Abstract
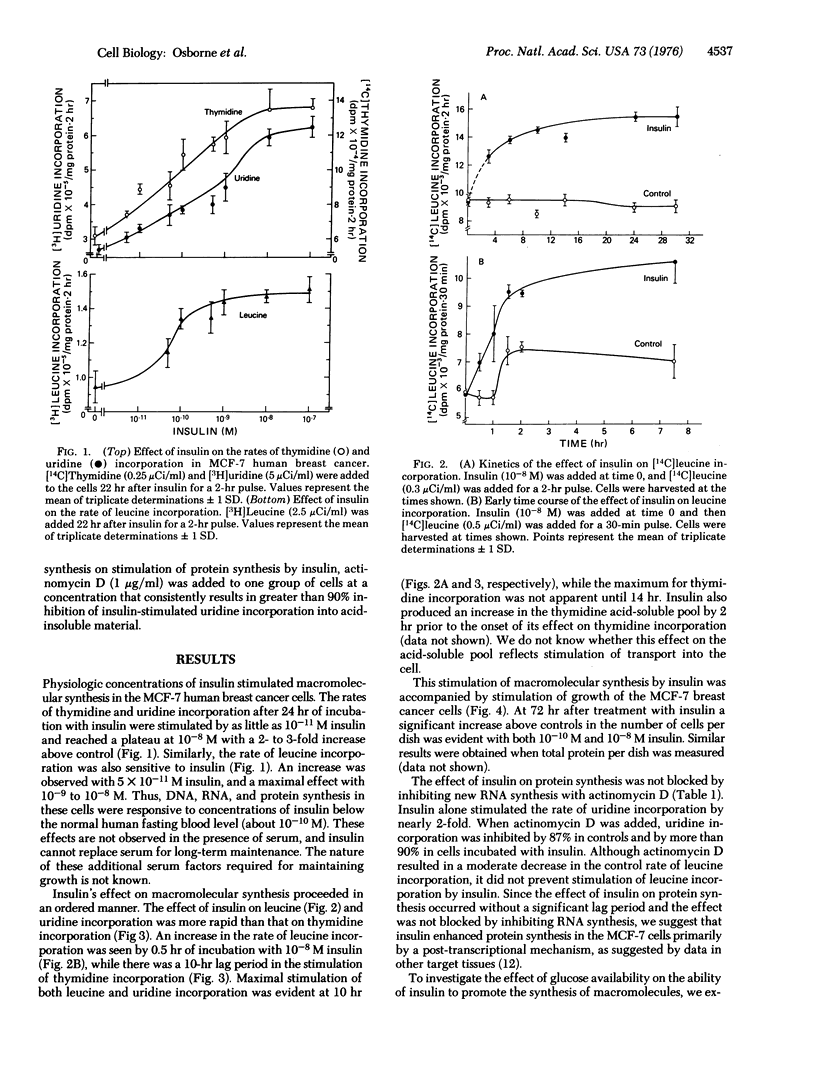
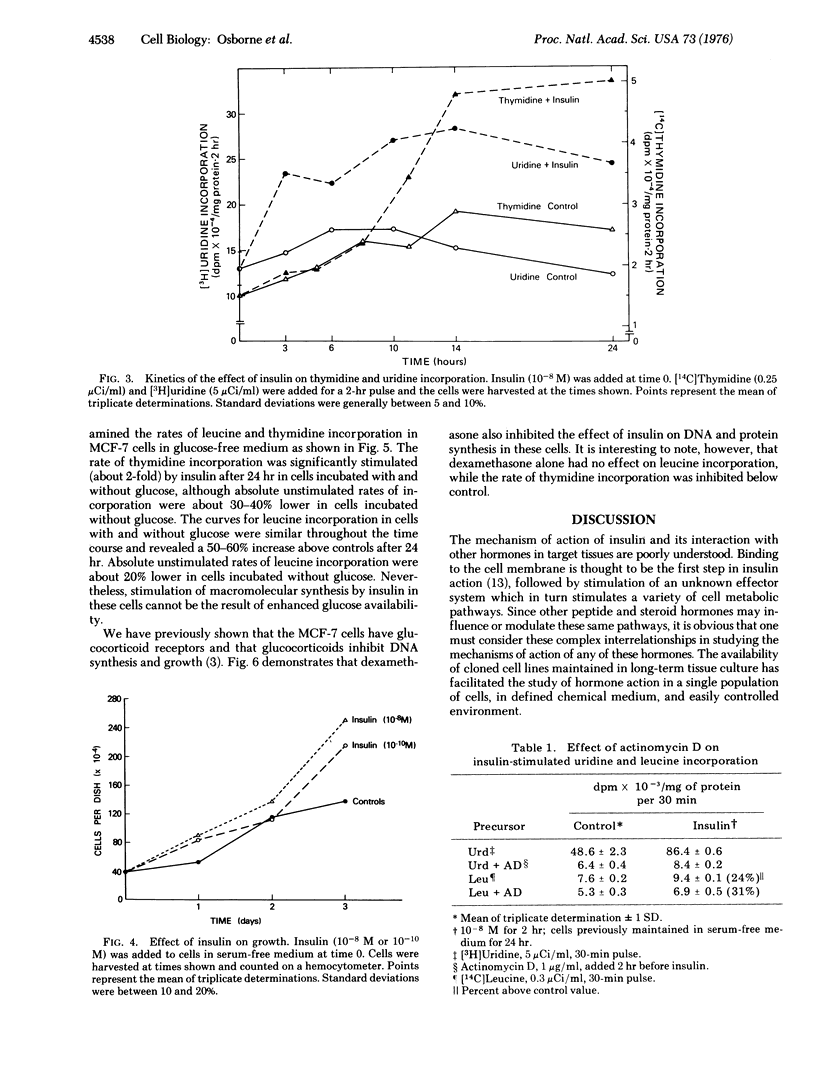
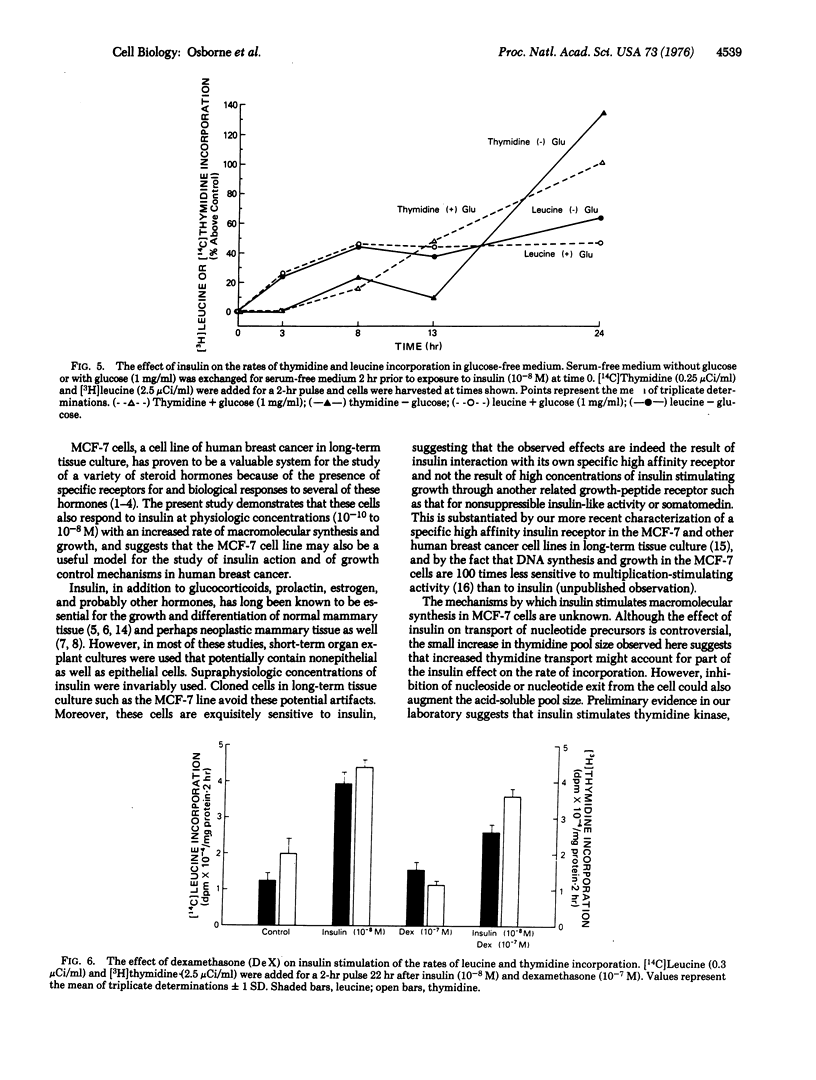
The mechanisms of steroid and peptide hormone action in human breast cancer are poorly understood. We have previously characterized a cell line of human breast cancer in long-term tissue culture that possesses various steroid hormone receptors and responses, providing a model for the study of steroid hormone action. The present studies describe a human breast cancer in vitro that responds to physiologie concentrations of insulin with an increased rate of macromolecular synthesis and growth. Thymidine and uridine incorporation in cells in serum-free medium are stimulated by 10(-11) M insulin and are maximal with 10(-8) M. Leucine incorporation is stimulated by 5 X 10(-11) M insulin and is maximal with 10(-9) M. Significant stimulation of uridine and leucine incorporation is evident by 3 hr and maximal by 10 hr. A 10-hr lag period exists for insulin stimulation of thymidine incorporation, which is maximal form 14 to 24 hr. The effect of 10(-8) M insulin on macromolecular synthesis is accompanied by a 69% increase above controls in the number of cells after 24 hr. The effect on macromolecular synthesis is observed in glucose-free medium. Insulin's effect on protein synthesis is not blocked by inhibition of RNA synthesis with actinomycin D. Glucocorticoids partially inhibit the action of insulin in these cells. This system provides a model for studying insulin action, and suggests that some human breast cancer may show growth regulation by insulin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks S. C., Locke E. R., Soule H. D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973 Sep 10;248(17):6251–6253. [PubMed] [Google Scholar]
- Ceriani R. L., Contesso G. P., Nataf B. M. Hormone requirement for growth and differentiation of the human mammary gland in organ culture. Cancer Res. 1972 Oct;32(10):2190–2196. [PubMed] [Google Scholar]
- Heuson J. C., Legros N., Heimann R. Influence of insulin administration on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in intact, oophorectomized, and hypophysectomized rats. Cancer Res. 1972 Feb;32(2):233–238. [PubMed] [Google Scholar]
- Heuson J. C., Legros N. Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer Res. 1972 Feb;32(2):226–232. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lippman M. E., Bolan G., Huff K. Human breast cancer responsive to androgen in long term tissue culture. Nature. 1975 Nov 27;258(5533):339–341. doi: 10.1038/258339a0. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature. 1975 Aug 14;256(5518):592–593. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- Manchester K. L. Effect of insulin on protein synthesis. Diabetes. 1972;21(2 Suppl):447–452. doi: 10.2337/diab.21.2.s447. [DOI] [PubMed] [Google Scholar]
- Rose H. N., McGrath C. M. Alpha-lactalbumin production in human mammary carcinoma. Science. 1975 Nov 14;190(4215):673–675. doi: 10.1126/science.1188362. [DOI] [PubMed] [Google Scholar]
- Skarda J., Green C. D., Aisbitt R. P., Barry J. M. Insulin does not stimulate DNA synthesis in cultured mammary tissue by enhancing glucose uptake. J Endocrinol. 1974 Jan;60(1):197–198. doi: 10.1677/joe.0.0600197. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Temin H. M. Purified multiplication-stimulating activity from rat liver cell conditioned medium: comparison of biological activities with calf serum, insulin, and somatomedin. J Cell Physiol. 1974 Oct;84(2):181–192. doi: 10.1002/jcp.1040840204. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- WOOL I. G., KRAHL M. E. Incorporation of C14-amino acids into protein of isolated diaphragms: an effect of insulin independent of glucose entry. Am J Physiol. 1959 May;196(5):961–964. doi: 10.1152/ajplegacy.1959.196.5.961. [DOI] [PubMed] [Google Scholar]
- Wool I. G. Relation of effects of insulin on amino acid transport and on protein synthesis. Fed Proc. 1965 Sep-Oct;24(5):1060–1070. [PubMed] [Google Scholar]
- Wool I. G., Stirewalt W. S., Kurihara K., Low R. B., Bailey P., Oyer D. Mode of action of insulin in the regulation of protein biosynthesis in muscle. Recent Prog Horm Res. 1968;24:139–213. doi: 10.1016/b978-1-4831-9827-9.50010-1. [DOI] [PubMed] [Google Scholar]


