Abstract
In this study, we undertook careful analysis of 13 quantitative physiological variables related to oropharyngeal swallowing from a sample of 42 subacute patients referred for dysphagia assessment. Each patient underwent videofluoroscopic swallowing examination in which they swallowed up to 5 boluses of 22% w/v ultra-thin liquid barium suspension administered by teaspoon. Our goal was to determine whether scores on thirteen kinematic or temporal parameters of interest were independently associated with the presence of penetration-aspiration in the final compiled dataset of 178 swallows. Participants were classified as aspirators, based on the presence of at least one swallow demonstrating a penetration-aspiration scale score ≥ 3. The parameters of interest included: six kinematic parameters for capturing hyoid position; three swallow durations (laryngeal closure duration; hyoid movement duration; upper esophageal sphincter (UES) opening duration); and four swallow intervals (laryngeal closure to UES opening; bolus dwell time in the pharynx prior to laryngeal closure; stage transition duration; and pharyngeal transit time). Mixed model repeated measures ANOVAs were conducted to determine the association between each parameter and aspiration status. Only one of the 13 parameters tested distinguished aspirators from non-aspirators: aspirators demonstrated significantly shorter UES opening duration. Additionally, a trend towards reduced maximum superior position of the hyoid was seen in aspirators. Limitations and future considerations are discussed.
Keywords: deglutition, deglutition disorders, dysphagia, swallowing, penetration, aspiration, impairment, kinematic, temporal, variability
Introduction
Videofluoroscopy (VF) allows direct and dynamic visualization of swallowing physiology. This radiographic procedure, combined with a standard protocol, is widely used to determine patient safety for oral intake, candidacy for swallowing treatment, and treatment outcome. Several types of physiological measures can be extracted from the VF including kinematic measures of structural displacement, temporal durations (i.e., the time required for a distinct physiological event to occur) and interval measures (i.e., the time lapse between two gestures in the swallow sequence) [1]. In addition, ratings of swallowing safety and efficiency can be made to capture a patient’s functional swallowing status [2]. The combined results of the VF exam have the potential to greatly impact a patient’s medical management and quality of life.
When a patient is observed to demonstrate a functional impairment, such as penetration or aspiration of material into the airway, the clinician must try to determine the reason for the problem in order to choose appropriate interventions. It is therefore important to understand the association between various possible physiological measures and penetration-aspiration, and to determine whether boundary values for these measures can be identified and validated with respect to their ability to dissociate functional from impaired swallowing. Previous work has reported mixed findings for associations between individual physiological measures and swallowing impairment. For example, one study reports that aspirators have significantly decreased hyoid excursion compared to non-aspirators [3] while another study finds no evidence of differences in hyoid excursion related to aspiration [4]. Some timing variables have been shown to differentiate patients who aspirate from those who do not, such as the initiation of laryngeal closure, stage transition duration and pharyngeal transit time [3, 5–7]; other timing measures, such as laryngeal closure duration, have been reported to not dissociate aspirators from non-aspirators [6, 7]. Power and colleagues [8] used discriminant analysis to demonstrate that they could predict aspiration status 73.11% of the time by combining the patient’s performance on three temporal measures: pharyngeal transit time, swallow response time, and laryngeal closure duration. In their study, aspiration status was as defined as at least one swallow with a score of ≥3 on the Penetration Aspiration Scale (PAS) [9] out of the six 5ml thin liquid boluses administered. The clear delineation of boundary values in specific physiological parameters of swallowing that characterize impairment is challenged by the fact that studies of healthy deglutition report wide ranges and considerable variability in both kinematic and temporal measures of swallowing [1].
In this manuscript, we explore a set of quantitative measures of swallowing physiology (both kinematic and temporal), to determine whether scores on any of these measures independently dissociate patients who aspirate ultra-thin liquid barium from those who do not. Using a sample of subacute hospital patients referred for VF, we operationally defined “aspirators” as patients who displayed at least one ultra-thin liquid swallow with a PAS score ≥ 3 [9]. The physiological variables of interest included six kinematic measures related to hyoid excursion: maximum anterior (i.e., X) position; maximum superior (i.e., Y) position; maximum XY hyoid position (i.e., the Pythagorean combination of maximum X and Y positions); anterior displacement (maximum X minus minimum X position); superior displacement (maximum Y minus minimum Y position) and hypotenuse displacement (i.e., XY, maximum minus minimum). In addition, seven temporal measures were explored. These included three event duration measures: hyoid movement duration (HMD); laryngeal closure duration (LCD); and upper esophageal sphincter opening duration (UESD); and four swallow interval measures: the time lapse between bolus entry into the pharynx and laryngeal closure, which we call bolus dwell time (BDT); swallow response time (SRT, also known as stage transition duration); pharyngeal transit time (PTT); and the time lapse between laryngeal closure and UES opening (LC-UES).
Our primary objective was to investigate the independent association between each of these dependent variables and penetration-aspiration, given a series of up to 5 repeated presentations of teaspoon-sized volumes of ultra-thin liquid barium. Our hypothesis was that participants with penetration-aspiration (henceforth referred to as “aspirators”) would show reductions in kinematic measures of hyoid excursion, slower durations and longer intervals than participants with safe swallowing (henceforth referred to as “non-aspirators”). This study was considered a preliminary exploration of these factors in an existing retrospective clinical dataset.
Materials and Methods
Participants
The swallowing data for this study were drawn from a retrospective database of VF recordings collected from 42 subacute patients (11 female) referred for VF between 2007 and 2010. While the exact etiology of each patient’s medical condition was not available in the database, the majority of patients were referred from stroke, acquired brain injury or geriatric rehabilitation units; consequently, the predominant etiology of dysphagia can be considered neurogenic. Male participants were 63.5 ± 18.2 years old (mean ± standard deviation) and female participants were 58.7 ± 17.6 years old.
VF Procedure
All VF exams were conducted using a Toshiba Ultimax (Toshiba America Medical Systems, Inc., Tustin, CA) fluoroscope in lateral view at 30 pulses per second, and were captured and recorded at 30 frames per second. A standard recipe was used to prepare 22% w/v barium suspension (Bracco Polibar® suspension diluted with water), which was the stimulus for all swallows extracted for this analysis. This concentration of barium has previously been labeled “ultra-thin” in the literature [10].
VF Post-processing
A minimum of 2 and maximum of 5 ultra-thin liquid single bolus clips were spliced out of the larger VF recordings for each participant. Clip boundaries were defined as starting 30 frames before the bolus passed the shadow of the mandible and ending 30 frames after the hyoid returned to rest with epiglottic return to upright (or when the fluoroscopy was turned off, if this occurred first). All instances of piecemeal deglutition (i.e., a single bolus partitioned in the oral cavity into multiple swallows) were excluded, resulting in a dataset of 178 videofluoroscopy clips for analysis. Information regarding the exact volume of the boluses administered was regrettably not available (given that this was a retrospective secondary analysis of a clinical dataset). Nevertheless, qualifying boluses were limited to those administered using a standard 5ml teaspoon, meaning that all boluses in this study were ≤ 5ml in volume.
Videofluoroscopy Ratings
The individual bolus clips were organized in random order and individually rated for penetration-aspiration status using the 8-point PAS [9] by an experienced clinically certified speech-language pathologist (first author) with inter-rater agreement ratings also being conducted by a second experienced, certified speech-language pathologist. All physiological measurements were conducted by a research assistant who was trained using a set of training swallow recordings to a level of excellent agreement with the first author. All raters were blinded to participant identity, so that they were not aware when two clips came from the same participant. Where a series of multiple swallows was employed to clear a single bolus (i.e., the entire bolus was swallowed from the oral cavity, with one or more subsequent clearing swallows), only the initial swallow in the series for that bolus was analyzed.
Aspiration status was determined using a binary reduction of the PAS. Any single swallow with a score ≥ 3 resulted in classification of the participant as an aspirator. If all the swallows collected from an individual resulted in PAS scores of 1 and/or 2, these participants were considered normal (non-aspirators), based on previous evidence that these scores are seen in healthy individuals [11, 12]
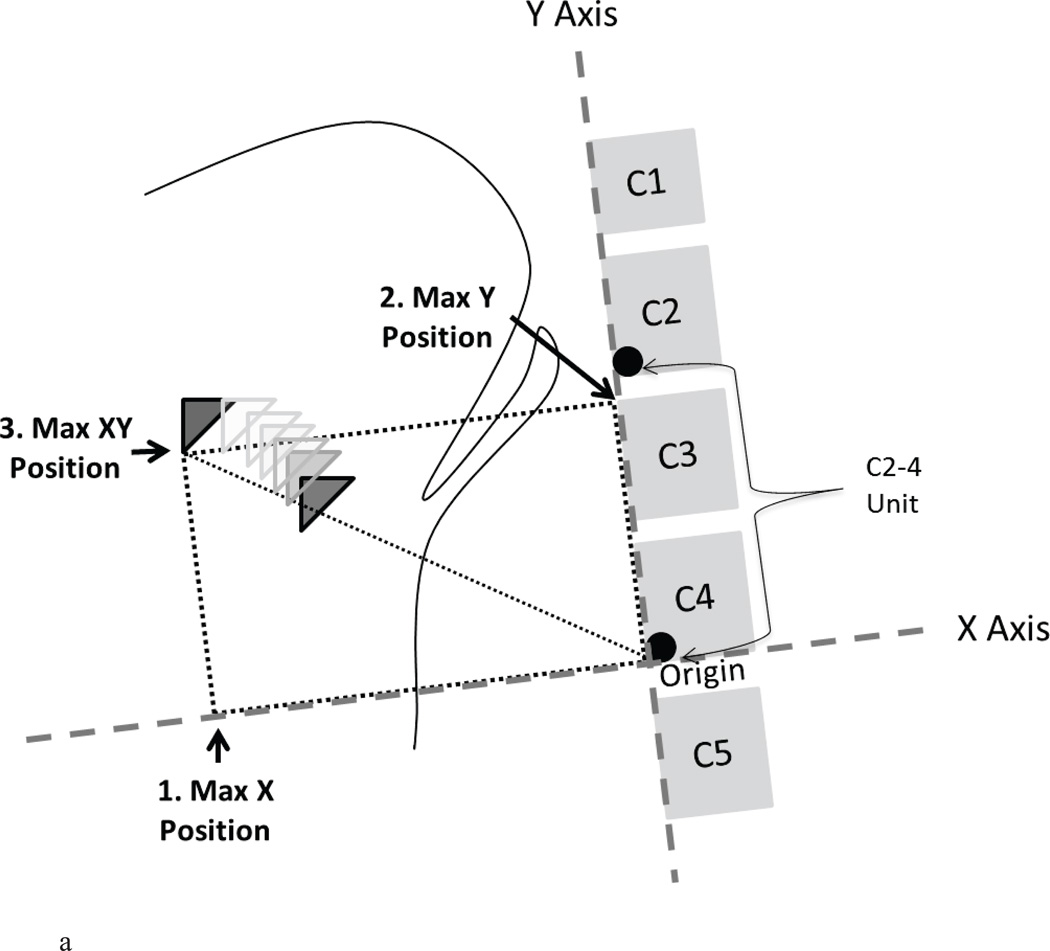
Hyoid position was measured according to procedures reported in detail elsewhere [13] and summarized here. The positions of the following structures were marked in each video frame with a movement-tracking software program: the anterior inferior corner of the C4 vertebra (origin); the anterior inferior corner of the C2 vertebra (Y vector); and the anterior inferior corner of the hyoid. All three of these points were measured in a Cartesian coordinate system with the Y-axis defined by the line running through the origin (C4) and the Y-Vector (C2), and the X-axis defined perpendicular to this line. The position of the hyoid in each frame was calculated based on its XY position relative to the origin (C4) within these participant-defined Cartesian coordinates. The positional data were scaled in cervical units (% C2–4 distance) to control for magnification artifact and sex-based differences in size across participants [13]. All measures were exported to a Microsoft Excel file with an embedded macro, which identified the maximum and minimum hyoid position values (in both the X and Y planes) between two user-defined boundary frames of interest (‘start’ and ‘end’). The ‘start’ frame was designated as 10 frames prior to hyoid movement onset and the ‘end’ frame was designated as 10 frames after the epiglottis returned to a vertical position. Between these boundary frames and using these positional data, the following hyoid measures were calculated in distances from the C4 origin and expressed in anatomically scaled units (See Figure 1):
Maximum X position;
Maximum Y position;
Maximum XY position;
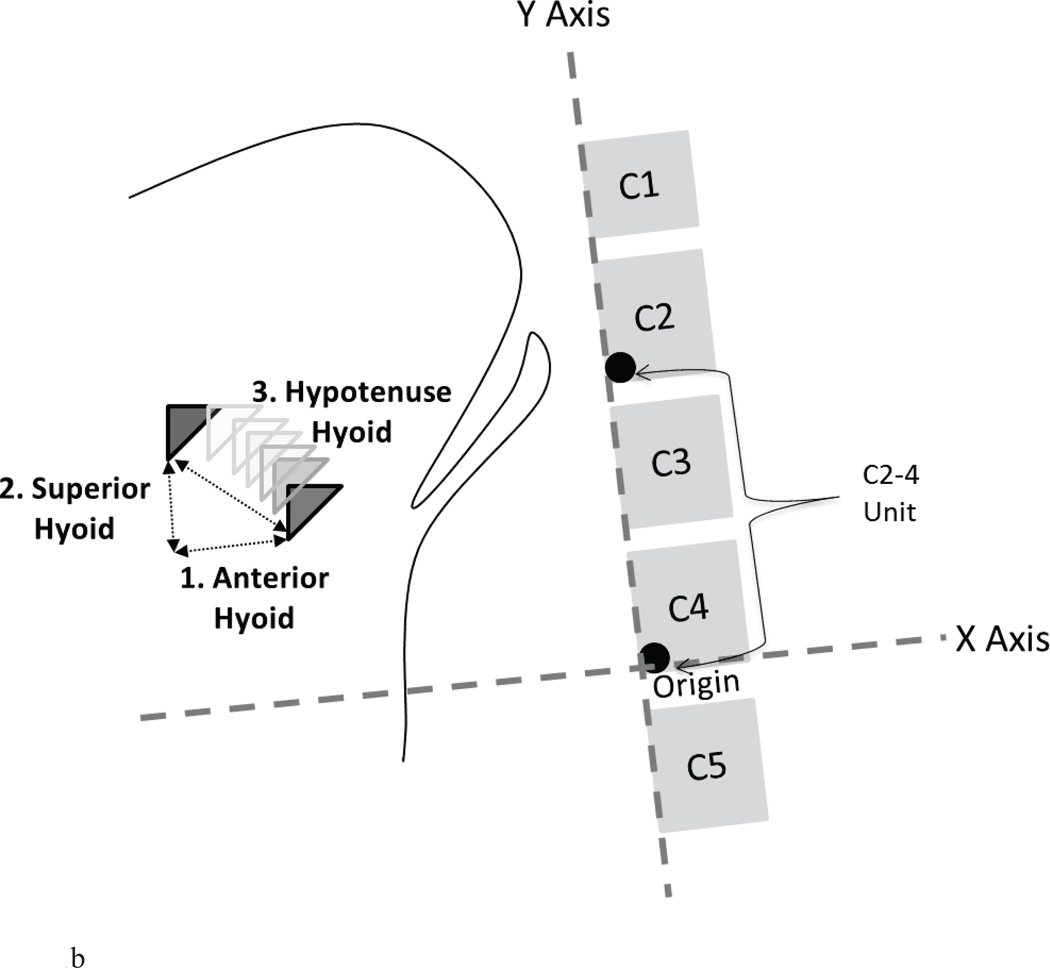
Anterior displacement (i.e., maximum X position minus minimum X position);
Superior displacement (i.e., maximum Y position minus minimum Y position);
Hypotenuse displacement (i.e., maximum XY position minus minimum XY position).
Figure 1.
a. Schematic of method for capturing maximum hyoid positions relative to the C4 origin (in %C2–4 units) with the X and Y axis rotated to the spine: maximum X position (1), maximum Y position (2) and maximum XY position (3).
b. Schematic of method of capturing maximum hyoid displacements (maximum – minimum positions) relative to the C4 origin (in %C2–4 units) with the X and Y axis rotated to the spine: anterior hyoid displacement (1), superior hyoid displacement (2) and hypotenuse hyoid displacement (3).
In order to make the temporal measures, the video clips were opened in ImageJ (National Institutes of Health, Bethesda, MD) and advanced frame-by-frame to identify the frames associated with seven specific timing events within each swallow:
onset of the hyoid movement burst;
bolus passing the ramus of mandible;
laryngeal vestibule closure;
laryngeal vestibule opening;
UES opening;
UES closure; and
hyoid return to rest at the end of the swallow.
Operational definitions for finding each frame have been reported elsewhere [1]. These seven frames were used to derive each of the 7 temporal variables of interest:
Hyoid Movement Duration (HMD): hyoid return to rest minus onset of the hyoid movement burst;
Laryngeal Closure Duration (LCD): laryngeal vestibule opening minus laryngeal vestibule closure;
UES Opening Duration (UESD): UES closure minus UES opening;
Bolus Dwell Time (BDT): laryngeal vestibule closure minus bolus passing ramus of mandible;
Stage Transition Duration (STD): hyoid movement onset minus bolus passing ramus of mandible;
Pharyngeal Transit Time (PTT): UES closure minus bolus passing ramus of mandible; and
Laryngeal Closure to UES opening (LC-to-UES): UES opening minus laryngeal vestibule closure.
While this is the first description of BDT to our knowledge, it is closely related to parameters reported by others [6, 14–16]. The units for all temporal measures were converted from frames to milliseconds (ms) by dividing the frame measures by 29.97 and multiplying by 1000.
Reliability
Ten percent of the swallows in the dataset were chosen at random and re-rated for all parameters by the original rater (intra-rater agreement) as well as by the first author (inter-rater agreement). Reliability was measured using two-way mixed intra-class coefficients (ICC) for consistency. Results appear in Table 1. Intra-rater reliability scores ranged from 0.76 to 0.99 and inter-rater reliability scores ranged from 0.74 to 1.00. With the exception of inter-rater reliability for UESD, all parameters demonstrated ‘excellent’ reliability. Regardless, intra-rater reliability for UESD still reached the high end of the ‘fair to good’ range (i.e., 0.40–0.75) [17].
Table 1.
Reliability results for all parameters.
| Parameter | ICC | 95%CI | ||
|---|---|---|---|---|
| Hyoid Positions (%C2–4) | Max X Hyoid | Inter-rater | 0.95 | (0.88–0.98) |
| Intra-rater | 0.98 | (0.80–0.96) | ||
| Max Y Hyoid | Inter-rater | 0.99 | (0.97–0.99) | |
| Intra-rater | 0.99 | (0.98–0.99) | ||
| Max XY Hyoid | Inter-rater | 0.97 | (0.94–0.99) | |
| Intra-rater | 0.98 | (0.94–0.99) | ||
| Hyoid Displacements (%C2–4) | Anterior Hyoid | Inter-rater | 0.85 | (0.62–0.94) |
| Intra-rater | 0.76 | (0.40–0.90) | ||
| Superior Hyoid | Inter-rater | 0.94 | (0.85–0.98) | |
| Intra-rater | 0.97 | (0.93–0.99) | ||
| Hypotenuse Hyoid | Inter-rater | 0.94 | (0.85–0.98) | |
| Intra-rater | 0.94 | (0.86–0.98) | ||
| Durations (ms) | Laryngeal Closure Duration | Inter-rater | 0.93 | (0.82–0.97) |
| Intra-rater | 0.98 | (0.96–0.99) | ||
| UES Opening Duration | Inter-rater | 0.74 | (0.38–0.89) | |
| Intra-rater | 0.89 | (0.73–0.95) | ||
| Hyoid Movement Duration | Inter-rater | 0.81 | (0.55–0.92) | |
| Intra-rater | 0.88 | (0.71–0.95) | ||
| Intervals (ms) | Laryngeal Closure to UES Opening | Inter-rater | 0.93 | (0.83–0.97) |
| Intra-rater | 0.89 | (0.74–0.96) | ||
| Pharyngeal Transit Time | Inter-rater | 1.00 | (0.99–1.00) | |
| Intra-rater | 0.98 | (0.96–0.99) | ||
| Stage Transition Duration | Inter-rater | 1.00 | (0.99–1.00) | |
| Intra-rater | 0.98 | (0.95–0.99) | ||
| Bolus Dwell Time | Inter-rater | 0.98 | (0.95–0.99) | |
| Intra-rater | 0.98 | (0.96–0.99) | ||
| PAS | Penetration-Aspiration Score | Inter-rater | 0.91 | (0.77–0.96) |
| Intra-rater | 0.96 | (0.91–0.99) | ||
Statistical Analyses
All statistical analyses were conducted using IBM SPSS Statistics Version 20. Two-tailed p-values < 0.05 were considered statistically significant. Frequencies for aspiration status were first tabulated by sex. Sex differences in the values for each of the 13 physiological parameters (dependent variables) were then explored using mixed-model repeated measures analyses of variance (ANOVA) with a between participant factor of sex. Given the fact that only one female participant was classified as an aspirator, and that none of the 13 dependent variables showed significant differences between male and female participants, we decided to eliminate sex as a factor from the subsequent analyses. The exploration of the association between aspiration status and the 13 physiological dependent variables was conducted using mixed-model repeated measures analyses of variance (ANOVA) with a between participant factor of aspiration status, and a repeated measures factor of bolus-number-within-participant. When significant main effects of aspiration status were identified, post hoc pairwise comparisons were conducted with Sidak adjustment for multiple comparisons. Effect sizes for pairwise comparisons were calculated using Cohen’s d [18]; according to this measure, values of 0.2–0.5 can be considered to show small effects, 0.5–0.8 show medium effects and values > 0.8 show large effects.
Results
In total, 13 participants (1 female) were identified as aspirators (at least one swallow with a PAS score of 3 or greater) and 29 participants (10 female) were identified as non-aspirators (all swallows with PAS scores of 1 or 2). Table 2 demonstrates the spread of best and worst PAS scores with grey shaded cells representing the aspirators in the sample. All participants had a least one PAS score of 2 or better. Table 3 and 4 details the descriptive statistics (using estimated marginal means) for the 6 kinematic parameters of interest (Table 3) in this study as well as the 7 temporal (duration and interval) measures of interest (Table 4). Detailed results for ANOVA analyses follow.
Table 2.
Crosstabulation of best Penetration-Aspiration Scale (PAS) scores seen per patient across all swallows by worst PAS scores seen.
| Aspiration Classification |
Worst PAS score across all swallows |
Best PAS Score across all swallows | |
|---|---|---|---|
| 2 | 1 | ||
| “Non-aspirators” (N = 29) | 1 | 0 | 24 |
| 2 | 0 | 5 | |
| “Aspirators” (N = 13) | 3 | 0 | 1 |
| 4 | 0 | 2 | |
| 5 | 0 | 1 | |
| 6 | 0 | 1 | |
| 7 | 0 | 1 | |
| 8 | 4 | 3 | |
| TOTAL (N = 42) | 4 | 38 | |
Table 3.
Descriptive statistics for 6 kinematic parameters of hyoid excursion.
| Parameter | Aspiration Classification |
Mean | 95% Confidence Interval |
Standard Deviation |
|
|---|---|---|---|---|---|
| Hyoid Positions (%C2–4) | Max X Hyoid | Non-aspirator | 135 | (132–139) | 19 |
| Aspirator | 136 | (128–144) | 22 | ||
| Max Y Hyoid | Non-aspirator | 91 | (84–98) | 36 | |
| Aspirator | 72 | (60–83) | 30 | ||
| Max XY Hyoid | Non-aspirator | 166 | (160–171) | 28 | |
| Aspirator | 156 | (146–166) | 26 | ||
| Hyoid Displacements (%C2–4) | Anterior Hyoid | Non-aspirator | 36 | (34–39) | 12 |
| Aspirator | 36 | (32–41) | 11 | ||
| Superior Hyoid | Non-aspirator | 51 | (48–54) | 16 | |
| Aspirator | 52 | (43–60) | 23 | ||
| Hypotenuse Hyoid | Non-aspirator | 63 | (60–67) | 17 | |
| Aspirator | 64 | (55–73) | 24 | ||
Table 4.
Descriptive statistics for 7 temporal parameters of swallowing.
| Parameter | Aspiration Classification |
Mean | 95% Confidence Interval |
Standard Deviation |
|
|---|---|---|---|---|---|
| Durations (ms) | Laryngeal Closure Duration | Non–aspirator | 555 | (510–599) | 217 |
| Aspirator | 578 | (465–690) | 296 | ||
| UES Opening Duration | Non–aspirator | 445 | (417–473) | 137 | |
| Aspirator | 395 | (336–453) | 153 | ||
| Hyoid Movement Duration | Non–aspirator | 1623 | (1472–1774) | 734 | |
| Aspirator | 1637 | (1354–1920) | 744 | ||
| Intervals (ms) | Laryngeal Closure to UES Opening | Non–aspirator | 3 | (−24–30) | 131 |
| Aspirator | 10 | (−33–54) | 114 | ||
| Pharyngeal Transit Time | Non–aspirator | 1358 | (1065–1652) | 1427 | |
| Aspirator | 949 | (751–1148) | 522 | ||
| Stage Transition Duration | Non–aspirator | 622 | (332–912) | 1408 | |
| Aspirator | 303 | (108–497) | 510 | ||
| Bolus Dwell Time | Non–aspirator | 1468 | (1170–1767) | 1447 | |
| Aspirator | 1122 | (887–1357) | 618 | ||
Kinematic Variables
Of the 6 hyoid excursion parameters studied, none showed significant differences between aspirators and non-aspirators at an alpha level of p<0.05. Maximum superior (Y) hyoid position showed a trend towards being lower in aspirating participants [F(1, 39.04) = 3.774, p = 0.059, Cohen’s d = 0.53, i.e., medium effect size]. None of the other hyoid parameters showed any differentiation between aspirators and non-aspirators under p < 0.1. There were no significant differences in kinematic variables across repeated boluses within participant and no interactions between aspiration status and the repeated measures factor.
Temporal Measures
Of the 7 temporal parameters studied, only one (UESD) showed discrimination potential between aspirators and non-aspirators in the form of a significant 2-way interaction between aspiration status and bolus-number-within-participant [F(4, 126.98) = 2.675, p = 0.035]. A main effect of bolus-number-within-participant was also significant for UESD [F(1, 126.98) = 7.61, p = 0.000]: longer UESD was seen for the 3rd and later boluses compared to the first bolus (Cohen’s d = 0.47, i.e., weak effect size). Post-hoc inspection of the data revealed that the extent to which UESD became progressively longer across successive boluses was much more marked in the aspirating participants (d = 1.45, i.e. strong effect) than in the non-aspirating participants (d = 0.41, i.e. weak effect). Effect sizes for the difference between aspirators and non-aspirators by bolus number ranged from strong (i.e. d = 0.93 for the first boluses to small (d = 0.45 and 0.5) for the 2nd and 3rd boluses, respectively and negligible (d = 0.05 and 0.11) for the fourth and fifth boluses per participant.
Discussion
The goal of this study was to investigate the independent association between 13 dependent physiological variables (including measures of swallowing kinematics and timing) and aspiration status, in patients who were given a series of up to 5 repeated presentations of teaspoon-sized volumes of ultra-thin liquid barium. Aspirators were defined as participants who had at least one PAS score of 3 or higher in their bolus series, whereas non-aspirators consistently demonstrated scores of 2 or better. It is important to note that aspiration-status was defined at the level of the participant rather than at the level of the swallow, similar to the approach taken in previous studies [3, 4, 8], but that every participant in the aspirator group had at least one swallow with a PAS score better than 3 (as shown in Table 2). Power and colleagues reported a similar finding [8], with 36.2% and 23.4% of their aspirating participants showing PAS scores ≥ 3 on only 1 or 2 out of 6 recorded swallows, respectively. The fact that aspiration may not be a consistent phenomenon across repeated swallows for a given patient underscores the importance of sampling more than one repetition per bolus condition to define a patient as an aspirator or non-aspirator. Although the definition of aspirator used both in our study and by others [8] is conservative (requiring only a single PAS score ≥ 3 to qualify for classification as an aspirator), this may also limit the extent to which kinematic analyses are likely to detect differences between groups, given that the swallows collected from the aspirator group contain examples that would be considered healthy.
With respect to swallow kinematics, we had hypothesized that aspirators would demonstrate reductions on measures of hyoid excursion. Previous literature demonstrates mixed findings in this respect. Bingjie and colleagues [3] reported reduced superior hyoid excursion in post-stroke aspirators compared with healthy controls, while Kim and McCullough [4] reported no significant difference in anterior or superior displacement of the hyoid between 10 post-stroke patients who aspirated and 31 post-stroke patients who did not. However, neither of these previous studies provides clear information on the rules used for classifying patients as aspirators versus non-aspirators. Further, neither of these studies employed scaling methodology to control for size-based variation in measures of hyoid excursion [13]. In general, our data found no difference in anatomically scaled measures of hyoid movement between aspirators and non-aspirators, with the exception of a trend (p=0.059) toward reduced maximal Y position of the hyoid in aspirators. This finding concurs with a previous report using anatomically scaled measures, which showed that significantly reduced hyoid excursion (below the first quartile boundary for the sample) was associated with aspiration compared to movement above that boundary [19]. It has recently been proposed that measurement of single point measures (e.g., maximal hyoid position) may be more reliable than displacement measures of hyoid movement (i.e., rest vs peak values) because this eliminates measurement error associated with selection of the rest frame [20]. Some support for this suggestion can be garnered from the present data, given that the only hyoid parameter showing predictive potential for aspirator status was a single point measure of maximum vertical position. This finding is of clinical importance and suggests that a threshold of approximately 80% of the C2–4 length scalar applied to measures of maximal Y hyoid position from the C4 origin (see Table 4) may divide safe from unsafe swallowing. Although further investigation with larger sample sizes will be required to confirm this finding, clinicians may want to consider interventions to improve the extent of hyoid movement in patients who display aspiration in conjunction with superior hyoid excursion below this threshold.
With respect to temporal measures of swallowing, we hypothesized that patients who aspirated would demonstrate reduced swallow durations and prolonged swallow intervals. A previous study exploring a number of temporal parameters found that patients who aspirated on thin and nectar-thick fluids showed longer durations of swallow response time, bolus dwell time and pharyngeal transit than non-aspirators, although the definition of aspirator status was viscosity-specific and more stringent than the one used in our study (requiring a score ≥ 6 on the PAS [21]. Of the 7 timing parameters studied, only UES opening duration discriminated between aspirators and non-aspirators. Aspirators displayed reductions in UES opening duration compared with non-aspirators, with this effect being most obvious on the first and second thin liquid boluses. This finding agrees with the study by Choi and colleagues [21], however it should be noted that UESD has been reported elsewhere to vary as a function of bolus volume [1] and in elderly patients compared to both age-matched and younger healthy controls for 1ml and 20ml liquid swallows [22]. In our study, UESD was found to be susceptible to variation across repeated boluses within participant and was also the variable showing the poorest inter-rater agreement during data processing. Both of these issues are theoretically likely to increase variability in a measure and lower the chances of a significant difference emerging between groups. In light of this, the significant find in our study and the concordance of findings across studies suggest that UES opening duration should be carefully examined when determining the reason for aspiration in a particular patient. We caution that reduced UESD is probably more likely to be associated with aspiration after the swallow, given that reduced UES opening duration is likely to leave post-swallow pharyngeal residue. Thus, it would be valuable for future studies to evaluate the direct association between UESD and aspiration at the level of the swallow, rather than the patient, taking into account the timing of the aspiration event relative to laryngeal closure and the contribution of post-swallow residue to the observed aspiration patterns.
It is interesting to note that despite well-established physiological dependencies between UES opening and anterior hyoid excursion (see for example, [23]), only one of these parameters was found to be predictive of aspiration when each parameter was tested in isolation. Other studies have also failed to demonstrate differences between aspirators and non-aspirators when individual parameters are analyzed in isolation [4, 6, 7]. Recent work by Power and colleagues [8] using discriminant analysis with temporal measures demonstrated that the best predictive models for aspiration might involve a combination of physiological parameters (pharyngeal transit time, swallow response time, and laryngeal closure duration). Unfortunately, our current study is under-powered to employ such statistical modeling (only 13/42 participants were classified as aspirators), however, we advocate for future research using discriminant analysis modeling techniques to include kinematic variables in addition to temporal variables.
There are several limitations to acknowledge with this study. First, this was a retrospective study conducted using an existing clinical dataset, and, as such, did not permit us to include examples of healthy volunteers (who were not referred for swallowing assessment) on the spectrum of aspirators and non-aspirators. Our sample (and aspirator status subgroups) lacked balance for sex, thereby limiting our ability to include this demographic parameter in the analysis. Further, strict volumetric control was not maintained in this retrospective clinical dataset, although all boluses included in this analysis were restricted to those administered by 5ml teaspoon, thus limiting the bolus volume to ≤ 5ml. Strict control of bolus volume would be particularly important for future investigations exploring direct relationships between kinematic measures and aspiration at the swallow level. It must also be acknowledged that certain choices in the design of this study and the selection of parameters for analysis may have limited our ability to find predictors of aspiration. Of these choices, the decision to classify aspiration status conservatively (i.e., PAS ≥ 3) and at the participant level may be most limiting. Unfortunately, our data set does not include a sufficient number of cases of PAS scores ≥ 6 to probe this further. Additionally, our protocol was limited to (up to) five ~ 5ml boluses of ultra-thin liquid per participant, excluding other volumes and textures. Certainly, alternative bolus conditions might have revealed impairment. As discussed, our analysis does not detail the timing of the aspiration event (i.e., before, during, or after the swallow) given the decision to classify aspiration status at the participant level; capturing such details would be important for future research. Finally, parameters that were not explored in this study may be important factors in determining the risk for aspiration, such as event sequencing, or the relationship between kinematics and residue. These were not studied, and we did not take into account the respiratory phase of the swallow and its possible relationship to aspiration.
Conclusions
In this retrospective sample of clinically-obtained videofluoroscopies comprised of patients swallowing up to 5 teaspoon-sized boluses of ultra-thin liquid barium, aspiration status was significantly independently associated with only one of thirteen physiological parameters tested: reduced duration of upper esophageal sphincter opening. A second parameter, of reduced maximum hyoid vertical position, showed a trend towards discriminating aspirators from non-aspirators. We advocate for further research using prospectively collected samples of patients with and without aspiration that address some of the limitations acknowledged here to guide clinicians and researchers to better understand the risks associated with various physiological parameters of swallowing (or combinations of parameters) and aspiration.
Acknowledgments
This work was a portion of the first author’s doctoral research for which she received funding from the Natural Sciences and Engineering Research Council (Canada) Create CARE program, the Ontario Student Opportunity Trust Fund and the Ontario Graduate Studies scholarship program. The second author holds a New Investigator award from the Canadian Institutes of Health Research. The authors would like to thank Sarah Hori, Chelsea Leigh and Clemence Tsang for assistance with data collection and analysis and acknowledge the support of Toronto Rehabilitation Institute who receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-term Care in Ontario. The views expressed do not necessarily reflect those of the ministry.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Molfenter SM, Steele CM. Variation in temporal measures of swallowing: Sex and volume effects. Dysphagia. 2013;28(2):226–233. doi: 10.1007/s00455-012-9437-6. [DOI] [PubMed] [Google Scholar]
- 2.Clavé P, De Kraa M, Arreola V, Girvent M, Farré R, Palomera E, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Alimentary Pharmacology and Therapeutics. 2006;24(9):1385–1394. doi: 10.1111/j.1365-2036.2006.03118.x. [DOI] [PubMed] [Google Scholar]
- 3.Bingjie L, Tong Z, Xinting S, Jianmin X, Guijun J. Quantitative videofluoroscopic analysis of penetration-aspiration in post-stroke patients. Neurol India. 2010 Jan-Feb;58(1):42–47. doi: 10.4103/0028-3886.60395. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, McCullough GH. Maximal Hyoid Excursion in Poststroke Patients. Dysphagia. 2010 Mar;25(1):20–25. doi: 10.1007/s00455-009-9224-1. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, McCullough GH. Stage transition duration in patients poststroke. Dysphagia. 2007;22(4):299–305. doi: 10.1007/s00455-007-9085-4. [DOI] [PubMed] [Google Scholar]
- 6.Park T, Kim Y, Ko D-, McCullough G. Initiation and duration of laryngeal closure during the pharyngeal swallow in post-stroke patients. Dysphagia. 2010;25(3):177–182. doi: 10.1007/s00455-009-9237-9. [DOI] [PubMed] [Google Scholar]
- 7.Power ML, Hamdy S, Singh S, Tyrrell PJ, Turnbull I, Thompson DG. Deglutitive laryngeal closure in stroke patients. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78(2):141–146. doi: 10.1136/jnnp.2006.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Power ML, Hamdy S, Goulermas JY, Tyrrell PJ, Turnbull I, Thompson DG. Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia. 2009;24(3):257–264. doi: 10.1007/s00455-008-9198-4. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 10.Fink TA, Ross JB. Are we testing a true thin liquid? Dysphagia. 2009;24(3):285–289. doi: 10.1007/s00455-008-9203-y. [DOI] [PubMed] [Google Scholar]
- 11.Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270–274. doi: 10.1007/s00455-006-9051-6. [DOI] [PubMed] [Google Scholar]
- 12.Allen JE, White CJ, Leonard RJ, Belafsky PC. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Dysphagia. 2010;25(4):347–348. doi: 10.1016/j.otohns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Molfenter SM, Steele CM. Teasing apart contributions to variability in hyoid movement in swallowing. Dysphagia. 2013 in press. [Google Scholar]
- 14.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: Single liquid swallows. J Appl Physiol. 2003;94(5):1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 15.Kang B, Oh B, Kim IS, Chung SG, Kim SJ, Han TR. Influence of Aging on Movement of the Hyoid Bone and Epiglottis during Normal Swallowing: A Motion Analysis. Gerontology. 2010;56(5):474–482. doi: 10.1159/000274517. [DOI] [PubMed] [Google Scholar]
- 16.Kendall KA, Leonard RJ, McKenzie SW. Sequence variability during hypopharyngeal bolus transit. Dysphagia. 2003;18(2):85–91. doi: 10.1007/s00455-002-0086-z. [DOI] [PubMed] [Google Scholar]
- 17.Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986. [Google Scholar]
- 18.Kotrlik J, Williams H. The Incorporation of Effect Size in Information Technology, Learning, and Performance Research. Inform Technol Learn Perform. 2003;21:1. [Google Scholar]
- 19.Steele CM, Bailey GL, Chau T, Molfenter SM, Oshalla M, Waito AA, et al. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36(1):30–36. doi: 10.1111/j.1749-4486.2010.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molfenter SM, Steele CM. Physiological variability in the deglutition literature: Hyoid and laryngeal kinematics. Dysphagia. 2011;26(1):67–74. doi: 10.1007/s00455-010-9309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi KH, Ryu JS, Kim MY, Kang JY, Yoo SD. Kinematic analysis of dysphagia: Significant parameters of aspiration related to bolus viscosity. Dysphagia. 2011;26(4):392–398. doi: 10.1007/s00455-011-9325-5. [DOI] [PubMed] [Google Scholar]
- 22.Kendall KA, Leonard RJ. Videofluoroscopic upper esophageal sphincter function in elderly dysphagic patients. Laryngoscope. 2002;112(2):332–337. doi: 10.1097/00005537-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97(6):1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]