurement for the primary outcomes analysis. CVP at 6 hours postoperatively also allowed for a period of optimization in the ICU after arrival from the operating room post-CABG and maximized data capture (CVP not measured following central venous catheter removal). Figure 2 displays unadjusted curves for predicted 30-day mortality stratified by cardiac index ≥ or < 2.0 L/min/m2. The shapes of the two curves (for those patients with cardiac index ≥ 2.0 L/min/m2 versus those with index < 2.0 L/min/m2) are approximately the same.
Figure 1.
Relationship between central venous pressure (CVP) at 6 hours and predicted probability of death through 30 days or in-hospital. Solid dark blue line represents point estimates and light blue shaded area represents 95% confidence interval.
Figure 2.
Relationship between central venous pressure (CVP) and predicted probability of death according to cardiac index (CI) ≥ 2 or < 2 L/min/m2 at 6 hours. The shapes of the curves are approximately the same indicating that the relationship between CVP and mortality is independent of cardiac index. Solid dark blue line represents point estimates and light blue shaded area represents 95% confidence interval.
Table 2 displays model results for the association between 6-hour postoperative CVP and mortality and the composite of mortality and renal failure. Tests for linearity of CVP on each outcome revealed a p-value for non-linearity between CVP at 6 hours and in-hospital/30 day mortality of 0.77. An unadjusted mortality model revealed an odds ratio (OR) of 1.5 (95% confidence interval [CI] 1.23, 1.87; p<0.0001) for increased mortality with every 5 mmHg increase in CVP. C-index for this unadjusted model was 0.62. For mortality or renal failure, the unadjusted model revealed an OR of 1.7 (95% CI 1.44, 1.99; p<0.001) for increased mortality or renal failure with every 1 mmHg increase in CVP (Table 2).
Table 2.
Models, unadjusted and risk-adjusted, estimating the odds of in-hospital / 30-day death and death or renal complications based on central venous pressure in the intensive care unit at 6 hours postoperatively
| N | Central Venous Pressure at 6 Hours |
OR | 95% CI | c-index | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | Chi-Square | p-value | |||||
| In-hospital / 30-day death | ||||||||
| Unadjusted model | 1834 | 0.083 | 0.021 | 15.29 | < 0.0001 | 1.52 | 1.23 – 1.87 | 0.62 |
| Adjusted model (age, perfusion time, diabetes, and lung disease) | 1497 | 0.044 | 0.024 | 3.27 | 0.071 | 1.25 | 0.98 – 1.59 | 0.72 |
| Adjusted model by cardiac index | 782 | 0.073 | 0.028 | 6.87 | 0.0088 | 1.44 | 1.10 – 1.89 | 0.62 |
| In-hospital / 30-day death or renal failure | ||||||||
| Unadjusted model | 1837 | 0.105 | 0.017 | 39.91 | <.0001 | 1.69 | 1.44 – 1.99 | 0.65 |
| Adjusted model (age, perfusion time, diabetes and lung disease) | 1500 | 0.086 | 0.019 | 20.82 | < 0.0001 | 1.54 | 1.28 – 1.86 | 0.68 |
| Adjusted model by cardiac index | 783 | 0.093 | 0.024 | 15.73 | < 0.0001 | 1.60 | 1.27 – 2.01 | 0.64 |
OR for 5 units increase in CVP at 6 Hours.
SE=standard error; OR=odds ratio; CI=confidence interval
One hundred forty (7.6%) patients suffered renal failure. The relationship between renal failure alone and CVP was non-linear and therefore a model was fitted with two linear splines for CVP < 9 mmHg and CVP ≥ 9 mmHg. An unadjusted model for renal failure alone revealed OR 6.9 (95% CI 2.59, 18.3; p<0.0001) for renal failure with every 5 mmHg increase in CVP among patients with 6 hour CVP <9 mmHg. For patients with 6 hour CVP ≥ 9 mmHg, the unadjusted OR was 1.3 (95% CI 1.06, 1.67; p=0.015) for renal failure with every 5 mmHg increase in CVP.
Risk-adjusted Models
A risk-adjusted mortality model revealed an OR of 1.05 (95% CI 1.0,1.1; p=0.07) for increased risk of death with every 5 mmHg increase in CVP at 6 hours. C-index was 0.72 (Table 2). Adjusting for cardiac index among the 782 patients with this value measured at 6 hours along with CVP, increasing CVP was associated with a significant risk of increased mortality: OR 1.4 (95% CI 1.10, 1.89; p=0.009) for every 5 mmHg increase in CVP.
The incidence of in-hospital / 30 – day death or renal failure remained significantly increased with increasing CVP after risk adjustment: OR 1.54 (95% CI 1.28, 1.86; p<0.0001) for every 5 mmHg increase in CVP. When also adjusting for cardiac index, the predictive value of CVP remained the same, with OR 1.6 (95% CI 1.27, 2.01; p<0.0001) for every 5 mmHg increase in CVP.
For renal failure alone, the risk-adjusted OR was 5.5 (95% CI 1.93, 15.5; p=0.001) with every 5 mmHg rise in CVP for patients with CVP < 9 mmHg. For patients with 6 hour CVP ≥ 9 mmHg, the risk-adjusted OR was 1.3 (95% CI 1.01, 1.65; p=0.045).
DISCUSSION
Central venous pressure (CVP), the downstream pressure of the systemic venous system, is frequently measured in patients undergoing CABG surgery. Despite often routine monitoring of CVP, a paucity of clinical data exists regarding the prognostic value of this measurement following CABG surgery. Existing literature focuses on the degree to which CVP gives information on right ventricular end-diastolic pressure and the limitations of CVP as a surrogate variable for left ventricular preload, but little consideration is given to whether this measurement has implications for clinical outcomes in the early postoperative CABG setting [7, 8, 9].
The results of this study indicate that CVP measured at 6 hours after surgery was associated with operative mortality and renal failure among a multi-institutional observational cohort of high-risk patients undergoing CABG surgery. This relationship persisted when adjusting for those key patient characteristics known to predict mortality following CABG [6]. Importantly, CVP was predictive of mortality and the composite of mortality and renal failure when controlling for cardiac index. The clinical importance of this finding relates to any health professional caring for the post-cardiac surgical patient, including critical care nursing and advanced practice providers who are often at the point of care and responsible to alert supervising physicians of patient status. An otherwise routine post-CABG surgery patient with a cardiac index > 2 but with an elevated CVP may deserve additional consideration.
Many post-cardiac surgery patients are being managed without a PA catheter or under “fast-track” protocols for early removal of PA catheters [1], however, CVP is typically able to be measured in these patients by way of a central venous catheter with its tip in the superior vena cava. The results of the present study indicate that CVP may be a useful predictor of patient outcome and the post-surgical patient with elevated CVP may warrant further workup to investigate the etiology (renal failure, pericardial tamponade, right heart failure, etc). Just as reduced cardiac index or alterations in systemic blood pressure may warrant notification by critical care nursing or advanced practice providers, CVP thresholds or trends may be incorporated in basic ICU protocols for notifying supervising physicians of the post-surgical patient who otherwise may be progressing as expected or according to a post-procedural “care map”.
The results of this study also suggest utility in the measurement of CVP in the post-CABG setting. Measurement of CVP at 6 hours postoperatively was chosen for this study because it allowed for a period of stabilization in the intensive care unit but was not so far out from surgery that central venous catheters were likely to have already been removed. We found that CVP at 6 hours correlated most strongly with outcome as opposed to CVP measured immediately upon postoperative ICU arrival, or the value of the difference between these values. Contemporaneous registry data has identified CVP as a strong independent predictor of outcomes among a diverse group of adult patients with cardiovascular disease [10], medically-managed heart failure patients with renal dysfunction [11], and patients with cirrhosis undergoing heart surgery [12]. Both the Mullens et al study of heart failure patients [11] and the Lopez-Delgado et al study of cirrhotic heart surgery patients [12] assessed CVP in the acute inpatient setting, although the patient populations were unique and sample sizes substantially smaller as compared to the present study.
The present study supports specifically the usefulness of CVP measurement during the early postoperative ICU recovery period among a multi-institutional cohort of CABG patients. Given the ease of measurement and ubiquitous nature of central venous catheters following CABG surgery, the present data support the routine assessment of CVP in this patient population. While considerable attention is given to cardiac index, CVP may provide additional information regarding right ventricular function. The present study supports clinical attention to high CVP values and consideration of overly aggressive volume resuscitation, pericardial tamponade, valvular regurgitation, right ventricular dysfunction, and other potential etiologies. Although the usefulness of further diagnostic studies or interventions based on CVP values remains to be validated in future studies, it remains our practice to measure and consider CVP values in decision-making for patients following CABG surgery.
The findings of this study should be viewed in the setting of several important limitations. This study evaluated associations between measured clinical variables and outcomes and the results herein may have been influenced by unmeasured variables. CVP measurements were not blind to clinicians. Therefore, response by clinicians to CVP values as part of patient care may have resulted in an underestimation of the association between CVP and outcome. While the present study is a large multi-institutional sampling of perioperative care in high-risk CABG patients, exact outcomes in any individual center may vary from our results. CVP values are affected by many conditions, including right ventricular dysfunction, tricuspid valve disease, cardiac tamponade, perioperative fluid administration, pericarditis, and renal failure. Etiology of elevated CVP was not able to be determined.
Conclusions
In this observational cohort, central venous pressure following CABG surgery was predictive of early mortality and renal failure, independent of cardiac index and other important clinical variables. Our study highlights the importance of using CVP routinely at 6 hours after ICU arrival, a simple and easily performed tool, to assess risk of early death and renal failure in patients undergoing CABG-surgery. Whether or not CVP can be used to guide therapy in this setting deserves further investigation.
REFERENCES
- 1.Schwann NM, Hillel Z, Hoeft A, Barash P, Mohnle P, Miao Y, Mangano DT. Lack of effectiveness of the pulmonary artery catheter in cardiac surgery. Anesth Analg. 2011;113:994–1002. doi: 10.1213/ANE.0b013e31822c94a8. [DOI] [PubMed] [Google Scholar]
- 2.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005 Oct 5;294(13):1664–1670. doi: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 3.Welke KF, Ferguson TB, Coombs LP, Dokholyan RS, Murray CJ, Schrader MA, Peterson ED. Validity of the Society of Thoracic Surgeons National Adult Cardiac Surgery Database. Ann Thorac Surg. 2004;77:1137–1139. doi: 10.1016/j.athoracsur.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Welke KF, Peterson ED, Vaughan-Sarrazin MS, O'Brien SM, Rosenthal GE, Shook GJ, Dokholyan RS, Haan CK, Ferguson TB., Jr Comparison of cardiac surgery volumes and mortality rates between the Society of Thoracic Surgeons and Medicare databases from 1993 through 2001. Ann Thorac Surg. 2007;84:1538–1546. doi: 10.1016/j.athoracsur.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Shahian DM, Jacobs JP, Edwards FH, Brennan JM, Dokholyan RS, Prager RL, Wright CD, Peterson ED, McDonald DE, Grover FL. The Society of Thoracic Surgeons National Database. Heart. 2013 Jan 18; doi: 10.1136/heartjnl-2012-303456. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Lopes RD, Williams JB, Mehta RH, Reyes EM, Hafley GE, Allen KB, Mack MJ, Peterson ED, Harrington RA, Gibson CM, Califf RM, Kouchoukos NT, Ferguson TB, Lorenz TJ, Alexander JH. Edifoligide and long-term outcomes after coronary artery bypass grafting: PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) 5-year results. Am Heart J. 2012 Sep;164(3):379–386. doi: 10.1016/j.ahj.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas PS, Edmunds LH, Sutton MS, et al. Unreliability of hemodynamic indexes of left ventricular size during cardiac surgery. Ann Thorac Surg. 1987;44:31–34. doi: 10.1016/s0003-4975(10)62352-1. [DOI] [PubMed] [Google Scholar]
- 8.Buhre W, Weyland A, Schorn B, et al. Changes in central venous pressure and pulmonary capillary wedge pressure do not indicate changes in right and left heart volume in patients undergoing coronary artery bypass surgery. Eur J Anaesthesiol. 1999;16:11–17. doi: 10.1046/j.1365-2346.1999.00406.x. [DOI] [PubMed] [Google Scholar]
- 9.Hartz A, Guse C, Kayser K, Kuhn E, Johnson D. Use of postoperative information to predict mortality rates for patients who have long stays in the intensive care unit after coronary artery bypass grafting. Heart Lung. 1998 Jan-Feb;27(1):22–30. doi: 10.1016/s0147-9563(98)90065-6. [DOI] [PubMed] [Google Scholar]
- 10.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009 Feb 17;53(7):582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 11.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Delgado JC, Esteve F, Javierre C, Perez X, Torrado H, Carrio ML, Rodríguez-Castro D, Farrero E, Ventura JL. Short-term independent mortality risk factors in patients with cirrhosis undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2012 Dec 12; doi: 10.1093/icvts/ivs501. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]