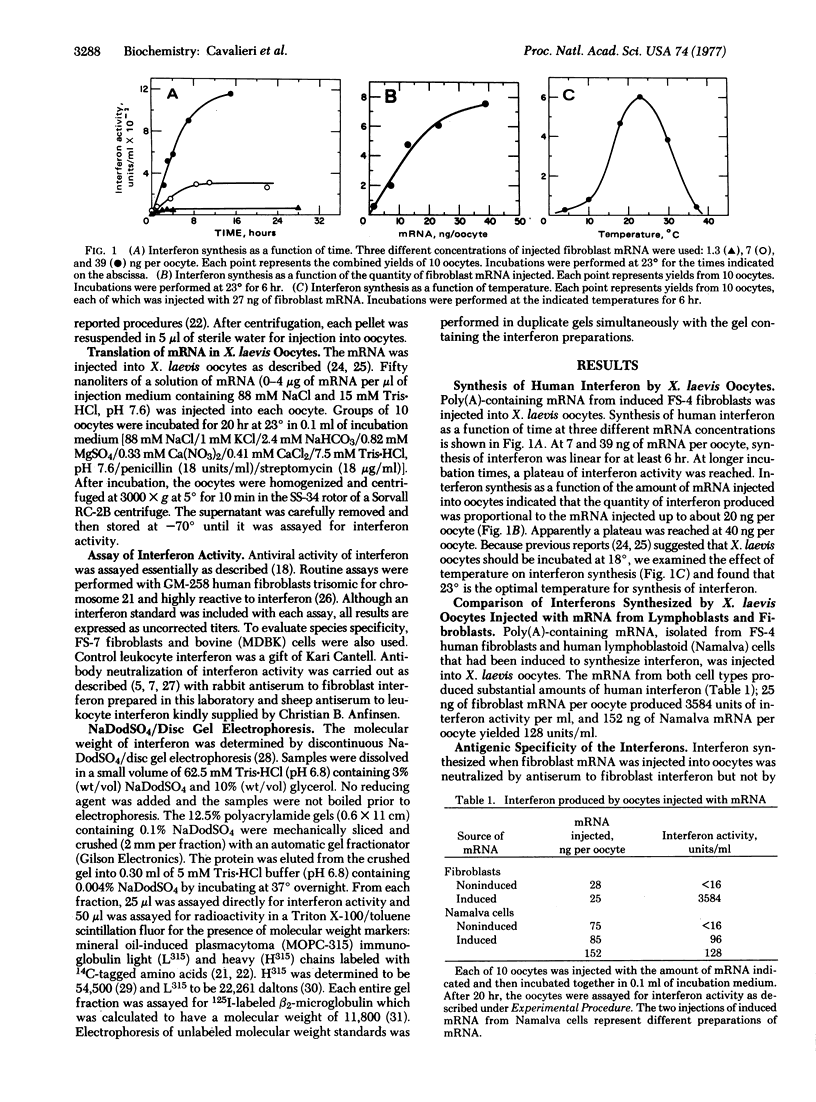
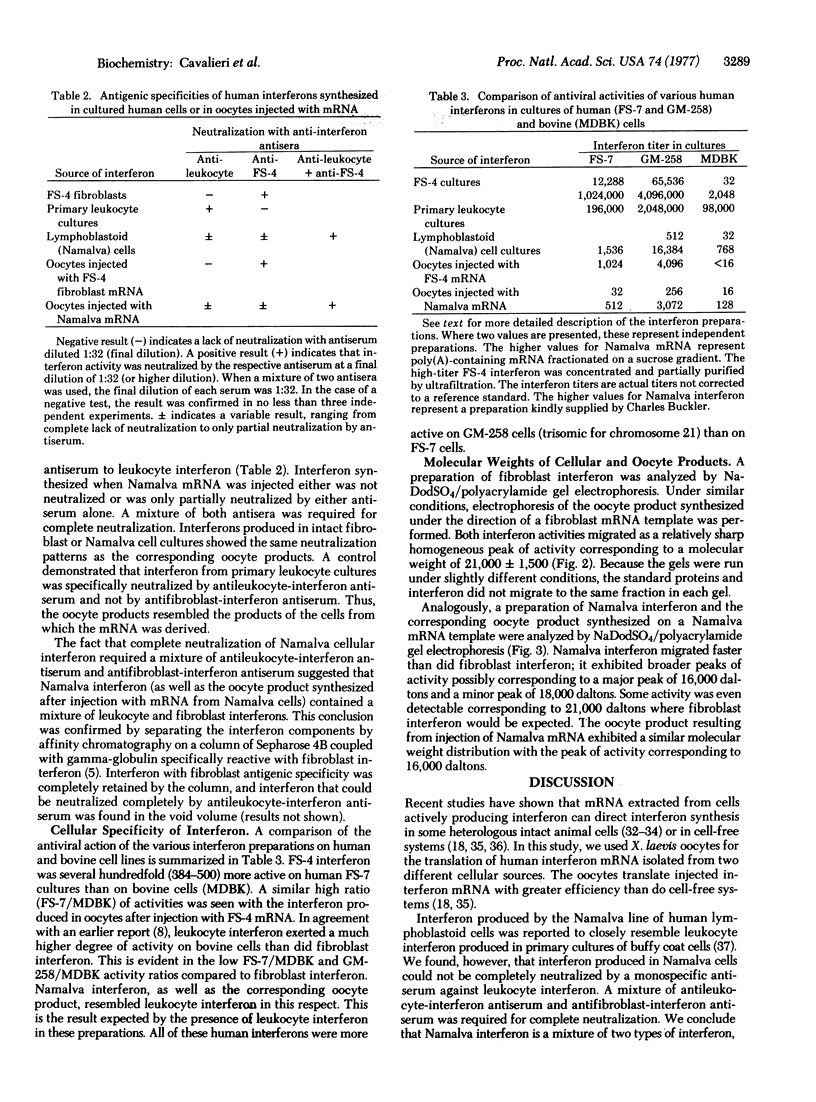
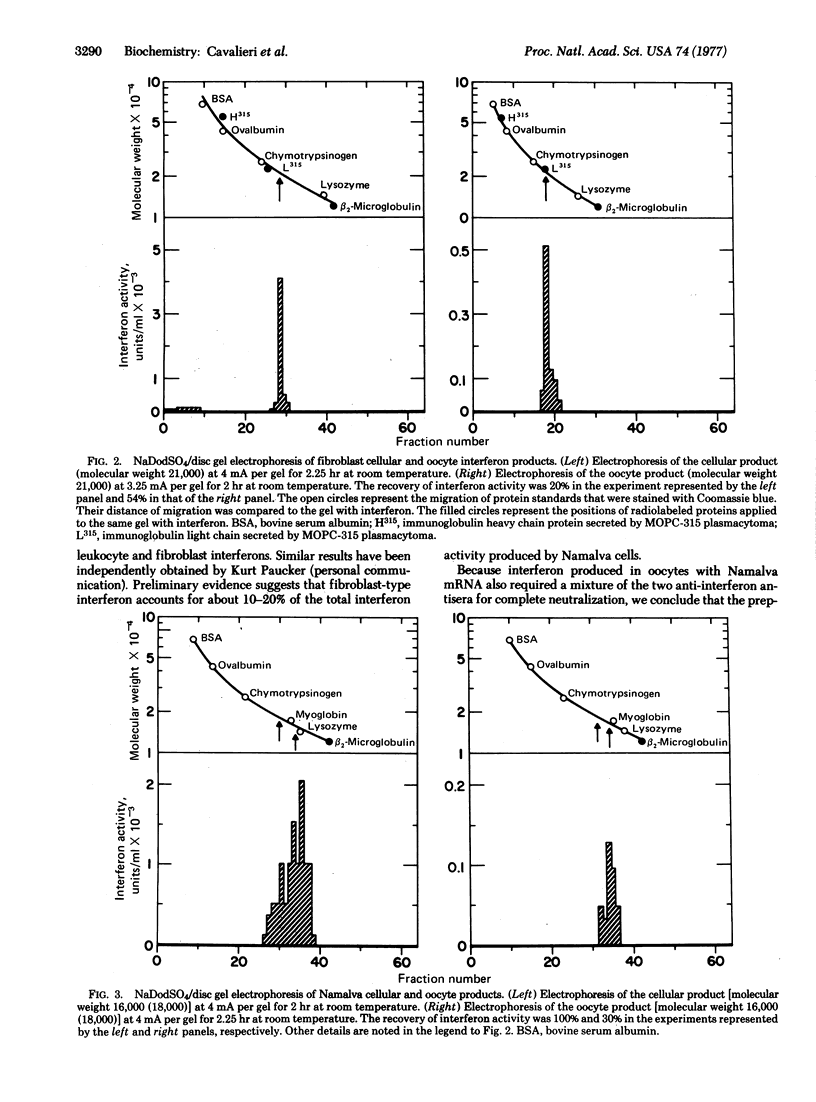
Abstract
Human fibroblasts and leukocytes produce interferons which may be distinguished by their antigenic and species specificity as well as by their molecular weight distributions. To elucidate the basis for these differences, we isolated mRNA from induced human fibroblasts and lymphoblastoid (Namalva) cells and studied the products of translation in Xenopus laevis oocytes. The mRNA from the respective cells yielded translation products, in oocytes, that were characteristic of the cells from which the mRNA was derived. We conclude that human cells contain at least two structural genes for interferon, coding for polypeptides differing in primary sequence. Fibroblasts synthesize a single species of interferon; lymphoblastoid cells synthesize two species, the fibroblast and leukocyte types.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg K., Ogburn C. A., Paucker K., Mogensen K. E., Cantell K. Affinity chromatography of human leukocyte and diploid cell interferons on sepharose-bound antibodies. J Immunol. 1975 Feb;114(2 Pt 1):640–644. [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Berns A. J., van Kraaikamp M., Bloemendal H., Lane C. D. Calf crystallin synthesis in frog cells: the translation of lens-cell 14S RNA in oocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1606–1609. doi: 10.1073/pnas.69.6.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Gurari-Rotman D., Ruegg U. T., Corley L., Anfinsen C. B. Apparent dispensability of the carbohydrate moiety of human interferon for antiviral activity. J Biol Chem. 1976 Mar 25;251(6):1659–1662. [PubMed] [Google Scholar]
- Colby C., Morgan M. J. Interferon induction and action. Annu Rev Microbiol. 1971;25:333–360. doi: 10.1146/annurev.mi.25.100171.002001. [DOI] [PubMed] [Google Scholar]
- Dugan E. S., Bradshaw R. A., Simms E. S., Eisen H. N. Amino acid sequence of the light chain of a mouse myeloma protein (MOPC-315). Biochemistry. 1973 Dec 18;12(26):5400–5416. [PubMed] [Google Scholar]
- Green M., Graves P. N., Zehavi-Willner T., McInnes J., Pestka S. Cell-free translation of immunoglobulin messenger RNA from MOPC-315 plasmacytoma and MOPC-315 NR, a variant synthesizing only light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):224–228. doi: 10.1073/pnas.72.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Zehavi-Willner T., Graves P. N., McInnes J., Pestka S. Isolation and cell-free translation of immunoglobulin messenger RNA. Arch Biochem Biophys. 1976 Jan;172(1):74–89. doi: 10.1016/0003-9861(76)90049-7. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Berman B., Ogburn C. A., Berg K., Paucker K., Vilcek J. Two antigenically distinct species of human interferon. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2185–2187. doi: 10.1073/pnas.72.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J., Falcoff E., Berman B. Suppression of human interferon production by inhibitors of glycosylation. Virology. 1975 Feb;63(2):475–483. doi: 10.1016/0042-6822(75)90320-7. [DOI] [PubMed] [Google Scholar]
- Ho M., Armstrong J. A. Interferon. Annu Rev Microbiol. 1975;29:131–161. doi: 10.1146/annurev.mi.29.100175.001023. [DOI] [PubMed] [Google Scholar]
- Jankowski W. J., Davey M. W., O'Malley J. A., Sulkowski E., Carter W. A. Molecular structure of human fibroblast and leukocyte interferons: probe by lectin and hydrophobic chromatography. J Virol. 1975 Nov;16(5):1124–1130. doi: 10.1128/jvi.16.5.1124-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Dombos L., Gothoskar B. Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus. Int J Cancer. 1972 Jul 15;10(1):44–57. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr Interferon: purification and initial characterization from human diploid cells. Proc Natl Acad Sci U S A. 1976 Feb;73(2):520–523. doi: 10.1073/pnas.73.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg L. H., Friedmann T. Relative quantitative assay of the biological activity of interferon messenger ribonucleic acid. J Gen Virol. 1975 May;27(2):225–238. doi: 10.1099/0022-1317-27-2-225. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Gurdon J. B., Crawford L. V. Translation of encephalomyocarditis viral RNA in oocytes of Xenopus laevis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3665–3669. doi: 10.1073/pnas.69.12.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar V. A., Gurdon J. B., Lane C. D., Marbaix G. Translational capacity of living frog eggs and oocytes, as judged by messenger RNA injection. J Mol Biol. 1971 Oct 14;61(1):93–103. doi: 10.1016/0022-2836(71)90208-7. [DOI] [PubMed] [Google Scholar]
- Pestka S., Hintikka H. Studies on the formation of ribonucleic acid-ribosome complexes. XVI. Effect of ribosomal translocation inhibitors on polyribosomes. J Biol Chem. 1971 Dec 25;246(24):7723–7730. [PubMed] [Google Scholar]
- Pestka S., McInnes J., Havell E. A., Vilcek J. Cell-free synthesis of human interferon. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3898–3901. doi: 10.1073/pnas.72.10.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Pitha P. M. The induction of interferon and its messenger RNA in human fibroblasts. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1023–1030. doi: 10.1016/s0006-291x(74)80082-3. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Premkumar E., Pitha P. M. Interferon activity produced by translation of human interferon messenger RNA in cell-free ribosomal systems and in Xenopus oöcytes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4881–4885. doi: 10.1073/pnas.72.12.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Desmyter J. Molecular heterogeneity of human leukocyte interferon: two populations differing in molecular weights, requirements for renaturation, and cross-species antiviral activity. Virology. 1975 Sep;67(1):68–73. doi: 10.1016/0042-6822(75)90403-1. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gresser I., Tovey M. G., Bandu M., Le Goff S. Identification of the cell multiplication inhibitory factors in interferon preparations as interferons. Nature. 1976 Jul 22;262(5566):300–302. doi: 10.1038/262300a0. [DOI] [PubMed] [Google Scholar]
- Strander H., Mogensen K. E., Cantell K. Production of human lymphoblastoid interferon. J Clin Microbiol. 1975 Jan;1(1):116–117. doi: 10.1128/jcm.1.1.116-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Schneider E. L., Tischfield J., Epstein C. J., Ruddle F. H. Human chromosome 21 dosage: effect on the expression of the interferon induced antiviral state. Science. 1974 Oct 4;186(4158):61–63. doi: 10.1126/science.186.4158.61. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Thang D. C., De Maeyer E., Montagnier L. Biosynthesis of mouse interferon by translation of its messenger RNA in a cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3975–3977. doi: 10.1073/pnas.72.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P., Pestka S. De novo synthesis and glycosylation of the MOPC-46B mouse immunoglobulin light chain in cell-free extracts. J Biol Chem. 1977 Jul 10;252(13):4474–4486. [PubMed] [Google Scholar]
- Underdown B. J., Simms E. S., Eisen H. N. Subunit structure and number of combining sites of the immunoglobulin A myeloma protein produced by mouse plasmacytoma MOPC-315. Biochemistry. 1971 Nov 23;10(24):4359–4368. doi: 10.1021/bi00800a002. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A., Yamazaki S. Antigenic, physicochemical, and biologic characterization of human interferons. Ann N Y Acad Sci. 1977 Mar 4;284:703–710. doi: 10.1111/j.1749-6632.1977.tb22006.x. [DOI] [PubMed] [Google Scholar]
- de Maeyer-Guignard J., de Maeyer E., Montagnier L. Interferon messenger RNA: translation in heterologous cells. Proc Natl Acad Sci U S A. 1972 May;69(5):1203–1207. doi: 10.1073/pnas.69.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]


