Abstract
Over the years, the role of B cells in the host immune response to malignancy has been overshadowed by our focus on T cells. Nevertheless, B cells play important roles as antigen-presenting cells and in the production of antibodies. Furthermore, B cells can function as effector cells that mediate tumor destruction on their own. This review will highlight the various functions of B cells that are involved in the host response to tumor.
Keywords: B cells, antibody, adoptive immunotherapy, tumor antigen
Introduction
A simple definition of B lymphocytes is a population of cells that express clonally diverse cell surface immunoglobulin (Ig) receptors recognizing specific antigenic epitopes.1 The identification of serum gammaglobulin as the source of antibodies was the initiating event for the discovery of antibody-producing cells. Plasma cells were suggested as a source of antibody as early as 1948.2 Plasma cells represent the terminally differentiated cells along the B cell development/maturation pathway. The discovery of B cells occurred in the mid-1960s simultaneously with the identification of T cells. Max Cooper and Robert Good described the functional distinction between cells in the chicken bursa of Fabricius (B cells) responsible for antibody production and cells that required an intact thymus (T cells) for manifestation of delayed-type hypersensitivity responses.3,4 Shortly thereafter, B cells were found to express surface Ig which could be used as markers to characterize these cells in the normal state as well as leukemic state.
Besides the role of B cells in antibody production (aka humoral immunity), B cells also mediate/regulate many other functions essential for immune homeostasis (Table 1). Of significant importance is the antigen-presenting role of B cells in the initiation of T cell immune responses.5-8 Depending upon the model system, B cells have different requisite roles for antigen-presentation to T cells. The congenital absence of B cells leads to abnormalities within the immune system which includes a decrease in thymocyte numbers and diversity, defects within spleen dendritic cell (DC) and T cell compartments, an absence of Peyer patch organogenesis and follicular DC networks, and an absence of macrophage subsets with decreased chemokine expression.1 Besides their role in the development of the immune system, B cells can exert modulatory effects on the immune system by the elaboration of cytokines.9-11 These effects can modulate T cell, DC and antigen-presenting cell function; regulate lymphoid tissue organization and neogenesis; as well as regulate wound healing and transplanted tissue rejection. B cells can play a significant role in infection and autoimmunity as regulatory cells (Bregs) via the elaboration of suppressive cytokines such as IL-10, TGF-β or IL-4.10,12,13
Abnormalities in B cell biology has resulted in clinicopathologic conditions that underscore the important role of B cells in maintaining normal physiology. Developmental blockade of B cells can lead to significant immunodeficiencies where Ig production is impaired and susceptibility to infection is high.14-16 Disruptions in the regulation of B cell reactivity can result in B cells capable of producing high-affinity effector antibodies that have autoreactivity. This can result in pathological autoimmune conditions such as rheumatoid arthritis. Dominant subclones of B cells with cytogenetic/molecular genetic abnormalities can exist from all developmental stages of the B cell lineage that give rise to leukemias and lymphomas.
B cells in cancer immunology
The role played by B cells in cancer immunology is complex and somewhat controversial. Depending upon their state of activation, B cells have had divergent roles on T cell differentiation and effector function. In tumor models, resting B cells have been reported to suppress T cell-mediated antitumor immunity.17-24 The mechanism by which suppression is mediated by resting B cells on the antitumor immune response is poorly understood. It appears to affect both CD4+ 20 and CD8+ 22,24 T cells. Elaboration of IL-10 by B cells23 and interaction with Tregs (CD4+CD25+ cells)24 have been implicated as potential mechanisms in tumor models. However, many of these studies have been performed using mice in which the immune system develops in the complete absence of B cells. These hosts are associated with a limited T cell repertoire25, lack follicular DCs and several macrophage populations26, and have enhanced IL-12 production by DC which can skew Th1 response.27 Hence, defining the role of B cells during tumor immunity has been difficult. Using a wild-type host, DiLillo et al. has recently shown that B cells are required for optimal CD4+ and CD8+ immunity induction.28 In their model, B cells were depleted in the wild-type host using an anti-CD20 monoclonal antibody (mAb) rather than using genetically B cell-deficient hosts; and found that B16 melanoma tumor growth was increased in the B depleted hosts associated with reduced effector-memory CD4+ and CD8+ immune T cell induction.
As opposed to resting B cells, several reports have indicated the efficacy of activated B cells in cellular immunotherapy of malignancies. Many of these reports have been focused on how activated B cells can be used as effective antigen presenting cells (APCs) for T cell sensitization. 29-35 Activated B cells have been reported to enhance the ability to generate tumor-infiltrating lymphocytes in a culture system involving anti-CD3 and IL-2.36 LPS activated B cells bound to anti-CD3 has been reported to mediate regression of B16 lung metastases after adoptive transfer.37 The mechanism by which the transferred B cells mediated tumor destruction is unknown, but most likely involved B-T cell interactions. The authors postulated that the activated B cells provided additional co-stimulatory signals to a host T cell response. Kawakami et al. reported that the administration of a DNA-based vaccine resulted in antitumor effects of established murine tumors that was mediated by both T and B cells.38 In that report, the role of B cells in mediating tumor regression was unknown. There is a paucity of information about the ability of B cells to directly kill tumor cells. The role of B cells in mediating tumor destruction via tumor-specific antibody production is well established and will be reviewed in a later section.
B cells as effector cells in adoptive immunotherapy
Recently, Li et al. reported that tumor-primed B cells activated in vitro with anti-CD40 mAb and LPS mediated tumor regression upon adoptive transfer into mice bearing either lung metastases or subcutaneous tumors.39 These B cells were harvested from tumor-draining lymph nodes (TDLN) of donor mice that had been inoculated with tumor cells in the flanks. As shown in Figure 1, activated TDLN B cells mediated significant regression of pulmonary metastases.39 The addition of TDLN T cells activated with anti-CD3 and anti-CD28 mAb in the adoptive transfer resulted in even greater tumor regression than either cell population alone (Figure 1).
Figure 1.
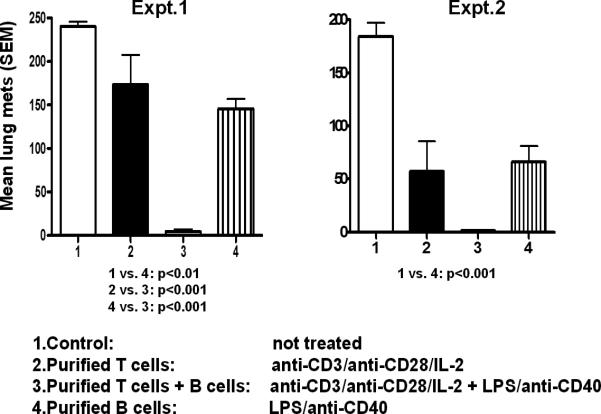
Activated B cells from tumor-draining lymph nodes (TDLN) mediate tumor regression after adoptive transfer in mice bearing 3-day established lung metastases. In these two separate experiments groups of mice (n=6) received activated T cells alone, B cells alone, or a combination of both. Mean number of pulmonary metastases (±SEM) at the end of the experiments are depicted in the graphs. The combination of T and B cells was superior to all other groups. (From Li et al.)39
The observation that activated B cells alone can mediate tumor regression in the adoptive immunotherapy of solid tumors is unique. It was shown to be a consistent finding with three different tumor histologies (i.e., sarcoma, melanoma and breast cancer).39,40 One potential mechanism by which activated B cells mediate tumor regression is via tumor cell cytotoxicity. Li and co-workers found that activated TDLN B cells can cause lysis of tumor cells in vitro as measured by a standard LDH release assay.40 Using the 4T1 mammary carcinoma murine tumor, 4T1 TDLN B cells activated with LPS and anti-CD40 were able to lyse 4T1 tumor cells, and not irrelevant Renca (renal cell carcinoma) or TSA (mammary adenocarcinoma) tumor cells (Figure 2). Hence, the B effector cells mediated tumor-specific cytotoxicity. This unique phenomenon was evident in the absence of antibody. The mechanism by which B effector cells lyse tumor cells is presently unknown. In a separate report, Kemp et al. found that anti-CD40 activated human B cells were able to mediate tumor cell killing via a TRAIL/Apo-2L-dependent mechanism.41
Figure 2.
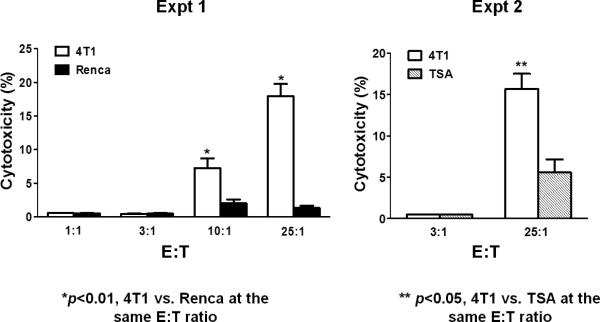
Activated B cells from TDLN can cause tumor cell cytolysis in an immunologically specific fashion. 4T1 TDLN B cells were secondarily activated with LPS/anti-CD40 and evaluated for cytotoxicity using an LDH release assay. Activated B cells were admixed with tumor cells at different effector: target (E:T) ratios and the amount of killing was assessed. 4T1 TDLN B cells were able to kill 4T1 tumor cells, and not the irrelevant Renca or TSA tumors as shown in these two separate experiments. (From Li et al.)40
Another potential mechanism by which B effector cells mediate tumor regression in adoptive immunotherapy is by presenting antigen to the host cellular immune system. As noted above, B cells play an important role as antigen-presenting cells for initiating T cell immune response. It was recently reported that the adoptive transfer of activated B cells specific for the 4T1tumor into tumor-bearing hosts resulted in the induction of systemic T cell immunity to 4T1 that could be detected in the peripheral blood and spleen.40 This is illustrated in Figure 3 where groups of 4T1-bearing mice received activated B cells, IL-2 treatment, or no treatment; and splenocytes were harvested to monitor for T cell immunity to 4T1. As can be seen, IFN-γ secretion was observed from spleen cells obtained from mice that received activated B cells and not from mice that received IL-2 or no treatment. The IFN-γ response was specific in response to 4T1 tumor cells and not to irrelevant TSA (mammary adenocarcinoma) or MT-901 (mammary carcinoma) tumor cells.
Figure 3.
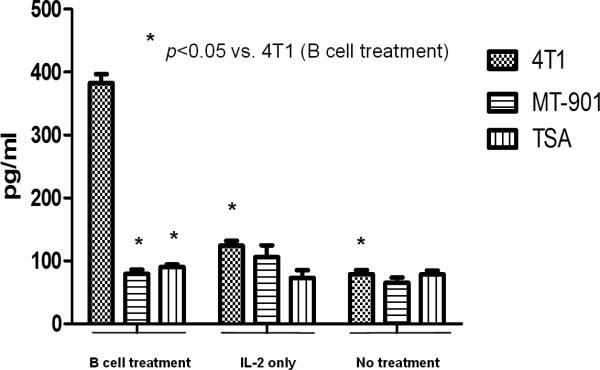
Activated B cells induce tumor reactive T cell responses in tumor-bearing hosts. Mice bearing 4T1 tumor were treated with activated B cells, IL-2 or no treatment. Spleens of the mice were harvested and splenic T cells activated in vitro. These activated splenic T cells were tested for tumor reactivity by measuring IFNγ release when mixed with irradiated tumor cells. Mice that received activated 4T1 TDLN B cells were found to have splenic T cells reactive to 4T1 tumor cells, and not to irrelevant tumor cells indicating the ability of activated B cells to present antigen to the secondary host. (Li et al.)40
B effector cells as producers of antigen-specific antibody
B cells have played a major role in the production of antibodies specific to tumor associated epitopes. Antibodies have played an increasing role in therapeutic applications in the treatment of cancers mainly by blockade of receptors important to tumor proliferation. An example of this is bevacizumab (Avastin®) which is a recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the biologic activity of human vascular endotherlial growth factor (VEGF). This antibody has been approved by the Food and Drug Administration (FDA) for the treatment of several solid malignancies (i.e., colorectal cancer, non-small cell lung cancer, glioblastoma and renal cell cancer). Another example is cetuximab (Erbitux®) which is a humanized mAb that blocks the epidermal growth factor receptor (EGFR) and is used in the treatment of colorectal cancer and head and neck cancer. More recently, ipilimumab (Vervoy®) was approved for the treatment of melanoma. This humanized mAb binds to CTLA-4, a molecule on activated T lymphocytes that down-regulates their function. By binding to CTLA-4, ipilimumab prevents blockade of the immune T cell response. The list of therapeutic monoclonal antibodies that modulate cell function and can directly or indirectly lead to tumor regression is growing.
Antibodies can also exert antitumor effects by other means. One mechanism is by antibody-dependent cell-mediated cytotoxicity (ADCC). This involves effector cell-mediated lysis of a target cell that has been bound by a specific antibody. ADCC is mediated by natural killer cells, neutrophils, T cells and macrophages. Lopez et al. described the ability of B cells from tumor-bearing hosts, but not normal non-tumor bearing hosts to mediate tumor lysis via ADCC.42 Another mechanism for tumor lysis involving antibody is known as complement dependent cytotoxicity (CDC). Antibody by itself is ineffectual in the lysis of target cells. However, antibody of the IgM and IgG classes can activate the complement system resulting in cell lysis. Rituximab (Rituxan®) is a humanized monoclonal antibody that binds to CD20 expressed on B cells. It was approved by the FDA for the treatment of refractory B cell lymphomas and is thought to mediate B cell antitumor effects via ADCC and CDC. Experimentally, complement-fixing tumor specific antibodies can induce regression of established human melanoma and breast tumors as reported using in vivo models. 31,32 Furthermore, activated B effector cells have been found to produce complement-fixing antibody that can mediate CDC. As shown in Figure 4, antibody produced by activated B cells from D5 melanoma TDLN or MCA 205 sarcoma TDLN mediated tumor-specific CDC. Both ADCC and CDC may represent additional mechanisms by which B effector cells mediate tumor regression in an adoptive immunotherapy setting.
Figure 4.
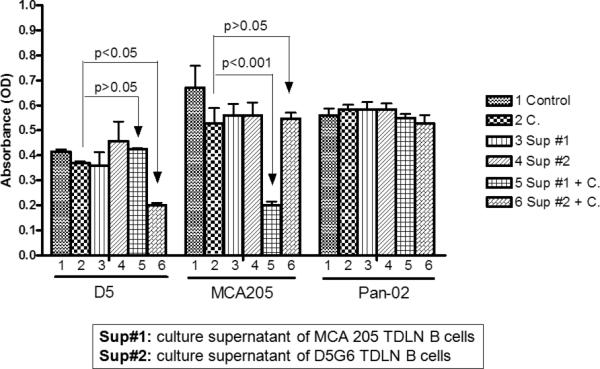
Activated TDLN B cells produce antibody that can mediate complement dependent cytotoxicity (CDC). Supernatants (Sup) from activation cultures of D5 and MCA 205 TDLN B cells were tested for their ability to mediate CDC in vitro. Pan-02 was used as an irrelevant tumor cell control. As can be seen, the supernatants contained antibodies that were able to specifically lyse the relevant tumor targets. (Li et al.)39
One additional advantage of developing B effector cells from TDLN is the opportunity to identify novel tumor antigens. The specificity of antibody generated by activated B effector cells demonstrated in Figure 4 indicates the binding of antibody to unique epitopes on the tumor cell surface. This is further illustrated in Figure 5, where IgG2b antibody isolated from the supernatant of MCA 205 TDLN B cells activated with LPS and anti-CD40 was examined for binding to MCA 205, D5 and Pan-02 (pancreatic carcinoma) tumor cells in vitro.39 By immunofluorescent staining, the IgG2b antibody bound only to MCA 205 tumor cells. Using immunoprecipitation techniques, antibody produced by tumor-specific B effector cells can be used to bind to lysates of the tumor cells and subsequently isolated by gel electrophoresis to identify unique bands that may represent potential tumor-associated antigens. An example of this is shown in Figure 6 where supernatants from activated D5 melanoma B effector cells was used to bind to D5, MCA 205 and Pan-02 tumor lysates and the immune complex was run in separate gel lanes. Supernatants from activated D5 melanoma T effector cells were used as additional controls. The unique bands associated with the D5 B cell supernatant lane represent unique D5 epitopes which can be further characterized by mass spectroscopy.
Figure 5.
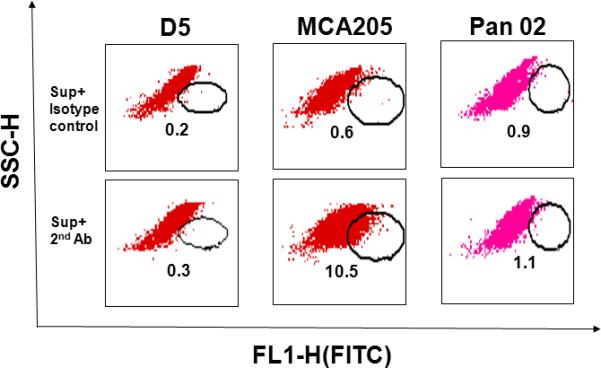
TDLN B cell-produced IgG2b binds specifically to tumor cells. D5, MCA 205, and Pan-02 tumor cells were incubated with the culture supernatant (Sup) of MCA 205 TDLN B cells. Bound IgG2b onto tumor cells was then detected by a secondary antibody, FITC-anti-mouse IgG2b. FITC-coupled isotype-matched control was used to measure background staining. (Li et al.)39
Figure 6.
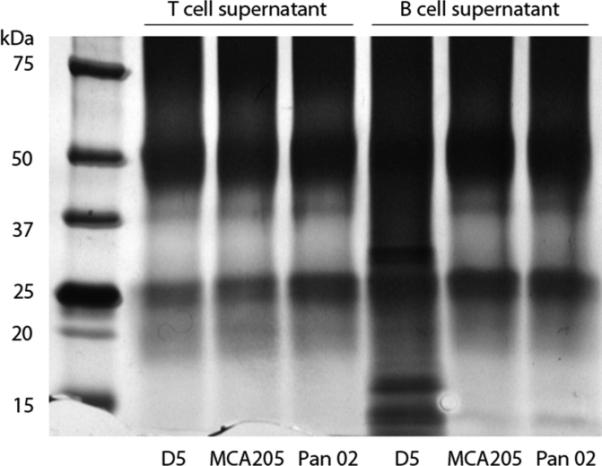
Antibodies produced by activated D5 TDLN B cells can bind to unique epitopes from the lysate of D5 tumor cells and not to other tumor lysates. Supernatants from activated D5 TDLN T cells and B cells were admixed with the lysates of D5, MCA 205 and Pan-02 tumor cells. The immunoprecipitates were then run on an SDS-PAGE gel. Unique bands were identified in the lane were antibodies produced by activated D5 TDLN B cells were admixed with D5 tumor lysate.(Namm et al. unpublished data).
Summary
The role of B cells in cancer immunotherapy has been overshadowed by the interest in developing T cell-mediated cellular responses. It is apparent that B cells can play a complementary role in the host response against tumor. An overview of how B cells can function in the therapeutic approach to cancer is depicted in Figure 7. Under appropriate conditions, B cells can mediate direct tumor cell killing independently or via antibody production. In addition, there appears to be an effective synergy between B cells and T cells to enhance the therapeutic efficacy of cancer immunotherapy. B cells can also exert regulatory functions via the release of cytokines. The role of these Breg cells in the host tumor environment remains to be defined.
Figure 7.
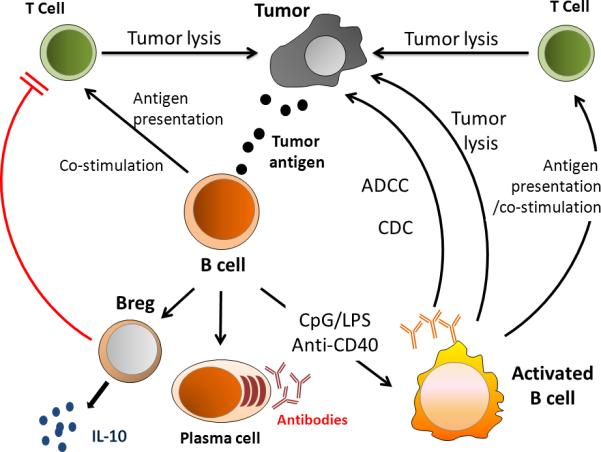
Schematic illustration of B cell interactions related to tumor. Abbreviations: CpG (cytosine phosphodiester guanine oligodeoxynucleotides); LPS (lipopolysaccharides); anti-CD40 (anti-CD40 monoclonal antibody); ADCC (antibody dependent cellular cytotoxicity); and CDC (complement dependent cytotoxicity).
Synopsis.
B cells play an important role in the host immune response to cancer. Methods to augment their function will be helpful in the design of novel immune treatments.
Acknowledgements
This work was supported in part by T32 CA009672, RO1 CA82529, and the Gillson Longenbaugh Foundation.
References
- 1.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–80. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagraeus A. The plasma cellular reaction and its relation to the formation of antibodies in vitro. J Immunol. 1948;58:1–13. [PubMed] [Google Scholar]
- 3.Cooper MD, Peterson RD, Good RA. Delineation of the Thymic and Bursal Lymphoid Systems in the Chicken. Nature. 1965;205:143–6. doi: 10.1038/205143a0. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MD, Raymond DA, Peterson RD, South MA, Good RA. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966;123:75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ron Y, De Baetselier P, Gordon J, Feldman M, Segal S. Defective induction of antigen-reactive proliferating T cells in B cell-deprived mice. Eur J Immunol. 1981;11:964–8. doi: 10.1002/eji.1830111203. [DOI] [PubMed] [Google Scholar]
- 6.Ron Y, Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987;138:2848–56. [PubMed] [Google Scholar]
- 7.Janeway CA, Jr., Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–5. [PubMed] [Google Scholar]
- 8.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 9.Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–82. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 10.Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol. 2005;175:7103–7. doi: 10.4049/jimmunol.175.11.7103. [DOI] [PubMed] [Google Scholar]
- 11.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. Regulation of IFN-gamma production by B effector 1 cells: essential roles for T-bet and the IFN-gamma receptor. J Immunol. 2005;174:6781–90. doi: 10.4049/jimmunol.174.11.6781. [DOI] [PubMed] [Google Scholar]
- 12.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–10. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 13.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Vetrie D, Vorechovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–33. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 15.Tsukada S, Saffran DC, Rawlings DJ, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–90. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 16.Bacchelli C, Buckridge S, Thrasher AJ, Gaspar HB. Translational mini-review series on immunodeficiency: molecular defects in common variable immunodeficiency. Clin Exp Immunol. 2007;149:401–9. doi: 10.1111/j.1365-2249.2007.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. J Immunol. 1978;121:359–62. [PubMed] [Google Scholar]
- 18.Monach PA, Schreiber H, Rowley DA. CD4+ and B lymphocytes in transplantation immunity. II. Augmented rejection of tumor allografts by mice lacking B cells. Transplantation. 1993;55:1356–61. [PubMed] [Google Scholar]
- 19.Chapoval AI, Fuller JA, Kremlev SG, Kamdar SJ, Evans R. Combination chemotherapy and IL-15 administration induce permanent tumor regression in a mouse lung tumor model: NK and T cell-mediated effects antagonized by B cells. J Immunol. 1998;161:6977–84. [PubMed] [Google Scholar]
- 20.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 21.Perricone MA, Smith KA, Claussen KA, et al. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother (1997) 2004;27:273–81. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–86. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 23.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 24.Watt V, Ronchese F, Ritchie D. Resting B cells suppress tumor immunity via an MHC class-II dependent mechanism. J Immunother (1997) 2007;30:323–32. doi: 10.1097/CJI.0b013e31802bd9c8. [DOI] [PubMed] [Google Scholar]
- 25.Joao C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–16. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 26.Crowley MT, Reilly CR, Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J Immunol. 1999;163:4894–900. [PubMed] [Google Scholar]
- 27.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–82. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–16. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–25. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 31.Kondo E, Topp MS, Kiem HP, et al. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J Immunol. 2002;169:2164–71. doi: 10.4049/jimmunol.169.4.2164. [DOI] [PubMed] [Google Scholar]
- 32.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 33.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 34.Van den Bosch GA, Ponsaerts P, Nijs G, et al. Ex vivo induction of viral antigen-specific CD8 T cell responses using mRNA-electroporated CD40-activated B cells. Clin Exp Immunol. 2005;139:458–67. doi: 10.1111/j.1365-2249.2005.02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung Y, Kim BS, Kim YJ, et al. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006;66:6843–50. doi: 10.1158/0008-5472.CAN-06-0889. [DOI] [PubMed] [Google Scholar]
- 36.Tamada K, Harada M, Okamoto T, et al. Specific antitumor activity of tumor-infiltrating lymphocytes expanded first in a culture with both anti-CD3 monoclonal antibody and activated B cells and then in a culture with interleukin-2. Cancer Immunol Immunother. 1995;41:339–47. doi: 10.1007/BF01526553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada M, Okamoto T, Kurosawa S, et al. The antitumor activity induced by the in vivo administration of activated B cells bound to anti-CD3 monoclonal antibody. Cell Immunol. 1995;161:132–7. doi: 10.1006/cimm.1995.1017. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami K, Terabe M, Kawakami M, Berzofsky JA, Puri RK. Characterization of a novel human tumor antigen interleukin-13 receptor alpha2 chain. Cancer Res. 2006;66:4434–42. doi: 10.1158/0008-5472.CAN-05-1265. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183:3195–203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, Li S, Chang AE. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clinical Cancer Research. 2011 doi: 10.1158/1078-0432.CCR-11-0207. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kemp TJ, Moore JM, Griffith TS. Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation. J Immunol. 2004;173:892–9. doi: 10.4049/jimmunol.173.2.892. [DOI] [PubMed] [Google Scholar]
- 42.Lopez DM, Blomberg BB, Padmanabhan RR, Bourguignon LY. Nuclear disintegration of target cells by killer B lymphocytes from tumor-bearing mice. FASEB J. 1989;3:37–43. doi: 10.1096/fasebj.3.1.2783411. [DOI] [PubMed] [Google Scholar]


