Abstract
Assessment of cognition is critical to providing optimal care to individuals with heart failure. Traditional neuropsychological batteries are time consuming. This pilot study obtained feasibility data for a brief neuropsychological battery. Mean administration time was 40 ± 7.5 minutes, averting subject fatigue, and the battery was sensitive to cognitive impairments.
1. Background and significance of problem
Although scientists have made great strides related to understanding the etiology and treatment of heart failure (HF), clinicians continue to struggle with the fact that individuals with HF have one of the highest 90-day readmission rates of any chronic illness. Inability to follow complex medical regimens and recognize worsening symptoms often contribute to the frequent rehospitalizations (Bennett, Pressler, Hays, Firestine, & Huster, 1997; Ekman, Fagerberg, & Skoog, 2001; Naylor, Stephens, Bowles, & Bixby, 2005; Zuccala et al., 2005). Further, researchers have designed successful interventions to decrease hospitalization rates only to have hospitalization rates increase following the conclusion of the study (Naylor et al., 2005). Cognitive impairment is now being examined as a potential contributor to self-management difficulty and frequent hospitalizations in the HF population, with current literature estimating that 28% to 58% of individuals with HF have impairment of one or more cognitive domains (Pressler, 2008). Unfortunately, there are no instruments with documented reliability and validity to evaluate cognitive impairment in the chronic HF population; therefore, the purpose of this study is to obtain feasibility data on a brief neuropsychological assessment that evaluates the most commonly impaired cognitive domains in the chronic HF population.
For example, executive function is a commonly impaired domain and encompasses the brain functions that allow individuals to process information and make decisions related to managing novel situations. Impairments in executive function cause decreases in functional status (Incalzi et al., 2003) and self-management ability (Dickson, Deatrick, & Riegel, 2008). When an individual with HF has worsening symptoms, intact executive function supports accurate processing of the worsening symptoms and concludes that contact with the health care provider is necessary. Impairment of executive function leads to the inability to correctly process the information and reach the desired conclusion (i.e., contacting the medical provider before symptoms deteriorate further). Therefore, intact cognitive function is paramount to an individual HF patient's overall ability to follow complex medical regimens, recognize worsening symptoms, and avoid frequent hospitalizations.
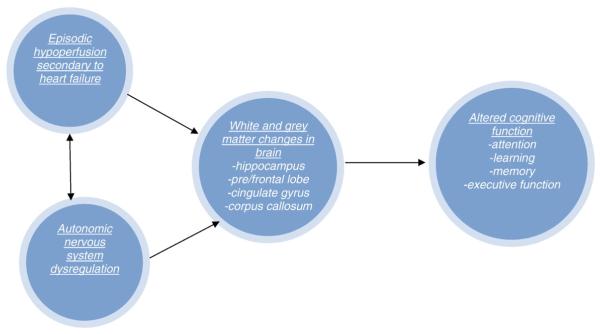
Research surrounding cognitive impairment in HF is still in its infancy, and therefore, why and when cognitive impairment occurs in the HF population and how cognitive impairment progresses are still very much unknown. Leading etiological hypotheses focus on the intermittent cerebral hypoperfusion and autonomic dysregulation that occur during the HF disease process (see Fig. 1). Briefly, this model suggests that intermittent cerebral hypoperfusion and autonomic dysregulation (i.e., sympathetic nervous system over stimulation) found in HF lead to changes in grey and white matter areas of the brain involved in various cognitive processes; these changes in the brain in turn affect the cognitive domains that allow an individual to receive, process, and recall information that is presented to them. Although not shown in the model, another arrow could be placed that illustrates how impairment in one's ability to receive, process, and remember information has many detrimental effects on one's functional status, including the ability to self-manage care. Unfortunately, the onset and trajectory of cognitive impairment in the HF disease process have yet to be examined in the literature, and there are no current hypotheses related to the trajectory of cognitive impairment in the HF population.
Fig. 1.
Model of cognitive impairment in chronic HF.
Clinicians understand that cognitive impairment is present in the HF population and that they need to be able to identify individuals with cognitive impairment to deliver interventions designed to compensate for cognitive impairment and prevent frequent hospitalizations; however, the science is lacking in terms of a feasible screening instrument or brief neuropsychological assessment that has been validated in the HF population. Multiple batteries have been used to measure cognitive function in HF (Almeida & Tamai, 2001; Bornstein, Starling, Myerowitz, & Haas, 1995; Gorkin et al., 1993; Hoth, Poppas, Moser, Paul, & Cohen, 2008; Incalzi et al., 2003; Schmidt, Fazekas, Offenbacher, Dusleag, & Lechner, 1991; Serber et al., 2008; Tanne et al., 2005; Trojano et al., 2003; Vogels, Oosterman, Laman, et al., 2008; Wolfe, Worrall-Carter, Foister, Keks, & Howe, 2006); however, none of the batteries used in these studies have documented reliability and validity in the chronic HF population. Few reviewed studies examined all six of the cognitive domains most often impaired in HF (i.e., attention, working memory, long-term memory, learning, executive function, and psychomotor speed), and administration time for some of the batteries lasted as long as 6 hours (Incalzi et al., 2003; Vogels, Oosterman, van Harten, et al., 2007), less than optimal for patients with HF who commonly experience fatigue. The purpose of this study was to obtain feasibility data for use of a brief neuropsychological test battery that measures all six of the cognitive domains in stable, community-dwelling individuals with chronic HF. Specific aims of this study are the following: (a) to describe administration time for a set of neuropsychological tests in individuals with chronic HF; (b) to describe subject perceptions for this set of neuropsychological tests; and (c) to document prevalence of cognitive impairment and cognitive preservation identified with the set of neuropsychological tests in a sample of individuals with chronic HF.
2. Methods
Institutional review board approval for the study was obtained for this descriptive study, which was part of a larger study designed to obtain psychometric data on the set of neuropsychological tests that were chosen as a battery for use in the chronic HF population. A convenience sample of stable community-dwelling ischemic and nonischemic New York Heart Association (NYHA) Class I–IV individuals with chronic HF were recruited from a large Midwestern HF clinic using the inclusion/exclusion criteria listed in Table 1.
Table 1.
Inclusion/Exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| >21 years old | Current diagnosis of neurological illness or dementia (e.g., Alzheimer’s dementia, Parkinson’s, epilepsy) |
| English speaking and writing | History of substance abuse/treatment |
| Duration of HF >6 months | History of stroke |
| Stable medication regimen for past 4 weeks | Hepatic insufficiency (AST/ALT more than twice normal limits) |
| Severe renal failure (serum creatinine N2.5 mg/dl) | |
| Anemia | |
| Left ventricular assist device | |
| History of the following within 3 months of study enrollment: | |
| Acute myocardial infarction | |
| Unstable angina | |
| Coronary artery bypass graft surgery | |
| percutaneous transluminal coronary angioplasty | |
| Biventricular pacemaker insertion | |
| History of hospitalization within past 4 weeks |
Note. AST = aspartate aminotransferase;ALT = alanine aminotransferase.
The final sample consisted of 26 stable NYHA class II–IV individuals with chronic HF (17 males, mean age = 69 ± 12 years, left ventricular ejection fraction = 41% ± 8%). Specific demographics for the sample are listed in Table 2.
Table 2.
Sample demographics (n = 26)
| Variable | Range | M (SD) |
|---|---|---|
| Age (years) | 32–86 | 69 (12) |
| No. of medications | 5–18 | 10 (3) |
| Previous education | 11–20 | 14 (2) |
| LVEF | 25–60 | 41 (8) |
| HF duration (months) | 6–380 | 92 (83) |
| brain natriuretic peptide | 20–628 | 121 (143) |
| glomerular filtration rate | 27–90 | 57 (18) |
3. Procedures
Prior to study data collection, the primary investigator (PI) completed appropriate training related to administration and scoring of the neuropsychological battery under the supervision of board-certified neuropsychologists. Following training, the PI completed several supervised administration and scoring sessions. As part of the supervised administration and scoring, interrater reliability was assessed between the PI and the consulting cognitive neuroscientist (98% agreement achieved on scoring).
Participants provided informed consent for the study. All participants were tested by the PI and completed the neuropsychological battery in a quiet and private examination room in the HF clinic. Following the neuropsychological testing, the subjects were interviewed and asked to respond to several questions (see Table 3). The questions were used to gain information regarding the subjective experience of neuropsychological testing and provide suggestions to help improve the neuropsychological test experience for the larger study.
Table 3.
Interview questions
| 1. | How did you feel about the amount of time needed to complete the testing today? |
| 2. | How did the testing session make you feel? |
| 3. | What did you think about the difficulty of the different tests? |
| 4. | Would you change anything about the testing session? |
4. Neuropsychological instruments used in the study
Consultation with clinical neuropsychologists at the PI's institution provided insight to instrument choice. The instrument battery was chosen based on ability to be administered in less than 40 minutes; ability to assess the domains most frequently impaired in HF, that is, attention, working memory, delayed memory, learning, executive function, and psychomotor speed; and availability of age- and education-adjusted normative data (as age and education have both been reported to have an influence on neuropsychological test scores). Table 4 defines each of the cognitive domains measured in this study and identifies the instrument chosen to evaluate each domain. All tests chosen for this study have documented reliability and validity in other chronic illness populations but have not been validated in the chronic HF population (Lezak, 2004).
Table 4.
Cognitive domains and corresponding instruments
| Cognitive domain | Domain function | Instrument/Scale |
|---|---|---|
| Attention | Allows individual to focus on information for further processing | RBANS Attention Index Scale Score |
| Working memory | Allows an individual to encode data and perform some operation on that data | Trail Making Test Part B |
| Immediate memory | Storing information for a short time (i.e., less than 5 minutes) | RBANS Immediate Memory Scale Score |
| Delayed memory | Storing information for a relatively long time (i.e., 20 to 30 minutes) | RBANS Delayed Memory Scale Score |
| Learning | Ability to process, store and recall information that is repeatedly presented | RBANS List Learning Score |
| Executive function | Individual’s ability to process information and respond to novel situations | Letter Fluency “D” |
| Psychomotor speed | Speed an individual can physically respond to a stimulus | Finger Tapping Test |
| Trail Making Test Part A |
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) contains 12 subsets (list learning, story memory, figure copy, line orientation, picture naming, semantic fluency, digit span, coding, list recall, list recognition, story memory, and figure recall) that contribute to five index scores (immediate memory, visual/spatial construction, language, attention, and delayed memory) and a general global score. It is a brief paper/pencil battery that has age- and education-adjusted normative data for patient with ages 20 to 89 years (Duff et al., 2003) and generally takes 20 to 30 minutes to complete. Patients are allowed to complete the test at their own pace and may take breaks if needed between subscales. The RBANS has age- and education-adjusted normative data from other populations, including geriatric, vascular dementia, and stroke (Randolph, 1998).
Trail Making Test Part A involves connecting a series of numbers (similar to a dot-to-dot puzzle), and Part B involves connecting a series of numbers alternating with letters (similar to a dot-to-dot puzzle except that the connections run in order of 1 to A, A to 2, 2 to B, B to 3, 3 to C, and so on). Each section (Part A and B) is taken independently of the other, and each is timed. Scoring is based on the amount of time required to finish each section. Trail Making Test Part A and B have normative data from a wide range of studies accounting for factors known to influence results (i.e., age and education level in clinical populations; Mitrushina, Boone, Razani, & D'Elia, 2005; Tombaugh, 2004).
Letter fluency (D words in 1 minute) requires subjects to generate words that begin with the letter “D” for 1 minute. The subject recites the words to the examiner, who records all the words and corrects for any rule violations (e.g., cannot use proper nouns or words beginning with a different letter). Scoring is based on the number of words generated in 1 minute. Letter fluency has been validated against neuroimaging in populations other than HF and was found to be associated with left hemisphere structures (Lezak, 2004).
The Finger Tapping Test is a task of “pure” psychomotor speed (Lezak, 2004) and involves a small, portable tapper that is easily transported and easily administered. The test is brief, less than 5 minutes, and subjects can take a break between test cycles. Scoring is based on number of times that the finger is tapped during the allotted time frame. Subjects perform the task with both dominant and nondominant hands over a period of 5 to 10 trials depending on variability of test results (Lezak, 2004). The trial scores for each hand are then averaged, providing researchers with a final score of psychomotor speed in the dominant and nondominant hands.
5. Statistical analysis
SPSS (Grad Pack v.17) was used to analyze descriptive statistics from the sample. RBANS Index and Total Scale scores, Trail Making Test Part A and Part B scores, and letter fluency raw scores were converted to Z scores. A Z score of −1.50 was used as the cutoff to define cognitive impairment (Mitrushina et al., 2005). The Finger Tapping Test scores were converted to percentiles, and a score falling below 8% was defined as cognitive impairment (Mitrushina et al., 2005).
6. Results
6.1. Aim 1
No subject refused to complete the testing session, and all subjects were able to complete the set of neuropsychological tests in its entirety. The mean time of administration for the complete battery was 38 ± 6.5 minutes, with a range of 25 to 50 minutes. One subject required a 5-minute break between the RBANS and Trail Making Tests.
6.2. Aim 2
In general, subjects reported that the length of time necessary to complete the testing was appropriate. None of the subjects reported that the battery was “too long” or that they were “tired out” from the testing session. Most subjects reported that the testing session was “fun.” Seven subjects stated that they felt like the “memory” tests were “hard”; however, their self-perceived memory impairment did not necessarily match their actual scores on the respective neuropsychological tests. Eight of the first 10 subjects' test scores on the Finger Tapping Test were confounded by arthritis or carpal tunnel disease, with 60% of the subjects scoring below the 42% cutoff for cognitive impairment secondary to inability to accurately complete the required trials. All of the first 10 subjects recommended an alternate form of psychomotor speed; therefore, because of subject burden, the Finger Tapping Test was dropped from the protocol and an alternate form of psychomotor speed (Trail Making Test Part A) was used.
6.3. Aim 3
Cognitive impairment noted in this sample included attention (27% prevalence), memory (35% prevalence, immediate; 23% delayed), and executive function (54% prevalence; see Table 5). Only one of the seven subjects who self-reported memory impairment and stated that the memory tests were “hard” was found to have impaired memory based on their neuropsychological test scores. Finally, the learning and visual/spatial/construction domains were preserved in this sample.
Table 5.
Results of Z scores for selected cognitive domains (n = 26)
| Test | Cognitive domain | Z-Score range | Frequency (%) subjects with Z <(–)1.5 (cognitive impairment) |
|---|---|---|---|
| RBANS Attention Index | Attention | −2.40 to 0.70 | 7 (27%) |
| RBANS Immediate Memory Index | Immediate memory | −2.30 to 1.00 | 9 (35%) |
| RBANS Delayed Memory Index | Delayed memory | −2.70 to 1.50 | 6 (23%) |
| Letter Fluency | Executive function | −3.40 to 1.10 | 14 (54%) |
7. Discussion
Fatigue is a common symptom among individuals with chronic HF; therefore, it is paramount to validate a set of neuropsychological tests that is brief enough to avoid increased fatigue that could potentially bias responses. The current battery of tests had an average administration time of less than 40 minutes, well below the longer time frames estimated in previous studies. Further, the testing was well tolerated by the sample, with only one subject requiring a 5-minute break between the RBANS and Trail Making Tests. No subjects felt that the testing time was “too long” or that it increased fatigue levels. All subjects were able to complete the battery, unlike other studies that reported as many as one third to one half the subjects being unable to complete some portions of the neuropsychological tests, potentially skewing results (Incalzi et al., 2003; Vogels, Oosterman, Laman, et al., 2008). Finally, no subjects reported that the set of neuropsychological tests was “too hard” or “too easy.” No studies were identified that evaluated time of completion or participant's subjective comments regarding the neuropsychological tests. Therefore, this study adds to the current literature by providing initial data regarding completion time and the subjective experience of individuals with chronic HF to neuropsychological testing.
Cognitive impairment documented with this set of tests was consistent with results from previous HF literature, with deficits in attention, memory, and executive function most frequently reported. With regard to prevalence of cognitive impairment in the HF population, this study is one of the few to evaluate cognitive function in stable, community dwelling individuals with HF. Therefore, these findings provide preliminary support for use of these neuropsychological tests in individuals with chronic HF.
8. Limitations
The results of this study are limited by several factors. First, the utilization of a small convenience sample limits generalizability of the findings. Future studies with larger sample sizes are warranted. The Finger Tapping Test was problematic. There were multiple complaints regarding the Finger Tapping Test among participants that increased variability of scores across the required 5 to 10 trials. Eight of the initial 10 subjects were unable to complete the desired number of trials due to complaints of carpal tunnel or osteoarthritis. Therefore, use of the Finger Tapping Test was discontinued, and an alternative assessment to measure psychomotor speed was used (i.e., Trail Making Test Part A).
9. Implications
In spite of the limitations, this set of neuropsychological tests was sensitive in terms of detecting cognitive impairment and feasible in terms of administration time and prevention of subject fatigue. Further reliability and validity documentation are needed with a larger sample. This data will allow clinicians and researchers to collaborate and move forward in two key areas. First, however, there is a need for an appropriate clinic screening instrument for use in patients with chronic stable HF. Although a 40-minute battery is appropriate for use in research studies, it remains impractical for routine use in a clinic setting. Individual tests from this set of instruments should be examined for their feasibility and sensitivity in a clinic setting. An appropriate screening instrument is necessary because cognitive impairment, especially the executive function domain, is not easily appreciated by medical providers during routine clinic visits and should be assessed in all individuals with HF. As an example, the population for this study consisted of stable individuals with HF (hospitalization within 4 weeks of study enrollment was an exclusion criterion), yet more than half the subjects had impairment of the executive function domain and were individuals who clinic nurses anecdotally reported as needing frequent telephone follow-up but would not have labeled cognitively impaired.
This feasibility study showed that these neuropsychological tests were practical for use; however, further reliability and validity testing is needed. Following validation, it would be important to design future research to examine the onset and trajectory of cognitive impairment and to further evaluate impaired and preserved cognitive domains in the chronic HF population. Finally, as scientists and clinicians become more familiar with which cognitive domains are impaired and which are preserved, interventions to compensate for impairment need to be developed and tested. For example, in this sample, visual/spatial memory was preserved; therefore, utilizing educational materials that are more visual (i.e., pictures and figures) may prove more beneficial than written out instructions. These types of interventions will not only decrease health care costs (e.g., decrease hospitalizations) but may also improve quality of life for individuals with chronic HF.
References
- Almeida OP, Tamai S. Congestive heart failure and cognitive functioning amongst older adults. Arqivos de Neuro-Psiquiatria. 2001;59(2-B):324–329. doi: 10.1590/s0004-282x2001000300003. [DOI] [PubMed] [Google Scholar]
- Bennett SJ, Pressler ML, Hays L, Firestine LA, Huster GA. Psychosocial variables and hospitalization in persons with chronic heart failure. Progress in Cardiovascular Nursing. 1997;12(4):4–11. [PubMed] [Google Scholar]
- Bornstein RA, Starling RC, Myerowitz P, Haas GJ. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurologica Scandinavica. 1995;91:260–265. doi: 10.1111/j.1600-0404.1995.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Dickson VV, Deatrick JA, Riegel B. A typology of heart failure self-care management in non-elders. European Journal of Cardiovascular Nursing. 2008;7(3):171–181. doi: 10.1016/j.ejcnurse.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Duff K, Patton D, Schoenberg MR, Mold J, Scott JG, Adams RL. Age- and education-corrected independent normative data for the RBANS in a community dwelling elderly sample. The Clinical Neuropsychologist. 2003;17(3):351–366. doi: 10.1076/clin.17.3.351.18082. [DOI] [PubMed] [Google Scholar]
- Ekman I, Fagerberg B, Skoog I. The clinical implications of cognitive impairment in elderly patients with chronic heart failure. Journal of Cardiovascular Nursing. 2001;16(1):47–55. doi: 10.1097/00005082-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Gorkin L, Norvell NK, Rosen RC, Charles E, Shumaker SA, McIntyre KM, et al. Assessment of quality of life as observed from the baseline data of the Studies of Left Ventricular Dysfunction (SOLVD) trial quality of life substudy. American Journal of Cardiology. 1993;71:1069–1073. doi: 10.1016/0002-9149(93)90575-w. [DOI] [PubMed] [Google Scholar]
- Hoth K, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac dysfunction and cognition in older adults with heart failure. Cognitive and Behavioral Neurology. 2008;21(2):65–72. doi: 10.1097/WNN.0b013e3181799dc8. [DOI] [PubMed] [Google Scholar]
- Incalzi RA, Trojano L, Acanfora D, Crisci C, Tarantino F, Abete P, et al. Verbal memory impairment in congestive heart failure. Journal of Clinical and Experimental Neuropsychology. 2003;25(1):14–23. doi: 10.1076/jcen.25.1.14.13635. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd Oxford University Press; New York: 2004. [Google Scholar]
- Mitrushina MN, Boone KB, Razani J, D'Elia LF. Handbook of normative data for neuropsychological assessment. 2nd Oxford University Press; New York: 2005. [Google Scholar]
- Naylor MD, Stephens C, Bowles KH, Bixby MB. Cognitively impaired older adults: From hospital to home. American Journal of Nursing. 2005;105(2):52–61. doi: 10.1097/00000446-200502000-00028. [DOI] [PubMed] [Google Scholar]
- Pressler SJ. Cognitive functioning and chronic heart failure: A review of the literature (2002–July 2007) Journal of Cardiovascular Nursing. 2008;23(3):239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- Randolph C. RBANS: Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio: The Psychological Corporation: 1998. [Google Scholar]
- Schmidt R, Fazekas F, Offenbacher H, Dusleag J, Lechner H. Brain magnetic resonance imaging and neuropsychologic evaluation of patients with idiopathic dilated cardiomyopathy. Stroke. 1991;22:195–199. doi: 10.1161/01.str.22.2.195. [DOI] [PubMed] [Google Scholar]
- Serber SL, Kumar R, Woo MA, Macey PM, Fonarow GC, Harper RM. Cognitive test performance and brain pathology. Nursing Research. 2008;57(2):75–83. doi: 10.1097/01.NNR.0000313483.41541.10. [DOI] [PubMed] [Google Scholar]
- Tanne D, Freimark D, Poreh A, Merzeliak O, Bruck B, Schwammenthal Y, et al. Cognitive functions in severe congestive heart failure before and after an exercise training program. International Journal of Cardiology. 2005;130(2):145–149. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Trojano L, Incalzi RA, Acanfora D, Picone C, Mecocci P, Rengo F. Cognitive impairment: Key feature of congestive heart failure in the elderly. Journal of Neurology. 2003;250:1456–1463. doi: 10.1007/s00415-003-0249-3. [DOI] [PubMed] [Google Scholar]
- Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, et al. Transcranial Doppler blood flow assessment in patients with mild heart failure: Correlates with neuroimaging and cognitive performance. Congestive Heart Failure (Greenwich, Conn) 2008;14(2):61–65. doi: 10.1111/j.1751-7133.2008.07365.x. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, Oosterman JM, van Harten B, Scheltens P, van der Flier WM, Schroeder-Tanka JM, et al. Profile of cognitive impairment in chronic heart failure. Journal of the American Geriatrics Society. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- Wolfe R, Worrall-Carter L, Foister K, Keks N, Howe V. Assessment of cognitive function in heart failure patients. European Journal of Cardiovascular Nursing. 2006;5(2):158–164. doi: 10.1016/j.ejcnurse.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Marzetti E, Cesari M, Monaco MR, Antonica L, Cocchi A, et al. Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. The American Journal of Medicine. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]