Abstract
Purpose
Thalassemia, a chronic blood disease, necessitates life-long adherence to blood transfusions and chelation therapy to reduce iron overload. We examine stability of Health-Related Quality of Life (HRQOL) in thalassemia and adherence to chelation therapy over time, especially after changes in chelator choice.
Methods
Thalassemia Longitudinal Cohort participants in the US, UK, and Canada completed the SF-36v2 (ages 14+), and the PF-28 CHQ (parents of children<14 years). Chelation adherence was defined as self-reported percent of doses administered in the last 4 weeks.
Results
258 adults/adolescents (mean 29.7 years) and 133 children (mean 8.5 years) completed a mean of 2.8 years follow-up. Children made few chelator changes, whereas a mean of 2.2 changes was observed among the 37% of adults/adolescents who made chelator changes, mainly, due to patient preference or medical necessity. Physical HRQOL improved among those with lower iron burden (better health status) at baseline who made a single change in chelator, but declined among participants with multiple changes and/or high iron burden (worse health status). Mental health improved among participants with lower iron burden, but iron overload was negatively associated with social functioning. Adherence did not significantly change over follow-up except for an increase after a change from DFO infusion to oral deferasirox (p=0.03). Predictors of lower adherence for adults/adolescents at follow-up included side effects, smoking, younger age, problems preparing DFO, increased number of days per week DFO prescribed, and lower physical QOL.
Conclusions
Strategies to balance medical needs with family, work, and personal life may assist in adherence.
Keywords: iron chelation, adherence, quality of life, thalassemia
INTRODUCTION
Thalassemia is a congenital blood disorder that is typically managed with chronic blood transfusions, typically every 2–4 weeks, which leads to progressive iron overload. Daily iron chelation therapy is prescribed to mitigate the iron overload and attempt to prevent progressive organ failure (heart, endocrine, liver). Medical advances have led to increased survival in patients with thalassemia; however complications from both the disease and from the chronic treatments remain common. Life expectancy is directly related to the quality of chelation therapy, and poor adherence to treatment increases the risk of complications and death.
The two most common chelation methods involve 1) subcutaneous or intravenous infusion of a chelator (deferoxamine [also called desferal or DFO]) for 8–12 hours a day, 5 to 7 days a week; and 2) ingestion of a daily oral chelator (deferasirox) dissolved in 4–6 oz of fluid. Other chelator options exist as second-line therapy (oral deferiprone) and in clinical trials. Combinations of chelators are often used in cases of high iron overload. Many patients prefer the less invasive oral chelation, but choice of chelator and dose is based on a combination of patient preference and medical necessity. Patients sometimes switch from one chelator to another for a variety of reasons, including increasing iron overload (i.e., worsening health status), side effects, and personal preference. Patients may change chelators multiple times to find the best option to suit their personal and medical needs.
The iron levels of thalassemia patients are regularly monitored in order to determine current treatment needs. Serum ferritin is typically measured every few months; liver iron concentration by MRI, SQUID, or biopsy, and cardiac T2* by MRI are typically performed annually. Worsening iron burden necessitates more aggressive treatment, such as increased dosage, adding an additional chelator, or changing to a different form of chelation that may prove more effective for the individual patient. In the case of improved iron levels, dosage may be lowered and patient preference in choice of chelator can be better accommodated. However, all forms of treatment can be time consuming, inconvenient, and result in unpleasant physical side-effects, all of which could potentially impact emotional functioning of patients [1].
Health Related Quality of Life (HRQOL) is a multi-dimensional concept that includes domains related to physical, mental, emotional and social functioning, and focuses on the impact health status has on quality of life [1]. There is little published data on HRQOL in adult or pediatric thalassemia, which is surprising given that individuals with chronic medical conditions are vulnerable to experiencing emotional difficulties, such as symptoms of anxiety and depression. Promoting healthy emotional functioning is important not only to psychological well-being, but also to physical health as it may impact adherence to medical regimens. Previous reports of baseline data from the Thalassemia Longitudinal Cohort (TLC) of the Thalassemia Clinical Research Network (TCRN) found high self-reported adherence to chelation therapy (75–90%), with higher adherence to oral chelation and after a change in chelator [2]. Adherence was highest in children and older adults.
Overall, adults and adolescents (age 14+) in the TLC reported lower HRQOL compared to US norms [3]. Female gender, older age, and increased disease complications or side effects from chelation were independently associated with lower HRQOL. In addition, 32% of participants indicated experiencing significant symptoms of anxiety and 11% significant symptoms of depression [4]. Females and older patients were more likely to experience these symptoms, which were also associated with self-report of difficulty with adherence and negatively associated with HRQOL. Furthermore, parents of children <14 years also reported impaired HRQOL compared to US norms, with family life especially impacted [5]. Oral chelation and feelings of success with chelation appeared to improve parental assessments. Other studies have also found impaired HRQOL in thalassemia in Italy [6–8], the UK [9], and in a small US cohort [10]. Only a single small (N=43) recent study in Italy [11] has examined change in HRQOL in thalassemia, finding improvement over 8 years, associated with a shift in the distribution of chelator choice over the same time period.
The aim of this study was to examine the stability of HRQOL and adherence to iron chelation therapy over time, especially after changes in chelator choice. We expect that most patients will not show significant differences in HRQOL or adherence over the 1–4 years of follow-up. However, a subset of patients may have a clinically significant change, potentially due to demographic and clinical factors such as changes in transfusion frequency, method of chelation, complications, or side effects. Additionally, emotional symptoms may affect adherence to therapy. Understanding change in HRQOL over time, and the factors associated with those changes, will help add to our understanding of the role clinical factors play in HRQOL and help us to better identity individuals at risk and to develop ways to maximize HRQOL and adherence to chelation therapy in patients with thalassemia.
METHODS
The TCRN was an NIH/NHLBI sponsored network of 16 thalassemia centers in the US, UK, and Canada. TLC eligibility included patients of all thalassemia syndromes who required at least annual monitoring for end-organ injury related to thalassemia. Baseline assessments were conducted from 2007–2009, with annual follow-up ongoing in 2012. Data on thalassemia and its complications are collected using clinical histories, standard-of-care procedures, and patient questionnaires. The protocol was approved by the TCRN Data and Safety Monitoring Board and by the ethical review boards of all TCRN institutions. Informed consent was obtained for all participants.
Participants aged 14 and older were asked to complete the hospital anxiety and depression scale (HADS) [12], as well as the SF-36v2 health survey [13], which measures HRQOL in 8 subscales and two summary scores: physical component and mental component. The subscales capture broad domains of health status considered important for individuals suffering from disease: physical functioning (a range of severe to minor physical limitations), role-physical (physical limitations in work and other activities), bodily pain, general health, vitality (energy level and fatigue), social functioning (health-related effects on quantity and quality of social activities), role-emotional (mental health-related limitations in work and other activities), and mental health.
Parents of children <14 years at baseline were asked to complete the PF-28 child health questionnaire (CHQ) [14], which uses 12 subscales and two summary scores to measure physical health, psychosocial health and family impact. The subscales capture broad domains of health status considered important for children suffering from disease: physical functioning (a range of severe to minor physical limitations), role-physical (physical limitations in school and other activities), general health, bodily pain, parental impact-time (parental limitations in personal time), parental impact-emotional (parental distress), role-emotional/behavior (emotional or behavior-related limitations in school and other activities), self esteem, mental health, general behavior, family activities (level of disruption to family), and family cohesion.
Higher scores indicate higher HRQOL for the SF-36 and CHQ and more symptoms of anxiety and depression on the HADS. We defined a clinically significant change as a difference of at least 2 points on any subscale for HRQOL [15] and a difference of at least one standard deviation for the HADS. This minimal clinically important difference can be loosely defined as the smallest change that is important, or seen by the patient as an improvement/decline.
For all participants, transfusion frequency, chelation history, and iron burden were recorded from chart review. Chelation history included chelator type, dosing, and dates prescribed. Chelation adherence was defined as percent of doses administered in the last 4 weeks (patient report of chelator use as number of doses taken in the past week and month) out of those prescribed (chart review). Problems with chelation were reported on a 5-point Likert scale, with higher numbers indicating more problems. Problems included remembering, preparing/taking, sticking yourself and wearing the pump (deferoxamine only), side effects, and feeling successful. Iron burden was measured by serum ferritin, liver iron concentration (LIC by FerriScan, MRI, SQUID, or liver biopsy), and cardiac T2* MRI.
Statistical Analysis
Number of chelator changes during follow-up was categorized as none, one, and more than one. Baseline demographics, chelators, and iron burden were compared by number of chelator changes using analysis of variance (ANOVA) to test means of continuous variables, Kruskal-Wallis tests of medians of continuous variables, and χ2 and Fisher exact tests of distributions of categorical variables. Repeated measures ANOVA, assuming a compound symmetric variance structure, was used to test for differences in adherence rates between chelators, as well as linear trends over time. Paired t-tests were used to examine differences in adherence rates at the annual study visits before and after a change in chelator. Multivariate repeated measures ANCOVA with backwards elimination was used to model predictors of chelation adherence. Possible predictors were: age, gender, race, country, number of transfusions in past year, smoking in the past year (yes/no), consumption of five or more alcoholic drinks in a single day in the last year (yes/no), SF-36 bodily pain scale, SF-36 physical and mental component summaries, HADS anxiety and depression scales, problems with side effects, and, for chelation with DFO only, problems preparing, sticking themselves, wearing their pump, and number of days per week of use. Paired t-tests and a repeated measures model with an autoregressive(1) correlation structure were used to analyze changes in HRQOL. Analysis of covariance (ANCOVA) controlling for baseline HRQOL was performed to test for associations between changes in HRQOL scales with demographic and clinical variables.
RESULTS
258 adults/adolescents and 133 children completed baseline and some follow-up assessments. These 391 participants had a mean of 2.8 years of follow-up (Table 1), with 13% having 1 year, 28% 2 years, 26% 3 years, and 33% 4 years. The age range studied was 0–58, with a diversity of genders and races (mainly white and Asian). The majority were chronically transfused β-thalassemia patients chelating with oral deferasirox.
Table 1.
Baseline Demographics for the Thalassemia Longitudinal Cohort (TLC) (N=391a) by Changes in Chelator Choice over Follow-up.
| Children < 14 years | Adolescents and Adults (Age 14+) | ||||
|---|---|---|---|---|---|
|
| |||||
| All N=133 |
No Change in Chelator N=162 |
One Change in Chelator N=42 |
Multiple Changes in Chelator N=54 |
p-valueb | |
| Age (years), mean (SD), range | 8.5 (3.6), 0.4 – 13.8 | 30.5 (11.0), 14.2 – 58.2 | 28.3 (10.3), 14.3 – 54.8 | 28.6 (9.0), 14.7 – 49.7 | 0.32 |
| Gender, N (%) | 0.56 | ||||
| Male | 65 (48.9%) | 82 (50.6%) | 18 (42.9%) | 24 (44.4%) | |
| Female | 68 (51.1%) | 80 (49.4%) | 24 (57.1%) | 30 (55.6%) | |
| Race (self-report), N (%) | 0.31 | ||||
| White | 40 (30.1%) | 92 (59.0%) | 23 (56.1%) | 23 (42.6%) | |
| Asian | 83 (62.4%) | 57 (36.5%) | 16 (39.0%) | 27 (50.0%) | |
| Other | 10 (7.5%) | 7 (4.5%) | 2 (4.9%) | 4 (7.4%) | |
| Country, N (%) | 0.008 | ||||
| US | 96 (72.2%) | 118 (72.8%) | 22 (52.4%) | 46 (85.2%) | |
| Canada | 37 (27.8%) | 19 (11.7%) | 11 (26.2%) | 4 (7.4%) | |
| UK | 0 (0.0%) | 25 (15.4%) | 9 (21.4%) | 4 (7.4%) | |
| Thalassemia diagnosis, N (%) | 0.015d | ||||
| β-thal transfused 8+ times | 47 (65.3%) | 132 (81.5%) | 29 (69.1%) | 42 (77.8%) | |
| β-thal transfused <8 times | 2 (2.8%) | 12 (7.4%) | 7 (16.7%) | 1 (1.9%) | |
| β-thal not transfused | 2 (2.8%) | 2 (1.2%) | 1 (2.4%) | 0 (0.0%) | |
| E-β-thal transfused 8+ times | 12 (16.7%) | 10 (6.2%) | 3 (7.1%) | 9 (16.7%) | |
| E-β-thal transfused <8 | 2 (2.8%) | 4 (2.5%) | 1 (2.4%) | 1 (1.9%) | |
| E-β-thal not transfused | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | |
| HbH | 3 (4.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| HbH Constant Spring | 2 (2.8%) | 1 (0.6%) | 1 (2.4%) | 0 (0.0%) | |
| HbH Dartmouth | 2 (2.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Alpha-thalassemia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.9%) | |
| Chelation, N (%) | <0.001d | ||||
| None | 31 (23.3%) | 11 (6.8%) | 5 (11.9%) | 16 (11.1%) | |
| Deferoxamine (DFO infusion) | 31 (23.3%) | 23 (14.2%) | 17 (40.5%) | 14 (25.9%) | |
| Deferasirox (oral) | 67 (54.0%) | 106 (65.4%) | 11 (26.2%) | 21 (38.9%) | |
| Deferiprone (oral) | 0 (0.0%) | 4 (2.5%) | 3 (7.1%) | 3 (5.6%) | |
| Deferoxamine + Deferasirox | 3 (2.3%) | 5 (3.1%) | 2 (4.8%) | 3 (5.6%) | |
| Deferoxamine + Deferiprone | 1 (0.8%) | 13 (8.0%) | 4 (9.5%) | 7 (13.0%) | |
| Iron Burden | |||||
| Ferritin (ng/mL), median, rangec | 1005.8, 83.5 – 5397.8.0 | 1411.0, 179.9 – 12062.0 | 1131.8, 255.3 – 5555.7 | 2147.2, 76.0 – 18,453.5 | 0.024 |
| Ferritin ≥ 2500 ng/mL | 10/123 (8.1%) | 37/157 (23.6%) | 10/42 (23.8%) | 24/52 (46.2%) | 0.006 |
| Liver Iron Concentration (LIC mg/g dw), median, rangec | 10.0, 1.1 – 46.9 | 7.8, 0.4 – 67.9 | 10.1, 0.9 – 40.6 | 12.1, 1.1 – 38.0 | 0.37 |
| LIC ≥ 15 mg/g dw | 12/50 (24.0%) | 20/87 (23.0%) | 5/16 (31.3%) | 11/29 (37.9%) | 0.27 |
| Cardiac T2* (ms), mean (SD), rangec | 31.5 (9.6), 6.8 – 46.9 | 28.8 (14.0), 3.2 – 62.0 | 24.7 (15.0), 4.0 – 56.0 | 17.4 (11.8), 4.9 – 49.0 | <0.001 |
| T2* < 20 ms | 2/25 (8.0%) | 25/85 (29.4%) | 7/16 (43.8%) | 25/37 (67.6%) | <0.001 |
| Follow-up (years), mean, median, range | 2.79, 3, 1–4 | 2.72, 3, 1–4 | 2.43, 2, 1–4 | 3.35, 4, 1–4 | 0.002 |
| Chelator changes, mean, median, range | 0.68, 0, 0–5 | 0, 0, 0–0 | 1, 1, 1–1 | 3.07, 3, 2–8 | NA |
26 of 417 participants were excluded due to lack of follow-up.
Among participants aged 14+. Analysis of variance (ANOVA) to test means of continuous variables, Kruskal-Wallace test of medians of continuous variables, χ2 and Fisher exact tests of distributions of categorical variables.
average over past year. Ferritin≥2500, LIC≥15, or T2*<20 are considered to be iron overload in need of aggressive treatment.
due to the inadequate sample size of some categories, only the largest 3 categories were used in statistical testing.
Preliminary HRQOL and Chelation Adherence Findings
Figure 1 summarizes previously reported results for HRQOL [3–5] and adherence to chelation therapy [2] using cross-sectional baseline data. HRQOL on the physical summary scale is lower than the norm of 50 for children (CHQ parent-report) and adults (SF36 self-report), especially older adults. On the other hand, HRQOL on the mental summary scale is only low for adults. Chelation adherence is high among children, likely due to parental involvement. However, adherence declines in young adulthood, especially for the more invasive DFO infusion. Adherence is increased among older thalassemia patients, potentially reflecting both maturation and the higher mortality rate among lower adherers.
Fig. 1.
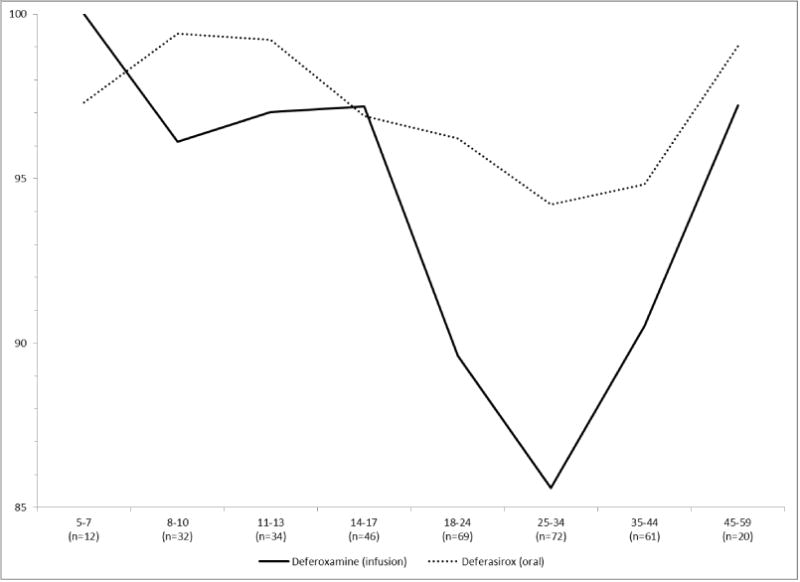
Summary of (a) HRQOL and (b) adherence over the lifespan of a thalassemia patient.
Changes in Chelator Choice over Follow-up
Few changes in chelators were made by children, and thus children were not divided into subcategories based on number of chelator changes (Table 1). Of the subgroup of 37% of adult/adolescent participants who made changes in chelators, we observed a mean of 2.2 changes (range 1–8, Table 1). Of those requiring more aggressive treatment due to high iron burden at baseline, defined as a mean ferritin over the past year of ≥ 2500 ng/mL, 48% changed chelators, with a mean of 2.4 chelator changes in this subgroup. Patients on DFO infusion at baseline were more likely to change chelators compared to those on oral deferasirox, as were patients with higher iron burden. Changes in chelator choice, as well as reasons for the changes, are examined in detail in Table 2. The most common changes were from DFO infusion to oral deferasirox and single chelator monotherapy to combination therapy. The most common reasons for changes were iron overload, patient preference, and participation in clinical trials. Of the participants with low baseline ferritin who made one chelator change, the most common change was from DFO infusion to oral deferasirox and due to the availability of a new commercial product (i.e., oral chelation by choice). This was in contrast to participants with high baseline ferritin who made one chelator change and those who made multiple changes, regardless of baseline iron status; these changes were most often to combination therapy for reasons of iron overload (i.e., medical necessity).
Table 2.
Changes in Chelator Choice and Reasons for Change in Adolescents/Adults (N=258).
| Chelation changes among participants with | a single change | multiple changes | ||
|---|---|---|---|---|
| Ferritin < 2500 ng/mLa N=32 |
Ferritin ≥ 2500 ng/mL N=10 |
Ferritin < 2500 ng/mLa N=28b |
Ferritin ≥ 2500 ng/mL N=24b |
|
| DFO Infusion to Oral Deferasirox | 8 (25%) | 2 (20%) | 14 (15%) | 7 (10%) |
| Oral Deferasirox to DFO Infusion | 3 (9%) | 0 (0%) | 14 (15%) | 9 (13%) |
| Single Chelator to Combination | 6 (19%) | 5 (50%) | 22 (23%) | 26 (37%) |
| Combination to Single Chelator | 5 (16%) | 1 (10%) | 17 (10%) | 14 (20%) |
| Any Chelation to No Chelation | 2 (6%) | 1 (10%) | 6 (6%) | 1 (1%) |
| No Chelation to Any Chelation | 5 (16%) | 0 (0%) | 8 (8%) | 2 (3%) |
| Other | 3 (9%) | 1 (10%) | 15 (15%) | 11 (15%) |
|
| ||||
| Reasons for change | ||||
|
| ||||
| High iron (requires more aggressive treatment) | 4 (17%) | 3 (33%) | 23 (29%) | 22 (40%) |
| Low iron (requires less aggressive treatment) | 2 (8%) | 0 (0%) | 8 (10%) | 1 (2%) |
| Participant or family decision | 4 (17%) | 1 (11%) | 13 (16%) | 6 (11%) |
| New commercial product available | 6 (25%) | 1 (11%) | 2 (3%) | 1 (2%) |
| Participation in a clinical trial | 1 (4%) | 2 (22%) | 14 (18%) | 12 (22%) |
| Illness or complication | 3 (12%) | 1 (11%) | 4 (5%) | 6 (11%) |
| Pregnancy or conception | 2 (8%) | 1 (11%) | 5 (6%) | 1 (2%) |
| Cardiac disease requiring intensification | 2 (8%) | 0 (0%) | 1 (1%) | 3 (5%) |
| Renal dysfunction | 0 (0%) | 0 (0%) | 4 (5%) | 0 (0%) |
| Insurance Issues | 0 (0%) | 0 (0%) | 5 (6%) | 0 (0%) |
| Improve Adherence | 0 (0%) | 0 (0%) | 0 (0%) | 3 (5%) |
includes participants without ferritin measurement at baseline. Categorization is as of baseline, and iron levels may have improved or declined over time.
96 chelator changes among 28 participants without high baseline ferritin; 70 chelator changes among 24 participants with high baseline ferritin.
Changes in Chelation Adherence over Follow-up
Self-reported adherence over the TLC study was high for all chelators (Table 3), and correlated significantly with iron levels. Adherence to oral deferasirox monotherapy was significantly higher than to DFO infusion (96% vs. 92%; p<0.001). Adherence to oral deferasirox on DFO/deferasirox combination therapy was lower than that of monotherapy (90% vs. 96%; p<0.001). Adherence to DFO infusion on DFO/deferiprone combination therapy was non-significantly lower than that of monotherapy or DFO/deferasirox combination therapy (88% vs. 92%; p=0.25). Adherence did not significantly change over follow-up except for an increase in adherence after a chelation change from DFO infusion to oral deferasirox (p=0.03, paired t-test). In qualitative manual examination of the reported adherence over time after this change in chelator, there was no evidence of a ‘honeymoon’ phase with temporary high adherence to the new chelator. In an open-ended comment section, many participants commented on the benefits of oral chelation and their improved adherence.
Table 3.
Adherence to Chelation in Adolescents/Adults.
| Chelation Adherence | ||
|---|---|---|
|
| ||
| Chelator | Na | % Adherenceb |
| Monotherapy | ||
| DFO (infusion) | 171 | 91.8 |
| Deferasirox (oral) | 494 | 96.2 |
| Deferiprone (oral) | 23 | 95.5 |
|
| ||
| DFO/Deferasirox combination therapy | ||
| DFO (infusion) | 49 | 91.2 |
| Deferasirox (oral) | 49 | 90.1 |
|
| ||
| DFO/Deferiprone combination therapy | ||
| DFO (infusion) | 80 | 87.8 |
| Deferiprone (oral) | 90 | 95.5 |
Sample size includes multiple observations per person over time. The same individual could be counted for different chelators if they changed chelators during the study.
Adherence to oral deferasirox was significantly higher than to DFO infusion (p<0.001, repeated measures ANOVA). Adherence to oral deferasirox on DFO/deferasirox combination therapy was lower than that of monotherapy (p<0.001). Adherence to DFO infusion on DFO/deferiprone combination therapy was non-significantly lower than that of monotherapy or DFO/deferasirox combination therapy (p=0.25).
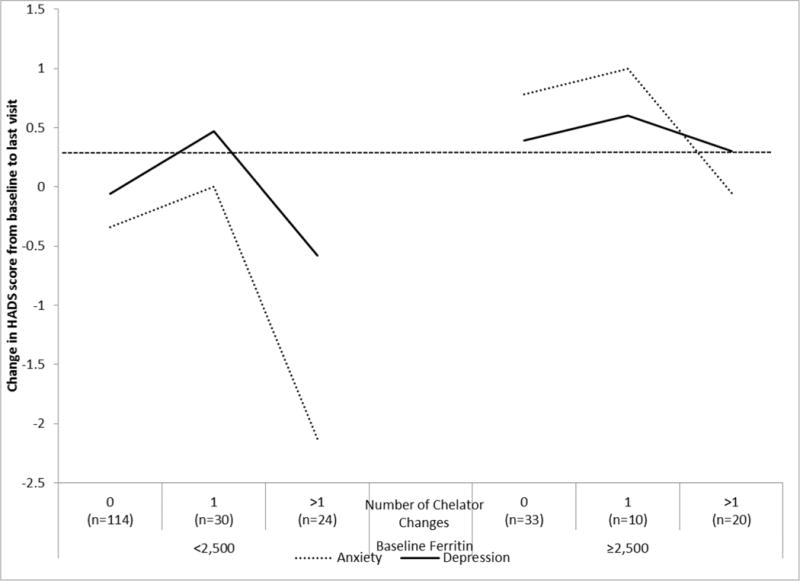
In longitudinal models of predictors of adherence for adults and adolescents (Table 4), smoking in the past year and increased problems with chelator side effects in the past year were independent predictors of decreased adherence to both DFO and deferasirox monotherapy. Insufficient sample size precluded analysis of predictors of other chelators, including combinations. Additional predictors of decreased adherence to DFO infusion were problems preparing the DFO and increased number of days per week prescribed. Additional predictors of increased adherence to oral deferasirox were older age and higher QOL (physical summary score). Chelator adherence was high for participants with low ferritin at baseline who made multiple changes in chelators (Figure 2a). Adherence to DFO infusion was low among participants with high ferritin at baseline, though small sample size precludes conclusions among those with chelator changes.
Table 4.
Predictors of monotherapy chelation adherence in adults and adolescentsa
| Predictors of deferoxamine (DFO infusion) adherence | Slope (SE) | p-value |
|---|---|---|
| Problems preparingb | −3.54 (1.40) | 0.013 |
| Problems with side effectsb | −2.10 (0.73) | 0.004 |
| Number of days per week | −2.36 (0.90) | 0.009 |
| Smoking in the past year | −11.70 (2.92) | <0.001 |
|
| ||
| Predictors of oral deferasirox adherence | Slope (SE) | p-value |
|
| ||
| Age | 0.14 (0.05) | 0.004 |
| Problems with side effectsb | −1.06 (0.39) | 0.007 |
| Smoking in the past year | −2.74 (1.37) | 0.046 |
| QOL physical component summaryc | 0.15 (0.06) | 0.018 |
predictors significant in bivariate analysis, controlling for age, at level 0.10 were entered into a multivariate analysis of covariance model with backwards elimination. For deferoxamine infusion adherence, predictors significant in bivariate analysis were gender, problems preparing their DFO, problems sticking themselves, problems wearing their pump, problems with side effects, number of days per week of deferoxamine use, smoking in the past year (yes/no), consumption of five or more alcoholic drinks in a single day in the last year (yes/no), SF-36 quality of life (QOL) mental component summary, and depression. For oral deferasirox adherence, predictors significant in bivariate analysis were age (quadratic effect), number of transfusions in the last year, problems with side effects, smoking in the past year (yes/no), bodily pain, SF-36 QOL physical and mental component summaries, anxiety, and depression.
problems measured on a 1–5 scale: never through a lot. Higher numbers indicate more problems.
Higher score indicates increased quality of life.
Abbreviations: SE=standard error
Fig. 2.
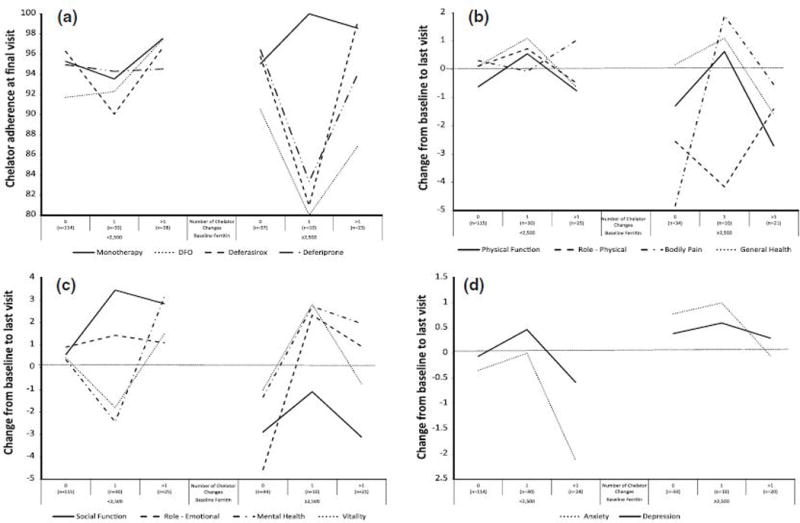
Adherence (a), change in SF-36 QOL physical (b) and mental scales (c), and HADS anxiety and depression (d) by number of chelator changes and baseline ferritin
Changes in HRQOL over Follow-up
In children, mean HRQOL remained stable over the study period. However on average each patient demonstrated a significant change in 8 of the 14 scales over the first year (Figure 3a). More changes were seen in the psychosocial health scales than in the physical health scales. General health, parental emotions and child behavior showed the most change. Increasing age was significantly associated with decreases in the psychosocial scales of the children as well as parental time. There was no significant association with gender, race, or clinical variables. Change in chelator was associated with a decrease in parental time and the psychosocial summary scale.
Fig. 3.
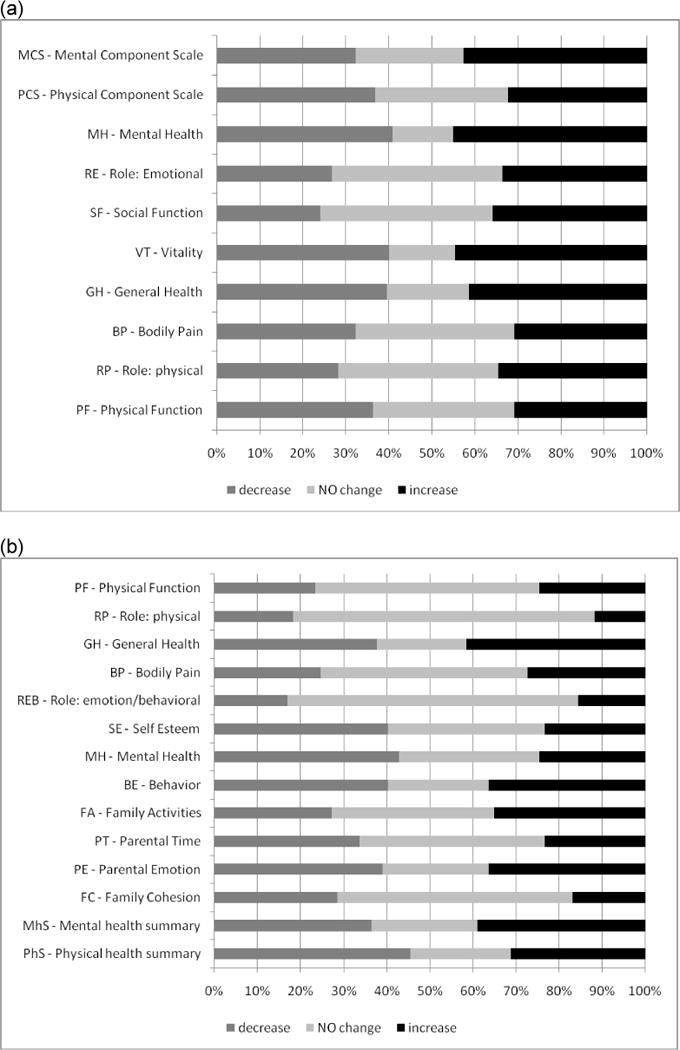
Percent of participants with clinically meaningful decrease (− 2 points), no change, or increase (+2 points) from baseline to 1-year follow-up in (a) SF-36 QOL scales for adults/adolescents (age 14+) and (b) CHQ QOL scales for children (<14 years)
In adults and adolescents, there were few population level changes in HRQOL over follow-up, but there were many individual changes in both directions. Mean SF-36 scores did not change significantly over a one year period, although social functioning showed an increase of 1.31 points (p=0.04). However all patients had a significant change in at least one SF-36 scale, with over 50% of patients reporting a significant increase or decrease in each domain (Figure 3b). 56% of patients reported their health was ‘about the same’, 14% somewhat worse, 19% somewhat better, and 11% much better, with 92% of patients reporting no new secondary complications. Gender, race, and change in number of transfusions were not significantly associated with change in HRQOL. Increasing age was significantly associated with decreased HRQOL in the following domains: physical function, bodily pain, vitality, social function, mental health, and physical summary scales, although only bodily pain showed a clinically significant change (+2.1 points/decade). A decrease in reported side effects from chelation was associated with an improvement in physical functioning (+2.8) and the mental summary score (+3.48). Development of a new complication was associated with a decrease in emotional role (−8.2). Finally, an increase in reported adherence was associated with a decrease in social functioning (−5.1), role emotional (−7.7) and the mental summary score (−5.4).
In comparison of HRQOL in adults/adolescents by number of chelator changes and baseline ferritin (Figure 2b,c), among those with low baseline ferritin, HRQOL on the physical scales improved among those who made a single change in chelator, declined among those with multiple changes, and remained stable among those with no change in chelators (Figure 2b). The changes in HRQOL were almost clinically significant (2 point difference) for the role physical scale. In contrast, HRQOL on this same scale (role physical) declined among participants with high ferritin. HRQOL on the other scales (physical functioning and general health) also declined among participants with high ferritin and multiple chelator changes. In terms of mental HRQOL, there was a modest improvement in role emotional among those with lower iron burden at baseline. Social functioning improved greatly with changes in chelators, but vitality and mental health declined among those who made a single change. Mental health was improved, however, among those with multiple changes. Among those with high iron burden at baseline, social functioning was impacted, as was role emotional for those who did not change chelator.
Changes in Anxiety and Depression over Follow-up
There was a significant time effect on anxiety (p=0.021) with anxiety dropping about one-quarter of a point per year, with no significant time effect for depression (p=0.62). For anxiety, there was a significant time interaction with age; subjects less than 18 years old experienced greater yearly decreases in anxiety as compared to subjects aged 18 or older. There were few clinically significant changes in anxiety or depression over a year of follow-up: 7.7% experienced increased and 12.7% decreased symptoms of anxiety, with 5.6% and 7.4% for depression. Anxiety was especially decreased among those with low baseline ferritin and multiple changes in chelators (Figure 2d).
DISCUSSION
Changes in chelator choice appear to have the potential for improvement in adherence and quality of life, although chelator change can have the opposite effect as well. Changing chelators was generally due to iron overload or patient preference. As expected, patients with high iron burden were more likely to be switching chelators based on medical necessity compared to patients with low ferritin, who were more likely to be switching based on personal preference. Those with multiple changes were also more likely to have done so due to medical necessity. Significant country differences are not well understood, but may relate to the availability of deferiprone. Intermittently transfused patients were more likely than chronically transfused patients to have exactly one switch as they do not require continual chelation.
Both iron overload and preference in chelator can be related to ability to adhere. As expected, there was little population level change in adherence or HRQOL over time. However, examination of subsets based on chelator changes and baseline ferritin was informative in identifying subpopulations with improvements or declines.
Subsets with improved adherence
Consistent with baseline findings from this study [2], self-reported adherence over time was high for all chelators, with higher adherence to oral chelators. As higher adherence was found among those who made multiple switches in chelators, patients may be seeking a routine that are able to adhere to, may better feel that they are in control of their medial regimen, or may understand better the need to adhere for medical reasons. The only case in which adherence significantly changed over follow-up was after a chelation change from DFO infusion to oral deferasirox; because a change in this direction is rarely due to medical necessity, we can conclude that changes in chelator choice appear to have the potential for improvement in adherence and that a change to oral chelation by choice is associated with improved self-reported adherence. Because oral deferasirox only became commercially available in the U.S. in 2006, a proportion of patients were certainly still switching from DFO infusion as the only previously available chelator to oral deferasirox after the start of the TLC in 2007.
Increased adherence was also associated with older age (maturity) and higher QOL on the physical component. Better physical component scores is complicated because it suggests that patients are better able to tend to the burden of their chelation routines, but non-adherence often leads to complications of iron overload that cause lower physical functioning. In the baseline study [2], bodily pain, a component of the physical component QOL scale, was associated with lower adherence to oral deferasirox and thus may help explain the direction of the current association. This requires further investigation that is beyond the focus of this study.
Subsets with declined adherence
Not surprisingly, problems preparing DFO and side effects of both chelator regimens were associated with lower adherence. Increased number of days per week of required DFO infusion was also negatively associated with adherence to this more burdensome regimen. Likewise, adherence to combination therapy was often lower than to single chelator monotherapy. These findings are concerning given that combination therapy and additional days for DFO infusion are sometimes prescribed due to prior non-adherence to therapy and the resultant iron overload. Physicians should be aware that prescription of additional therapy can be associated with declined adherence and counsel patients accordingly about the medical necessity for such prescriptions. Strategies for maintaining balance with a busy work and family life could be useful as well. Consistent with previously reported baseline results [2], smoking was an independent predictor of decreased adherence, suggestive of poor health decision-making overall.
Subsets with improved HRQOL
Change in chelators were positively associated with social functioning, as was. mental health with multiple chelator changes; perhaps patients were able to find and settle into a regimen that works for them. Additionally, changing from a chelator with unpleasant side effects may have played a role as decreased reports of chelator side effects were associated with improved quality of life.
Subsets with declined HRQOL
Although change in chelators can have a positive impact on social functioning, it was also associated with declined mental health. Although difficult to interpret, it is possible that the effect may have been transient as patients adjust to their new chelation regimen. One could suppose that patients with concerns either begin to feel successful with their new chelator long-term or they choose to switch back. This same effect was seen in children, with change in chelator associated with a decrease in parental time and the psychosocial summary scale. Again, this change could be temporary, as parents also need to adjust to a new routine.
Unfortunately, an increase in reported chelation adherence was associated with a decrease in social functioning and mental health. This is perhaps not surprising as use of chelators is disruptive to daily life. On the other hand, while lower chelation adherence may benefit quality of life in the short term, it will also likely lead to long-term consequences of greater iron overload that could drastically affect lifestyle. In fact, development of new complications, which is often due to inadequate long-term chelation adherence, was associated with declined quality of life. Additionally, among those with high iron burden, which may result from inadequate chelation, social functioning was lower, as well as time for work and other activities (role physical).
Conclusions
In this large international, racially diverse cohort across a range of ages, we found few changes in chelation adherence or quality of life at the population level. However, changes in both directions were evident in subsets of the population. As this broad initial study considered only change in chelator at any point, rather than specific timing relative to quality of life measures, it would be worth looking in more detail at annual changes and the timing relative to chelator changes, which might help sort out causation among the potentially complex bidirectional relationships in this study. Additionally, use of a convenience sample may have introduced selection bias toward more compliant patients, and an observational cohort may introduce confounding due to unmeasured factors. Further multivariate modelling with inclusion of potential confounders is warranted. A generic measure of HRQOL may have also missed factors specific to thalassemia and further study of newly developed thalassemia-specific HRQOL instruments is suggested. Furthermore, although self-reported adherence may underestimate true adherence, the significant correlation with iron levels was reassuring. The high adherence reported in this study for some participants could also reflect concurrent enrollment in chelator clinical trials (e.g., Novartis) and the subsequent emphasis on adherence and close monitoring. Future study should include additional validated measures of adherence beyond the 4-week recall considered here.
Change to oral chelation appeared especially beneficial to both adherence and quality of life in general, but some patients may experience conflicted feelings about trying something new. In order to maximize adherence, patients may benefit not only from counseling about the medical necessity of chelation, but also discussion of the trade-off between short-term and long-term quality of life. Although chelation may be difficult to maintain in the face of a busy lifestyle, inadequate chelation can lead to considerably poorer quality of life later on. Strategies to help balance medical needs with family, work, and personal life may assist in adherence. Thalassemia patients sometimes change chelators multiple times to find the best option to suit their personal and medical needs. It is important for providers to monitor the effect on quality of life and adherence in order to maximize health outcomes.
Acknowledgments
This work was supported by the following NIH-NHLBI cooperative agreements: U01-HL65232 and NIH/NCRR UL1-RR-024134 to the Children’s Hospital of Philadelphia, U01-HL72291 and by Harvard Catalyst CTSC U-01RR025758 to Children’s Hospital, Boston, U01-HL65233 to University Health Network Toronto General Hospital, UL1RR024131-01to Children’s Hospital & Research Center Oakland, U01-HL65244 and CTSC UL1-RR024996 to Weill Medical College of Cornell University, and U01-HL65238 to New England Research Institutes. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI.
Appendix 1
The following institutions and researchers contributed to the Thalassemia Clinical Research Network Thalassemia Longitudinal Cohort data reported in this paper.
Children’s Hospital, Boston: Ellis Neufeld, MD, PhD, Principal Investigator, Jennifer Braunstein, NP, Research Nurse, Amber Smith, Study Coordinator, Latoya Lashley, Study Coordinator; Satellite: University of Texas Southwestern Medical Center at Dallas, Charles Quinn, MD, MS, Principal Investigator, Deborah Boger, RN, MSN, PNP, Study Coordinator, Leah Adix, Study Coordinator, Sandra Richardson, Study Coordinator; Children’s Healthcare of Atlanta, Jeanne Boudreaux, MD, Principal Investigator, Leann Hassen, Study Coordinator; Baylor College of Medicine, Brigitta Mueller, MD, Principal Investigator, Bogden Dino, Study Coordinator. Weill Medical College of Cornell University: Patricia Giardina, MD, Principal Investigator, Dorothy Kleinert, RN, Research Nurse; Satellite: Winthrop University Hospital, Mark Weinblatt, MD, Principal Investigator, Linda Skelly, Study Coordinator. The Children’s Hospital of Philadelphia: Janet Kwiatkowski, MD, Principal Investigator, Marie Martin, RN, Research Nurse, Sage Green, Study Coordinator; Satellite: Children’s Memorial Hospital, Chicago, IL, Alexis Thompson, MD, Principal Investigator, Janice Beatty, RN, Research Nurse, Diane Calamaras, RN, CPNP, Research Nurse, Pauline Hess, study coordinator. Children’s Hospital at Oakland: Elliott Vichinsky, MD, Principal Investigator, Dru Foote, NP, Research Nurse, Nancy Sweeters, Study Coordinator, Olivia Vega, Study Coordinator; Satellites: Children’s Hospital of Los Angeles, Thomas Coates, MD, Principal Investigator, Susan Carson, RN, Research Nurse, Eun Ha Pang, Study Coordinator, Rachna Khanna, Study Coordinator; Stanford Hospital, Michael Jeng, MD, Principal Investigator, Kokil Bakshi, Clinical Research Associate; Children’s and Women’s Health Center of British Columbia, John Wu, Principal Investigator, Heather McCartney, RN, Research Nurse, Colleen Fitzgerald, Study Coordinator, Stephanie Badour, Study Coordinator. Toronto General Hospital, Toronto, Ontario, Canada: Nancy F. Olivieri, MD, Principal Investigator, Vivek Thayalasuthan, Study Coordinator; Satellite: Hospital for Sick Children, Isaac Odame, MD, Principal Investigator, Manuela Merelles-Pulcini, RN, Study Coordinator. University College London, John Porter, MD, Principal Investigator, Cindy Bhagwandin, Study Coordinator; Satellite: Whittington Hospital, Farrukh Shah, MD, Principal Investigator. NHLBI oversight, Kathryn Hassell, MD. Data Coordinating Center: New England Research Institutes, Sonja McKinlay, PhD, Principal Investigator, Lisa Virzi, RN, MS, MBA, Project Director, Felicia Trachtenberg, PhD, Senior Statistician.
References
- 1.U.S. Department of Health and Human Services. Healthy People 2020. Washington, DC: Office of Disease Prevention and Health Promotion. Available at http://www.healthypeople.gov/2020/about/qolwbabout.aspx. Accessed 1/30/14. [Google Scholar]
- 2.Trachtenberg F, et al. Iron chelation adherence to deferoxamine and deferasirox in thalassemia. Am J Hematol. 2011;86(5):433–6. doi: 10.1002/ajh.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobota A, et al. Quality of life in thalassemia: a comparison of SF-36 results from the thalassemia longitudinal cohort to reported literature and the US norms. Am J Hematol. 2011;86(1):92–5. doi: 10.1002/ajh.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mednick L, et al. Symptoms of depression and anxiety in patients with thalassemia: prevalence and correlates in the thalassemia longitudinal cohort. Am J Hematol. 2010;85(10):802–5. doi: 10.1002/ajh.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita R, Sobota A, Trachtenberg F, Xu Y, Quinn CT, Vichinsky EP, Haines D, Kleinert DA, Schilling L, Pakbaz Z, Boudreaux J, Odame I, Thompson AA, Giardina PJ, Kwiatkowski JL, Neufeld EJ. The Impact of the Child with Thalassemia on the Family: Parental Assessments. 2013 in press. [Google Scholar]
- 6.Messina G, et al. Psychosocial aspects and psychiatric disorders in young adult with thalassemia major. Intern Emerg Med. 2008;3(4):339–43. doi: 10.1007/s11739-008-0166-7. [DOI] [PubMed] [Google Scholar]
- 7.Scalone L, et al. Costs, quality of life, treatment satisfaction and compliance in patients with beta-thalassemia major undergoing iron chelation therapy: the ITHACA study. Curr Med Res Opin. 2008;24(7):1905–17. doi: 10.1185/03007990802160834. [DOI] [PubMed] [Google Scholar]
- 8.La Nasa G, et al. Long-term health-related quality of life evaluated more than 20 years after hematopoietic stem cell transplantation for thalassemia. Blood. 2013;122(13):2262–70. doi: 10.1182/blood-2013-05-502658. [DOI] [PubMed] [Google Scholar]
- 9.Payne KA, et al. Iron chelation therapy: clinical effectiveness, economic burden and quality of life in patients with iron overload. Adv Ther. 2008;25(8):725–42. doi: 10.1007/s12325-008-0085-z. [DOI] [PubMed] [Google Scholar]
- 10.Payne KA, et al. Clinical and economic burden of infused iron chelation therapy in the United States. Transfusion. 2007;47(10):1820–9. doi: 10.1111/j.1537-2995.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 11.Gollo G, et al. Changes in the quality of life of people with thalassemia major between 2001 and 2009. Patient Prefer Adherence. 2013;7:231–6. doi: 10.2147/PPA.S42133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snaith R, Zigmond A. The Hospital Anxiety and Depression Scale Manual. London, UK: nferNelson; 1994. [Google Scholar]
- 13.Ware JE, et al. User’s Manual for the SF-36v2 Health Survey. 2. Lincoln, RI: Quality Metric Incorporated; 2007. [Google Scholar]
- 14.Landgraf JM, Abetz L, Ware JE. Third Printing. Lincoln, RI: Quality Metric Incorporated; 2006. Child Health Questionnaire (CHQ): A User’s Manual. [Google Scholar]
- 15.Cook CE. Clinimetrics Corner: The Minimal Clinically Important Change Score (MCID): A Necessary Pretense. J Man Manip Ther. 2008;16(4):E82–3. doi: 10.1179/jmt.2008.16.4.82E. [DOI] [PMC free article] [PubMed] [Google Scholar]


