Abstract
IMPORTANCE
Antidepressant medication (ADM) is efficacious in the treatment of depression, but not all patients achieve remission and fewer still achieve recovery with ADM alone.
OBJECTIVE
To determine the effects of combining cognitive therapy (CT) with ADM vs ADM alone on remission and recovery in major depressive disorder (MDD).
DESIGN, SETTING, AND PARTICIPANTS
A total of 452 adult outpatients with chronic or recurrent MDD participated in a trial conducted in research clinics at 3 university medical centers in the United States. The patients were randomly assigned to ADM treatment alone or CT combined with ADM treatment. Treatment was continued for up to 42 months until recovery was achieved.
INTERVENTIONS
Antidepressant medication with or without CT.
MAIN OUTCOMES AND MEASURES
Blind evaluations of recovery with a modified version of the 17-item Hamilton Rating Scale for Depression and the Longitudinal Interval Follow-up Evaluation.
RESULTS
Combined treatment enhanced the rate of recovery vs treatment with ADM alone (72.6% vs 62.5%; t451 = 2.45; P = .01; hazard ratio [HR], 1.33; 95% CI, 1.06–1.68; number needed to treat [NNT], 10; 95% CI, 5–72). This effect was conditioned on interactions with severity (t451 = 1.97; P = .05; NNT, 5) and chronicity (χ2 = 7.46; P = .02; NNT, 6) such that the advantage for combined treatment was limited to patients with severe, nonchronic MDD (81.3% vs 51.7%; n = 146; t145 = 3.96; P = .001; HR, 2.34; 95% CI, 1.54–3.57; NNT, 3; 95% CI, 2–5). Fewer patients dropped out of combined treatment vs ADM treatment alone (18.9% vs 26.8%; t451 = −2.04; P = .04; HR, 0.66; 95% CI, 0.45–0.98). Remission rates did not differ significantly either as a main effect of treatment or as an interaction with severity or chronicity. Patients with comorbid Axis II disorders took longer to recover than did patients without comorbid Axis II disorders regardless of the condition (P = .01). Patients who received combined treatment reported fewer serious adverse events than did patients who received ADMs alone (49 vs 71; P = .02), largely because they experienced less time in an MDD episode.
CONCLUSIONS AND RELEVANCE
Cognitive therapy combined with ADM treatment enhances the rates of recovery from MDD relative to ADMs alone, with the effect limited to patients with severe, nonchronic depression.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00057577
There is a growing consensus1 that simply reducing depressive symptoms (response) is not sufficient and that full normalization (remission) should be the goal of acute treatment. Practitioners are encouraged to switch or augment treatments until remission is achieved or all reasonable alternatives have been exhausted. Sustained remission (recovery) is better still, and it is recommended2 that patients in remission continue to receive treatment until they pass the period of risk for relapse.
Antidepressant medication (ADM) is the most common treatment for depression3 and is especially recommended for patients whose condition is more severe.4 One-third of all patients will achieve remission with any given ADM, but half of these patients will experience relapse during continuation treatment before they achieve recovery.5 Cognitive therapy (CT) is as efficacious as ADM alone,6 and combining the 2 increases response rates, with estimates of the increase ranging from 6% to 33%.7–11
Most randomized clinical trials do not reflect the aims of personalized medicine. Randomized clinical trials usually test a single ADM delivered for a brief duration, whereas patients in clinical practice can receive treatment for as long as necessary with whatever medications are required to yield the desired result.12 Similarly, CT is delivered in a brief time-limited format in most randomized clinical trials, even though patients with comorbid Axis II disorders need longer treatment.13 Studies in which practitioners are not permitted to adapt treatment to meet the needs of the patient likely underestimate what could be achieved using the best clinical practice.14 We sought to determine whether combining CT with ADM enhances recovery when treatment is personalized.
Methods
Patients
The study was conducted at outpatient clinics at the University of Pennsylvania, Philadelphia; Rush Medical Center, Chicago, Illinois; and Vanderbilt University, Nashville, Tennessee. Institutional review boards at the respective institutions approved the protocol, and an independent data safety monitoring board monitored study implementation. Participants were recruited from persons who sought treatment at the clinics in these institutions. Written informed consent was obtained prior to any research activity. Participants received financial compensation for completing the assessments but not for the treatment. The Structured Clinical Interviews for DSM-IV diagnosis (Axis I and Axis II) were used to establish diagnostic eligibility.15,16
The sample comprised 452 adult outpatients. Inclusion criteria were (1) DSM-IV major depressive disorder (MDD)17 either chronic (episode duration ≥ 2 years) or recurrent (with an episode in the past 3 years if only the second episode), (2) 17-item Hamilton Rating Scale for Depression (HRSD) score of 14 or more, (3) age 18 years or older, (4) English speaking, and (5) willing and able to provide informed consent. Exclusion criteria were (1) history of bipolar disorder or nonaffective psychosis, (2) substance dependence in the past 3 months, (3) DSM-IV Axis I disorders requiring nonprotocol treatment, (4) DSM-IV Axis II disorders poorly suited to study treatments (antisocial, borderline, and schizotypal), (5) suicide risk requiring immediate hospitalization, (6) medical condition precluding the use of study medications (including pregnancy), (7) current medications that induce depression, or (8) mandated treatment or compensation issues.
Procedures
Figure 1 depicts the study design and patient flow. The sample size was set to detect differences of 15% or greater (α = .05; β = 0.20) based on previous findings.18 A total of 2097 potential participants were screened in person or by telephone; 1178 were invited for diagnostic interviews. Of those, 452 patients met all entry criteria and were randomly assigned (1:1 ratio) to receive ADM alone (n = 225) or ADM plus CT (n = 227). The project statistician (R.G.) generated randomization schedules for each site stratified on sex, marital status, symptom severity, recurrence, chronicity, and comorbid Axis II disorder. Project coordinators at each site were able to access these assignments only after each patient was screened into the project and provided informed consent. Intake ran from July 24, 2002, through February 22, 2006; the last patient completed continuation treatment in July 2009. (A 3-year follow-up will be reported.)
Figure 1.
Consolidated Standards for Reporting of Trials Diagram of Patient Flow Through the Study
MDD indicates major depressive disorder; SCID, Structured Clinical Interview for DSM-IV.15
Acute treatment lasted until the patient met the criteria for remission, defined as 4 consecutive weeks of minimal symptoms; continuation treatment lasted to the point of recovery, defined as another 26 consecutive weeks without relapse. Patients did not need to maintain the symptom levels required for remission to meet the criteria for recovery. Participants who experienced relapse during continuation were required to meet remission criteria again before they were eligible to meet the criteria for recovery. Patients who did not meet the symptomatic criteria for remission within 18 months of treatment were removed from the study and referred for other treatment, as were patients who did not meet criteria for recovery within 36 months. Patients who met only the symptomatic criterion for remission at month 18 (or recovery at month 36) continued treatment until it was determined whether they also met the temporal criteria. Thus, up to 19 months were allowed for remission and up to 42 months for recovery.
Measures
The 17-item HRSD,19 modified to include increases in sleep, appetite, and weight,20 was used to assess depression severity. The Longitudinal Interval Follow-up Evaluation (LIFE) was used to provide retrospective assessments of diagnostic status across time.21 Both instruments were conducted at least biweekly through week 4, every 4 weeks through week 20 of acute treatment, and every 8 weeks thereafter through the end of continuation treatment. Trained interviewers blind to treatment condition conducted the evaluations. All evaluations were recorded, and a subset was rated across sites to establish interrater reliability. An intraclass correlation coefficient of 0.96 was obtained for the 17-item total HRSD score (n = 24); major depressive episode designation on the LIFE scale yielded a κ value of 0.80 (n = 12).22
Outcome Criteria
Full remission was defined as HRSD scores of 8 or less and LIFE ratings of 2 or less for 4 consecutive weeks. After month 12, these criteria were relaxed such that 4 weeks of HRSD scores of 12 or lower or LIFE ratings of 3 or lower were sufficient to meet the criteria for partial remission. Relapse was defined as 2 consecutive weeks of HRSD scores of 16 or more or LIFE scores of 5 or more. Serious adverse events (SAEs) were reported to the respective institutional review boards and to the data safety monitoring board as they occurred. Serious adverse events were defined as any untoward event that compromised the patient’s health including death for any reason, suicide attempt, psychiatric or medical hospitalization, and pregnancy or motor vehicle crash while receiving study medications.
Treatment Procedures
Pharmacotherapy
A principle-based algorithm was implemented that could involve up to 4 different classes of ADMs and any of the augmenting or adjunctive agents commonly used in clinical practice. Dosages were raised as rapidly as possible and kept at maximum tolerated levels for at least 4 weeks. Treatment in patients who exhibited only a partial response was augmented with additional medications, and treatment in those who showed minimal response (or little additional response following augmentation) was switched to another ADM. Most patients were given multiple trials with easier-to-manage selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors before treatment was switched to more difficult-to-manage tricyclic antidepressants or monoamine oxidase inhibitors. Patients who experienced remission usually received the same medications during treatment continuation, but the prescribing practitioners were free to adjust the doses and augment or switch medications as needed to forestall relapse. The goal was to provide personalized antidepressant therapy using the best clinical practice. These principles were followed in both treatment conditions. A detailed account of the medications used is beyond the scope of this article and will be subsequently reported.
The protocol called for patients to meet with their prescribing practitioner weekly for the first month, biweekly thereafter during acute treatment, and monthly during continuation. The initial session lasted 30 to 45 minutes, with subsequent sessions approximately 20 minutes. Ten board-certified psychiatrists and 7 psychiatric nurse practitioners with prescribing privileges provided pharmacotherapy (including J.D.A. and J.Z.). Sessions followed the protocol developed by Fawcett and colleagues23 for the Treatment of Depression Collaborative Research Program. Dr Fawcett oversaw the training and provided consultation throughout the study. Three of us served as the medical directors and provided supervision at the respective sites (J.D.A., R.C.S., and J.Z.). Pharmacotherapy sessions focused on (1) medication management including education about medications, dosage schedules, and adverse effects; and (2) clinical management, including a review of the patient’s functioning in major life spheres and brief supportive counseling.
Cognitive Therapy
Twelve doctoral-level psychologists, 1 psychiatrist, and 1 nurse practitioner provided CT (including P.R.Y.). The therapists met weekly for 90 minutes at each site to review cases, with on-site supervision provided by 3 of the authors (R.J.D., P.R.Y., and S.D.H.). The therapists followed the procedures outlined in the original treatment manual for CT of depression,24 augmented when indicated for patients with comorbid Axis II disorders.25 The protocol called for 50-minute sessions to be held twice weekly for at least the first 2 weeks, at least weekly thereafter during acute treatment, and then at least monthly during continuation. Therapists were free to vary the session frequency to meet the needs of the patient.
Statistical Analysis
Survival analyses were used to model treatment outcomes. In conventional survival analyses, censoring because of attrition is assumed to be unrelated to treatment or patient characteristics and therefore independent of time to the event.26 However, when attrition precludes the occurrence of the event, as it did in this trial, it is a competing risk that can bias estimates of the time to remission or recovery.27 We therefore adopted the subdistribution hazard model developed by Fine and Gray28 to account for the possible nonindependence of the censoring mechanism. The weighted partial likelihood estimation directly assesses the intervention and moderation effects for the target event even in the presence of a competing and possibly informative relationship between multiple competing events. The basic model included main effects for site, treatment, and their interaction. Main effects and treatment interactions for each of the stratification variables were estimated in the full models and retained in the final models only if significant. All models were implemented in SAS, version 9.3 (SAS Institute Inc) using the algorithm developed by Zhang and Zhang29 for the subdistribution hazard model. Significance was determined using 2-tailed, unpaired t tests. To characterize the clinical significance of the findings, we computed the number needed to treat (NNT) ratio, a metric used in evidence-based medicine to estimate the number of persons who would need to receive the intervention to produce 1 additional positive outcome.30 Mantel-Haenszel χ2 analysis was used to test for treatment differences in the frequency of relapses and SAEs.
Results
Baseline Characteristics
A total of 452 patients were randomized: 151 at the University of Pennsylvania, 151 at Rush University, and 150 at Vanderbilt University. Baseline HRSD score means did not differ significantly as a function of treatment condition or site (overall mean [SD], 22.1 [4.2]; range, 14–33). The Table gives descriptive statistics for the baseline variables. No significant differences between the conditions were observed in these variables, but there were some significant between-site differences.
Attrition and Termination
Of the randomized patients, 102 (22.6%) did not complete treatment: 95 dropped out and 7 were withdrawn by the staff (excessive substance use, 5; manic episode, 2). Attrition was nearly twice as likely to occur during acute treatment (n = 67) than during continuation (n = 35). Attrition rates were lower in the ADM plus CT group than in the ADM-alone group (18.9% vs 26.8%; t451 = −2.04; P = .04; hazard ratio [HR], 0.66; 95% CI, 0.45–0.98). Patients with Axis II disorders were more likely to drop out irrespective of their condition (27.4% vs 18.4%; t451 = 2.09; P = .04). Patients who did not achieve remission by month 18 (n = 28) or recovery by month 36 (n = 17) were terminated from the study. Termination rates did not differ significantly by condition (ADM plus CT, 8.8%; ADM alone, 11.1%; χ1 = 0.62; P = .43).
Remission
Remission rates were high and did not differ significantly as a function of treatment (full remission of 63.6% for ADM plus CT vs 60.3% for ADM alone by month 12; t451 = 0.57; P = .58; and full or partial remission of 80.1% for ADM plus CT vs 77.2% for ADM alone by month 18; t451= 0.87; P = .38). Median time to remission was shorter with ADM plus CT than with ADM alone (week 33 vs week 38), but this difference also was not significant. Patients in the ADM plus CT group evidenced fewer relapses than did patients in the ADM-alone group (71 relapses in 48 patients vs 80 relapses in 54 patients, respectively), but this difference was not significant.
Recovery
Recovery rates were higher with ADM plus CT than with ADM alone (72.6% vs 62.5%; t451 = 2.45; P = .01; HR, 1.33; 95% CI, 1.06–1.68; NNT, 10; 95% CI, 5–72) and lower for patients with vs those without comorbid Axis II disorders irrespective of treatment condition (61.2% vs 73.5%; t451 = 2.81; P = .01; HR, 1.40; 95% CI, 1.11–1.77). The main effect of treatment on recovery was conditioned on interactions with severity (t451 = 1.97; P = .05; NNT, 5) and chronicity (χ2 = 7.46; P = .02; NNT, 6). There were no other significant main effects or interactions of treatment condition with the other stratification variables or with site (all P > .05).
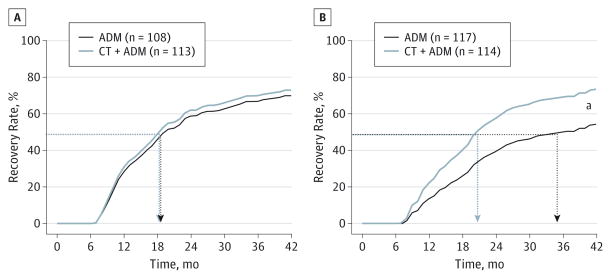
Figure 2 displays the severity by treatment interaction. Recovery rates for patients with low-severity MDD (intake HRSD median, <22) were similar in the 2 conditions (72.9% vs 69.8% [n = 221]; t220 = 0.58; P = .56; HR, 1.10; 95% CI, 0.80–1.51; NNT, 32; 95% CI, 7–211). For patients with high-severity MDD, the rate was higher with ADM plus CT compared with ADM alone (73.4% vs 54.3% [n = 231]; t230 = 3.25; P = .001; HR, 1.71; 95% CI, 1.24–2.37; NNT, 5; 95% CI, 3–15). Patients with nonchronic MDD also evidenced a higher recovery rate with ADM plus CT compared with ADM alone (76.7% vs 59.2%; n = 280; t279 = 3.47; P = .001; HR, 1.69; 95% CI, 1.26–2.27; NNT, 6; 95% CI, 4–15). No significant difference was observed in patients with chronic MDD (63.2% with CT plus ADM vs 70.2% with ADM alone; n = 172; t171 = −1.07; P = .28; HR, 0.82; 95% CI, 0.57–1.18; NNT, −14.08; 95%, CI, −21 to −5).
Figure 2.
Time to Recovery as a Function of Severity by Condition
Recovery was defined as 6 months without relapse following remission. A, Low-severity major depressive disorder (MDD), defined as an HRSD score of less than 22 at intake. B, High-severity MDD, defined as an HRSD score of 22 or greater at intake. ADM indicates antidepressant medication; CT+ ADM, cognitive therapy combined with ADM; HRSD, Hamilton Rating Scale for Depression; and dashed lines, median time to recovery (50th percentile).
aP < .001.
We followed up this pattern of findings with an investigation of the relationship between the 2 moderators in their effect on treatment. The test of the 3-way interaction (severity by chronicity by treatment) did not indicate a significant difference, but it was grossly underpowered.31 We therefore conducted an exploratory analysis to determine whether severity and chronicity contributed independently to the increments observed or whether the effects of each depended on the other. We divided the sample into 4 subgroups defined by severity and chronicity and obtained a significant 4 (subgroup) × 2 (treatment) interaction (χ3, 10.41; P = .02). In both low-severity subgroups, as well as in the high-severity chronic subgroup, small, nonsignificant treatment effects were obtained (P = .32, P = .42, and P = .92, respectively). In the nonchronic severe subgroup, the difference in recovery rate between ADM plus CT (n = 71) and ADM alone (n = 75) was large and significant (81.3% vs 51.7%; n = 146; t145 = 3.96; P = .001; HR, 2.34; 95% CI, 1.54–3.57; NNT, 3; 95% CI, 2–5) and remained so after Bonferroni correction. Figure 3 depicts treatment effects on recovery, conditioned on chronicity, among patients with more severe depression.
Figure 3.

Time to Recovery as a Function of Chronicity by Condition Within High Severity
Recovery was defined as 6 months without relapse following remission. A, High-severity chronic major depressive disorder (MDD), defined as an HRSD score of greater than 22 at intake and episode duration of 2 years or more. B, High-severity nonchronic MDD, defined as an HRSD score of 22 or greater at intake and episode duration of less than 2 years. ADM indicates antidepressant medication; CT+ ADM, cognitive therapy combined with ADM; HRSD, Hamilton Rating Scale for Depression; and dashed lines, median time to recovery (50th percentile).
aP < .001.
Safety
Patients experienced fewer SAEs with ADM plus CT compared with ADM alone (49 vs 71; χ1 = 5.76; P = .02). The largest categories were psychiatric hospitalization (19 vs 29) and medical hospitalization (22 vs 31). Seven patients made suicide attempts: 3 in the ADM plus CT group (twice by 1 person) and 4 in the ADM-alone group. There were no completed suicides. There was no significant difference in the rate at which those SAEs occurred once time to recovery was taken into account.
Discussion
Combining CT with ADM enhanced the rate of recovery compared with ADM alone in a sample of patients with chronic or recurrent nonpsychotic MDD and minimal exclusions for other psychiatric and medical comorbidities. The modest (10%) increment observed is low in the range of comparable trials7–10 but similar to the one other study11 that also followed a more flexible medication algorithm. Doing so may leave little room for CT to enhance recovery.
The magnitude of this increment nearly doubled for patients with more severe depression or nonchronic MDD episodes, but there was little evidence of benefit for patients with less severe or chronic MDD. These findings are consistent with those from earlier trials. Thase and colleagues32 found that patients with severe recurrent depression were particularly likely to benefit from combined treatment relative to psychotherapy alone, and Kocsis and colleagues11 found no advantage for combined treatment relative to algorithm-guided treatment among patients with chronic depression. In the present study, exploratory analyses suggested that this increment was larger still in the one-third of the patients with MDD that was both more severe and nonchronic. Patients with chronic depression and those with nonchronic and less severe depression (each approximately one-third of the sample) showed evidence of little increment from combining CT with ADM. It may be that only patients with more severe MDD need CT to be added to ADM and that those with chronic MDD are unable to benefit from its addition.
Moderators identified in the present investigation could be used prescriptively to guide a more efficient allocation of treatment resources.33 Given the higher cost of combined treatment, it might be reserved for patients with nonchronic, more severe depression. Such a recommendation would be consistent with the goals of personalized medicine; patients are given what they most need, and costly resources are reserved for those likely to benefit from them. Patients with comorbid Axis II disorders evidenced higher rates of attrition and lower rates of recovery than did those without comorbid Axis II disorders irrespective of treatment condition. We had hoped that using a version of CT adapted to the specific needs of such patients would boost response, but clearly more needs to be done.25 Patients who received the combined treatment experienced fewer SAEs (including hospitalizations) but largely because they spent less time in MDD episodes.34 The fact that 7 patients made suicide attempts and that 48 were hospitalized for psychiatric reasons indicates that we were providing therapy for clinically representative patients.14
The study has strengths and limitations. Treating MDD to a fixed outcome rather than for a fixed duration and following a principle-driven algorithm rather than limiting the medications used is more representative of clinical practice than is the typical approach used in randomized clinical trials. Limitations include (1) the exclusion of patients with nonchronic first-episode MDD, which precluded the opportunity to test for interactions involving chronicity and recurrence; (2) the absence of another psychotherapy or psychotherapy control, in combination with medications, to test for the specificity of CT in accounting for the combined treatment advantage; (3) the absence of a psychotherapy-only condition, which limits the generalizability of the findings to patients receiving CT with concurrent ADM; (4) the lack of blinding for patients and treatment providers to the condition, which may have contributed to the superiority of combined treatment; and (5) the lack of a formal cost-benefit analysis.
Moderation always implies differential mediation.35 Our findings suggest that CT engages different mechanisms than ADM but that it likely does so only in some patients. Identifying these mechanisms may suggest ways to enhance treatment response. Future combinatorial trials should include comparisons with CT alone to examine the viability of each monotherapy, especially given evidence that CT effects persist beyond the end of treatment.36
Conclusions
Cognitive therapy combined with medication treatment enhanced rates of recovery relative to medications alone, with the effect limited to patients with severe nonchronic depressions. Combined treatment also reduced the frequency of severe adverse events, but largely because it reduced time in episode.
Table.
Baseline Characteristicsa
| Characteristic | No. (%) of Patients | |||||
|---|---|---|---|---|---|---|
| Total (N = 452) | Pennsylvania (n = 151) | Vanderbilt (n = 150) | Rush (n = 151) | Combined Therapy (n = 227) | ADM (n = 225) | |
| HRSD score, mean (SD) | 22.1 (4.2) | 21.9 (4.3) | 22.3 (4.3) | 22.0 (4.0) | 21.9 (4.0) | 22.2 (4.4) |
| Female sex | 266 (58.8) | 75 (49.7)* | 96 (64.0)† | 95 (62.9)† | 130 (57.3) | 136 (60.4) |
| Age, mean (SD), y | 43.2 (13.1) | 45.8 (13.9)† | 44.4 (12.3)† | 39.2 (12.2)* | 43.3 (12.9) | 43.0 (13.4) |
| Race/ethnicity | ||||||
| White | 388 (85.8) | 127 (84.1) | 130 (86.7) | 131 (86.8) | 194 (85.5) | 194 (86.2) |
| Hispanic | 27 (6.0) | 6 (4.0)* | 6 (4.0)* | 15 (9.9)† | 17 (7.5) | 10 (4.4) |
| College graduate | 218 (48.2) | 86 (57.0)† | 57 (38.0)* | 75 (49.7)* | 118 (52.0) | 100 (44.4) |
| Income <$40 000/y | 265 (58.6) | 86 (57.0) | 95 (63.3) | 84 (55.6) | 137 (60.4) | 128 (56.9) |
| Married or cohabitating | 168 (37.2) | 53 (35.1) | 59 (39.3) | 56 (37.1) | 85 (37.4) | 83 (36.9) |
| Unemployed | 140 (31.0) | 47 (31.1) | 48 (32.0) | 42 (27.8) | 77 (33.9) | 61 (27.1) |
| Chronic MDDb | 159 (35.2) | 53 (35.1)† | 88 (58.7)‡ | 18 (11.2)* | 78 (34.4) | 81 (36.0) |
| Recurrent MDDb | 376 (83.2) | 124 (82.1)*† | 120 (80.0)* | 132 (87.4)† | 190 (83.7) | 186 (82.7) |
| Age at onset, mean (SD), y | 23.8 (12.7) | 22.0 (13.1)* | 24.0 (13.4)*† | 25.5 (11.3)† | 24.6 (13.0) | 23.0 (12.4) |
| No. of episodes, mean (SD) | 7.8 (18.0) | 6.8 (11.4)* | 3.4 (6.2)* | 13.2 (27.5)† | 7.4 (16.7) | 8.2 (19.3) |
| Prior ADM | 303 (67.0) | 100 (66.2) | 104 (69.3) | 100 (66.2) | 152 (67.0) | 151 (67.1) |
| Melancholicb | 179 (39.6) | 48 (31.8)* | 69 (46.0)† | 62 (41.0)*† | 88 (38.8) | 91 (40.4) |
| Atypicalb | 96 (21.2) | 30 (19.9) | 38 (25.3) | 28 (18.5) | 47 (20.7) | 49 (21.8) |
| Other Axis I disorderb | 226 (50.0) | 63 (41.7)* | 90 (60.0)† | 76 (50.3)*† | 114 (50.2) | 115 (51.1) |
| Any Axis II disorderb | 225 (49.8) | 53 (35.1)* | 79 (52.7)† | 93 (61.6)† | 113 (49.8) | 112 (49.8) |
Abbreviations: ADM, antidepressant medication; HSRD, Hamilton Rating Scale for Depression; MDD, major depressive disorder; Pennsylvania, University of Pennsylvania; Rush, Rush University; Vanderbilt, Vanderbilt University.
When all 3 sites differ from one another, they each have a different symbol: lowest (*), intermediate (†), and highest (‡). When the lowest site differs from the other 2 sites and they do not differ from one another: lowest (*) and each of the 2 highest (†). When the 2 lowest sites do not differ from each other but each differs from the highest: each of the 2 lowest (*) and highest (†). When the lowest site differs from the highest site but the intermediate site does not differ from either of the other 2 sites: lowest (*), intermediate (*†), and highest (†).
According to DSM-IV criteria.
Acknowledgments
Funding/Support: This study was supported by grants MH60713 and MH01697 (K02) (Dr Hollon), MH60998 (Dr DeRubeis), and MH060768 (Drs Fawcett and Zajecka) from the National Institute of Mental Health. Wyeth Pharmaceuticals provided venlafaxine, and Pfizer Inc provided sertraline for the trial.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Additional Information: Robert J. DeRubeis, PhD (University of Pennsylvania), Jan Fawcett, MD (University of New Mexico), and Steven D. Hollon, PhD (Vanderbilt University), were the principal investigators, and Jay D. Amsterdam, MD (University of Pennsylvania), John Zajecka, MD (Rush University), and Richard C. Shelton, MD (Vanderbilt University), were the coprincipal investigators. Drs DeRubeis and Hollon oversaw the implementation of CT at University of Pennsylvania and Vanderbilt University, respectively, and Dr Young did the same at Rush University. Dr Fawcett oversaw the implementation of pharmacotherapy across the study, and Drs Amsterdam, Zajecka, and Shelton supervised the implementation of pharmacotherapy at the respective sites.
Author Contributions: Drs Hollon and DeRubeis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hollon, DeRubeis, Fawcett, Amsterdam, Shelton, Zajecka.
Acquisition, analysis, or interpretation of data: Hollon, DeRubeis, Fawcett, Amsterdam, Shelton, Young, Gallop.
Drafting of the manuscript: Hollon, DeRubeis, Amsterdam, Zajecka, Gallop.
Critical revision of the manuscript for important intellectual content: Hollon, DeRubeis, Fawcett, Amsterdam, Shelton, Zajecka, Young.
Statistical analysis: Hollon, DeRubeis, Zajecka, Gallop.
Obtained funding: Hollon, DeRubeis, Fawcett, Amsterdam, Shelton, Zajecka.
Administrative, technical, or material support: Hollon, DeRubeis, Fawcett, Amsterdam, Shelton, Zajecka, Young.
Study supervision: Hollon, DeRubeis, Fawcett, Amsterdam, Zajecka, Young.
Additional Contributions: Brent Freeman, BA, and Bernadette Kooi, MS (University of Pennsylvania), Debra Klbecka, RN, and Matthew Marasco, BA (Rush University), and Margaret L. Lovett, MEd (Vanderbilt University), served as the study coordinators. Giampaolo Gallo, MD, Moira Molloy, MSN, Bobbie Posmontier, PhD, Nancy Rutherford, MSN, Irene Soeller, CRNP, Jeffrey Staab, MD, and Jay D. Amsterdam, MD (University of Pennsylvania), Jagannath Devulapally, MD, Corey Goldstein, MD, Ian Mackey, MD, William Miles, MD, Raj Tummale, MD, and John Zajecka, MD (Rush University), and Virginia Gardner, MSN, Jennifer Scroggie, MSN, Sandra Seidel, MSN, and Vatsal Thakkar, MD (Vanderbilt University), served as study pharmacotherapists. Julie Jacobs, PhD, Cory P. Newman, PhD, and Rita Ryan, PhD (University of Pennsylvania), David C. Clark, PhD, Kristen Flynn, PhD, John Larson, MD, Patricia Meaden, PhD, Chad Owen, PsyD, and Paula R. Young, PhD (Rush University), and Laurel L. Brown, PhD, Kirsten Haman, PhD, Karl N. Jannasch, PhD, Sandra Seidel, MSN, and Dorothy D. Tucker, PhD (Vanderbilt University), served as the cognitive therapists. Kirsten L. Haman, PhD, oversaw the training of the clinical interviewers. All of the above were reimbursed for their participation. William T. McKinney, MD (Northwestern University [emeritus]), Irene Elkin, PhD (University of Chicago [emerita]), Robert Gibbons, PhD, and Mark Siegler, MD (University of Chicago), and Burt Jensen (Federal Bureau of Investigation [retired]) served on the independent data safety monitoring board. Members of the board received an honorarium for their participation. DatStat developed the data entry and patient management system used to conduct the study.
Conflict of Interest Disclosures: Dr Shelton reports being a consultant to Bristol-Myers Squibb, Cerecor, Inc, Cyberonics, Inc, Forest Pharmaceuticals, Janssen Pharmaceutica, Medtronic, Inc, Naurex, Inc, Pamlab, Pfizer Inc, Ridge Diagnostics, Shire Plc, and Takeda Pharmaceuticals and receiving grant or research support from Assurex Health, Bristol-Myers Squibb, Elan Corp, Forest Pharmaceuticals, Janssen Pharmaceutica, Jazz Pharmaceuticals, Naurex, Inc, Novartis Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab, and Takeda Pharmaceuticals. Dr Zajecka reports receiving grant or research support from Alkermes, Allergan, AstraZeneca, Cyberonics, Euthymics, ElMindA, Forest Pharmaceuticals, the Cheryl T. Herman Foundation, Hoffman-LaRoche, Naurex, Inc, Otsuka, the National Institutes of Health, Shire Plc, and Takeda Pharmaceuticals; serving as a consultant or on the advisory board of Abbvie, Avanir (Depression Data Safety Monitoring Board), Eli Lilly & Company, Forest Pharmaceuticals, Lundbeck, Pamlab, Shire Plc, and Takeda Pharmaceuticals; and receiving other financial support from the Cheryl T. Herman Foundation. No other disclosures were reported.
References
- 1.Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Kraemer HC, Sackeim HA, et al. ACNP Task Force. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 3.Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265–1273. doi: 10.1001/archgenpsychiatry.2010.151. [DOI] [PubMed] [Google Scholar]
- 4.Psychiatry Online. Practice guideline for the treatment of patients with major depressive disorder: 3rd rev. Washington, DC: American Psychiatric Association; 2010. [Accessed October 9, 2012]. American Psychiatric Association Practice Guidelines. http:www.psychiatryonline.com/pracGuide/pracGuideTopic_7.aspx. [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163 (11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 6.Hollon SD, Jarrett RB, Nierenberg AA, Thase ME, Trivedi M, Rush AJ. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455–468. doi: 10.4088/jcp.v66n0408. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn IM, Bishop S, Glen AI, Whalley LJ, Christie JE. The efficacy of cognitive therapy in depression: a treatment trial using cognitive therapy and pharmacotherapy, each alone and in combination. Br J Psychiatry. 1981;139:181–189. doi: 10.1192/bjp.139.3.181. [DOI] [PubMed] [Google Scholar]
- 8.Hollon SD, DeRubeis RJ, Evans MD, et al. Cognitive therapy and pharmacotherapy for depression: singly and in combination. Arch Gen Psychiatry. 1992;49(10):774–781. doi: 10.1001/archpsyc.1992.01820100018004. [DOI] [PubMed] [Google Scholar]
- 9.Murphy GE, Simons AD, Wetzel RD, Lustman PJ. Cognitive therapy and pharmacotherapy: singly and together in the treatment of depression. Arch Gen Psychiatry. 1984;41(1):33–41. doi: 10.1001/archpsyc.1984.01790120037006. [DOI] [PubMed] [Google Scholar]
- 10.Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- 11.Kocsis JH, Gelenberg AJ, Rothbaum BO, et al. REVAMP Investigators. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry. 2009;66 (11):1178–1188. doi: 10.1001/archgenpsychiatry.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201–204. doi: 10.1176/ajp.2007.164.2.201. [DOI] [PubMed] [Google Scholar]
- 13.Fournier JC, DeRubeis RJ, Shelton RC, Gallop R, Amsterdam JD, Hollon SD. Antidepressant medications versus cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124–129. doi: 10.1192/bjp.bp.107.037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westen D, Novotny CM, Thompson-Brenner H. The empirical status of empirically supported psychotherapies: assumptions, findings, and reporting in controlled clinical trials. Psychol Bull. 2004;130(4):631–663. doi: 10.1037/0033-2909.130.4.631. [DOI] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 2001. [Google Scholar]
- 16.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II, Version 1.0) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 18.Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(2):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimherr FW, Amsterdam JD, Quitkin FM, et al. Optimal length of continuation therapy in depression: a prospective assessment during long-term fluoxetine treatment. Am J Psychiatry. 1998;155(9):1247–1253. doi: 10.1176/ajp.155.9.1247. [DOI] [PubMed] [Google Scholar]
- 21.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 22.Fleiss JL, Cohen J. Statistical Methods for Rates and Proportions. New York, NY: John Wiley & Sons; 1973. [Google Scholar]
- 23.Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH NIMH Treatment of Depression Collaborative Research Program. Clinical management—imipramine/placebo administration manual. Psychopharmacol Bull. 1987;23(2):309–324. [PubMed] [Google Scholar]
- 24.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guilford Press; 1979. [Google Scholar]
- 25.Beck AT, Freeman A, Davis DD. Cognitive Therapy of Personality Disorders. 2. New York, NY: Guilford Press; 2003. [Google Scholar]
- 26.Greenhouse JB, Stangl D, Bromberg J. An introduction to survival analysis: statistical methods for analysis of clinical trial data. J Consult Clin Psychol. 1989;57(4):536–544. doi: 10.1037//0022-006x.57.4.536. [DOI] [PubMed] [Google Scholar]
- 27.Szychowski JM, Roth DL, Clay OJ, Mittelman MS. Patient death as a censoring event or competing risk event in models of nursing home placement. Stat Med. 2010;29(3):371–381. doi: 10.1002/sim.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 29.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Kazdin AE, Bass D. Power to detect differences between alternative treatments in comparative psychotherapy outcome research. J Consult Clin Psychol. 1989;57(1):138–147. doi: 10.1037//0022-006x.57.1.138. [DOI] [PubMed] [Google Scholar]
- 32.Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54(11):1009–1015. doi: 10.1001/archpsyc.1997.01830230043006. [DOI] [PubMed] [Google Scholar]
- 33.DeRubeis RJ, Cohen ZD, Forand NR, Fournier JC, Gelfand LA, Lorenzo-Luaces L. The Personalized Advantage Index: translating research on prediction into individualized treatment recommendations. A demonstration. PLoS One. 2014;9(1):e83875. doi: 10.1371/journal.pone.0083875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.March J, Silva S, Petrycki S, et al. Treatment for Adolescents With Depression Study (TADS) Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS): randomized controlled trial. JAMA. 2004;292(7):807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 35.Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- 36.Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? a meta-analysis. BMJ Open. 2013;3(4):e002542. doi: 10.1136/bmjopen-2012-002542. [DOI] [PMC free article] [PubMed] [Google Scholar]