Abstract
Background
Stress has been shown to suppress ovulation in experimental models, but its effect on human reproduction at the population level is unclear.
Methods
Healthy women (n=259), aged 18–44 years from Western New York, were followed for two menstrual cycles (2005–2007). Women completed daily perceived stress assessments, a 4-item Perceived Stress Scale (PSS-4) up to four times each cycle, and a 14-item PSS at baseline. Mixed model analyses were used to assess effects of stress on log reproductive hormone concentrations and sporadic anovulation.
Results
High versus low daily stress was associated with lower estradiol (-9.5%; 95% confidence interval (CI)= -15.6% to -3.0%), free estradiol (-10.4% [-16.5% to -3.9%]), and LH (-14.8% = [-21.3% to -7.7%]), and higher FSH (6.2% [2.0% to 10.5%]) after adjusting for age, race, percent body fat, depression score, and time-varying hormones and vigorous exercise. High versus low daily stress was also associated with lower luteal progesterone (-10.4% [-19.7% to -0.10%]) and higher odds of anovulation (adjusted OR = 2.2 [95% CI=1.0 to 4.7]). For each unit increase in daily stress level, women had a 70% higher odds of an anovulatory episode (OR=1.7 [1.1 to 2.4]). Similar but attenuated results were found for the association between the PSS-4 and reproductive hormones, while null findings were found for the baseline PSS.
Conclusion
Daily perceived stress does appear to interfere with menstrual cycle function among women with no known reproductive disorders, warranting further research to explore potential population-level impacts and causal biologic mechanisms.
Stress among women is common, with 23% of U.S. women reporting high levels of stress and nearly 69% reporting stress levels above what is considered healthy.1 Psychosocial or perceived stressors, defined as challenges that individuals view as taxing or exceeding their coping abilities,2 are of particular concern for women's health, as they can increase the risk of adverse health outcomes, including cardiovascular disease, depression, and autoimmune disorders.2 Among premenopausal women, prolonged physical stressors such as excessive dieting or intensive exercise have been linked to reproductive dysfunction (e.g., hypothalamic amenorrhea),3 but the effects of short-term perceived stress on reproductive hormones and ovulatory function among healthy eumenorrheic women are unknown.
Multiple pathways have been proposed linking perceived stress with reproductive function, including activation of the hypothalamic pituitary adrenal (HPA) axis leading to a delay or inhibition of the luteinizing hormone (LH) surge3, and activation of the sympathetic medullar system leading to altered blood flow through the fallopian tubes and interrupted gamete transport.4 While both nonhuman primate and experimental studies in humans have helped inform mechanistic hypotheses,5, 6 few studies have assessed the effects of stress on reproductive hormones and menstrual cycle function among premenopausal women; none of these prospectively assessed perceived stress or used more than one stress instrument.7, 8 Global measures of psychosocial stress, such as the Perceived Stress Scale (PSS),9 have been used for decades to study relationships between stress and adverse health outcomes. However, recent evidence indicates that daily perceived stress captured via diary designs have the added advantage of not only reducing memory bias by minimizing the time elapsed between an experience and the account of the experience, but also of capturing within-person changes in perceived stress over time.10,11 Additionally, capturing perceived stress via a daily diary is particularly relevant when exploring the temporal relationship between stress and menstrual cycle function.12
To better understand the potential effects of multiple psychosocial stressors on reduced female fecundity, 4, 13 larger population-based studies with repeated measurements of daily stressors, hormones, and ovulation over more than one menstrual cycle are needed. To address this gap, we evaluated the association between perceived stress, captured via both a daily diary and the PSS, and reproductive function among a cohort of healthy premenopausal women.
Methods
The BioCycle Study, conducted in 2005–2007, was a prospective cohort study that followed 259 premenopausal women from Western New York State for up to two menstrual cycles. The study size was based on power to detect differences in oxidative stress levels with endogenous reproductive hormone levels and antioxidants, the primary study outcome.14 The study population and methods have been previously described in detail.14 Briefly, women aged 18–44 years with self-reported cycle lengths of 21–35 days for the past 6 months, and no previous diagnosis of gynecological or chronic disease, were included in the study. Women using long-acting hormone contraception within the past 12 months, or oral contraceptives or other hormone supplement within the past 3 months, were excluded, as were women who were pregnant or breastfeeding within the past 6 months. Baseline urine hCG pregnancy tests (Quick Vue, Quidel Corporation, San Diego, CA, USA) were performed to verify that potential study participants were not pregnant. Additionally, women could not have a self-reported body mass index (BMI) < 18.5 or >35.0 kg/m2 at screening.14 Of 449 women who were screened, 318 met the eligibility criteria, and 276 of these enrolled. Seventeen women (6%) withdrew before completing the study. Women who withdrew were not markedly different from women who completed the study, although a greater percentage of Asians (19%) than of blacks (6%) or whites (3%) withdrew. Reasons for withdrawal included scheduling conflicts (n = 8), loss to follow-up (n = 4), inability to tolerate blood draws (n = 3), inability to participate because of illness (n = 1), or loss of interest (n = 1).14 The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. Written informed consent was obtained from all participants.
Women provided fasting blood specimens at up to 8 visits/cycle for one (n = 9) or two (n = 250) menstrual cycles, with visit timing assisted by the use of fertility monitors, which tracked urinary levels of estrone-3-glucuronide and LH.15 Scheduled visits corresponded to menstruation, mid- and late-follicular phases, LH/follicle-stimulating hormone (FSH) surge, expected ovulation, and early-, mid-, and late-luteal phases. Serum estradiol (E2), progesterone, LH, FSH, and sex hormone binding globulin (SHBG) were measured via Immulite 2000 Solid Phase competitive chemiluminescent enzymatic immunoassay. Albumin was measured by the Beckman LX20 auto analyzer using bromcresol purple methodology. Free E2 was calculated from total E2, SHBG, and albumin concentrations.16 All biochemical analyses were conducted by Kaleida Health Laboratories in Buffalo, New York. The coefficients of variation for these assays were as follows: <10% for E2, SHBG, and insulin; <5% for LH, FSH, and albumin; and <14% for progesterone. Study protocol compliance was high, with 94% of the participants completing 7 or 8 visits each cycle. At the late-luteal phase visit for each cycle, all women completed a urine hCG pregnancy test; no women were pregnant.14
Sporadic anovulation was defined as any cycle with a peak progesterone concentration ≤5 ng/mL (1 ng/mL = 3.18 nmol/L) and no observed serum LH peak on the mid- or late luteal phase visits.17 Participants recorded menstrual bleeding via a daily diary. Menses length was defined as a bleeding episode that included ≥ 2 days of bleeding in a 3-day interval preceded by at least two bleed-free days 18 and total blood loss on each bleeding day was estimated using a standard algorithm.19 Cycle length was calculated as the number of days between the first day of menstrual bleeding and the onset of the next menses for each cycle. Day of ovulation among ovulatory cycles was assigned based on dates and levels of the LH peak using the fertility monitor compared with the observed LH maximum value in serum and the first day of progesterone rise. For anovulatory cycles, the day of ovulation was set at day 14 for comparison purposes only. Follicular phase length was defined as day 1 of bleeding to the day of ovulation. Luteal phase length was defined as day of ovulation through the day before the next menses.
Participants prospectively recorded stress levels (not stressful [1], a little stressful [2], very stressful [3]) using a daily diary over the course of the two menstrual cycles. Participants also completed a 14-question PSS (PSS-14) assessment at baseline to measure stress in the previous month; and a 4-item PSS (PSS-4) assessment at 4 of the clinic visits corresponding to menstruation, the mid-follicular phase, expected ovulation, and mid-luteal phase to measure stress in the previous week. The PSS measured the degree (via a five-point scale from never to very often) to which situations in one's life are appraised as stressful.9 The majority of participants (89%) completed ≥75% of their daily diaries. All but one woman completed the baseline PSS-14 assessment, while 90% of women completed all four PSS-4 assessments during each cycle.
Four 24-hour dietary recalls were administered at the same clinic visits as the 4-item PSS. Food and beverage intake information was collected, and nutrient data were analyzed using the Nutrition Data System for Research (version 2005; Nutrition Coordinating Center, University of Minnesota). Total energy intake (kcals/day), fiber (grams/day), alcohol (grams/day), caffeine (mg/day), and the alternate Mediterranean Diet Score20 were calculated for each dietary assessment. Minutes of vigorous exercise, daily sexual activity (defined as vaginal intercourse), sleep duration, cigarette use, and medication use were assessed from the daily diary. Total caffeine intake was calculated as the sum of dietary and medication-based caffeine.21
Age, race, income, education level, sexual and reproductive history, and depression (Center for Epidemiologic Studies Depression Scale, CES-D) were obtained at baseline using standard questionnaires.14, 22 Race was considered because previous research has shown that reproductive hormone metabolism and perceived stress vary by race.23, 24 At the end of the follow-up period, total percent body fat was measured using duel energy X-ray absorptiometry scans.
Two of the 509 cycles (<1%) were missing daily diary stress information and were excluded from all analyses. Descriptive statistics were compared among tertiles of daily stress averaged over the study period by using ANOVA for normally distributed continuous variables and the Wilcoxon-Mann-Whitney test for non-normally distributed continuous variables. Fisher's exact tests were used for categorical variables.
Linear mixed models were used to evaluate the association between daily stress and log serum concentrations of E2, progesterone, LH and FSH, both over the entire cycle and restricted to follicular and luteal phases, including all cycles. We analyzed cycle phases separately because previous research has shown stress to be associated with changes in phase-specific hormone concentrations.7 We averaged daily stress levels reported for the 5 days before each clinic visit (hereafter referred to as “recent daily stress”), as this reduced intra-individual variability and was comparable to the recall window for the PSS-4. Daily, PSS-4, and PSS-14 stress levels were analyzed as continuous and dichotomous measures (high stress, > the median; low stress, ≤ median). For better comparison between continuous stress measures with different scales, results are presented as a one-standard-deviation increase in stress level for each measure.
Our final models were adjusted for age, percent body fat, race, baseline depression score, and time-varying vigorous exercise. Because E2, progesterone, LH, and FSH concentrations change over the cycle in response to complex feedback mechanisms with other hormones and because that fluctuation in these hormones may change stress vulnerability,25 traditional regression adjustment for other hormone concentrations across the cycle may be inadequate. Additionally, time-varying exercise could be both a cause and consequence of time-varying perceived stress. Therefore, we also conducted analyses using marginal structural models that adjusted for other reproductive hormones and exercise through stabilized inverse-probability-of-exposure weights 26, 27 to appropriately account for time-varying confounding by hormones and exercise affected by prior stress levels. Weighted linear mixed-effects models with random intercepts were used to estimate the parameters of the marginal structural models.
Generalized linear mixed models with random intercepts were used to estimate odds ratios (ORs) for the association between each measure of stress (average daily stress, PSS-4, and PSS-14 scores; analyzed continuously and dichotomously [above and below the median]) and anovulation, while adjusting for age, percent body fat, race, vigorous exercise, and depression score. We assessed daily and PSS-4 stress measures, along with vigorous exercise, up until ovulation so as to best preserve the temporal cause and effect order.
We conducted several sensitivity analyses to evaluate the robustness of our initial results. To assess if there remained residual confounding, we additionally adjusted for other baseline and time-varying factors such as sleep duration, sexual activity, pain or antibiotic medication use, caffeine and alcohol intake, cigarette use, nulligravidity, total energy and fiber intake, and the alternate Mediterranean Diet Score, and we compared the resulting effect estimates with our original analysis. We also compared our final adjusted model to one excluding baseline depression score to evaluate its impact on our findings. Average stress levels over the past 2 days instead of 5 days before each clinic visit, and stress reported on each of the 5 days preceding the clinic visit, were examined to see how varying the exposure window affected our reproductive hormone estimates. Effect modification was evaluated for age quartiles (18–20, >20–24, >24–35, >35 years) and race (white, black, Asian, and other) by fitting multiplicative interaction terms of these factors with perceived stress in multivariate models.
In regard to the effect of stress on ovulatory function, sensitivity analyses were conducted to assess the effect of stress on anovulation using a more conservative definition that included any cycle with a peak progesterone concentration ≤3 ng/mL and no observed serum LH peak on the mid- or late luteal phase visit.17 Additionally, we conducted a case-crossover analysis among the women with both an ovulatory and anovulatory cycle during the study period (n=21) who recorded daily stress during the first half of their menstrual cycle. Conditional logistic regression was used to calculate the matched OR for anovulation by dichotomized stress levels while adjusting for time-varying factors within the cycle.28, 29 Case-crossover studies control for baseline factors by design; thus age, percent body fat, race, and baseline depression score were not included in our adjusted analyses. All analyses were performed in SAS, version 9.3, software (SAS Institute, Inc., Cary, North Carolina).
Results
Over the study period, women in the BioCycle Study reported 59% of days as not stressful, 32% as a little stressful, and 8% as very stressful, according to the daily diaries. Geometric mean concentrations, averaged over the menstrual cycle, of total and free estradiol were 87.1pg/mL (95% confidence interval [CI] = 83.6 to 90.8; range, 30.6 - 212.2) and 1.3pg/mL (CI = 1.2 to 1.3; range = 0.5 - 3.8) respectively; progesterone 1.4ng/mL (CI = 1.4 to 1.5; range = 0.2 - 3.1); LH, 6.4 ng/mL (CI = 6.2 to 6.7; range = 2.1 - 16.6); and FSH, 5.2 mIU/mL (CI = 5.0 to 5.4; range = 1.9 - 13.5). Average daily stress was positively associated with depressive symptoms (P<0.001), level of education (P=0.02), and having at least one anovulatory cycle (P=0.04) (Table 1). No associations were found between daily stress and demographic, lifestyle, dietary, and other menstrual cycle characteristics.
Table 1. Characteristics of women participating in the BioCycle Study by tertile of average daily stress (n=259 women)a.
| Daily Stress Tertile | ||||
|---|---|---|---|---|
| Low (1-1.31) (n=87) | Moderate (>1.31–1.63) (n=87) | High (>1.63–2.73) (n=85) | P | |
| Demographic/Lifestyle | ||||
| Age (years); mean (SD) | 28.3 (8.4) | 27.0 (8.0) | 26.5 (8.2) | 0.34 |
| Race; no. (%) | 0.22 | |||
| White | 51 (33) | 47 (31) | 56 (36) | |
| Black | 20 (39) | 22 (43) | 9 (18) | |
| Asian | 10 (27) | 12 (32) | 15 (41) | |
| Other | 6 (35) | 6 (35) | 5 (29) | |
| Hispanic; no. (%) | 5 (6) | 3 (3) | 3 (4) | 0.80 |
| Income; no. (%) | 0.06 | |||
| <$39,999 | 45 (52) | 45 (52) | 28 (33) | |
| $40 000–74 999 | 20 (23) | 24 (28) | 28 (33) | |
| >75 000 | 22 (25) | 18 (21) | 29 (34) | |
| Education; no. (%) | 0.02 | |||
| ≤ High School | 14 (16.1) | 15 (17.2) | 4 (4.7) | |
| Post-secondary | 73 (83.9) | 72 (82.8) | 81 (95.3) | |
| Married; no. (%) | 24 (27.6) | 20 (23.0) | 22 (25.9) | 0.78 |
| Percent body fat; mean (SD) | 29.4 (5.9) | 29.1 (6.1) | 30.0 (5.8) | 0.95 |
| Hormonal contraception ever; no. (%) | 47 (34) | 49 (35) | 44 (31) | 0.83 |
| Nulligravid; no. (%) | 57 (32) | 64 (36) | 56 (32) | 0.33 |
| Nulliparous; no. (%) | 60 (32) | 68 (36) | 61 (32) | 0.15 |
| Depression score; median (IQR) | 3 (0-6) | 4 (1-8) | 6 (3-11) | <0.0 01 |
| Current smoker; no. (%) | 14 (33) | 13 (31) | 15 (36) | 0.89 |
| Sleep (hours); mean (SD) | 7.3 (0.7) | 7.4 (0.7) | 7.2 (1.4) | 0.72 |
| Vigorous exercise (min/day); median (IQR) | 8.2 (0.7 -20.0) | 10.7 (3.2 -17.5) | 10.6 (2.8 - 22.3) | 0.46 |
| Pain medication use; no. (%) | 55 (31.4) | 67 (38.3) | 53 (30.3) | 0.07 |
| Antibiotic medication use; no. (%) | 7 (38.9) | 3 (16.7) | 8 (44.4) | 0.24 |
| Sexual activity; no. (%) | ||||
| Never | 15 (25) | 22 (37) | 22 (37) | |
| Previous, not current | 27 (42) | 20 (31) | 17 (27) | |
| Sexually active (<1/week) | 22 (32) | 23 (33) | 24 (35) | 0.63 |
| Sexually active (≥1/week) | 23 (37) | 20 (32) | 20 (32) | |
|
| ||||
| Diet | ||||
| Total energy (kcal); mean (SD) | 1550.2 (345.8) | 1684.9 (368.8) | 1604.6 (378.4) | 0.05 |
| Alcohol (g); median (IQR) | 0.08 (0.02, 3.9) | 0.07 (0.02, 2.3) | 0.14 (0.02, 4.2) | 0.49 |
| Mediterranean Diet Score; mean (SD) | 2.7 (0.8) | 2.8 (0.9) | 3.0 (1.1) | 0.12 |
| Fiber (g/day); mean (SD) | 12.8 (4.1) | 13.6 (6.1) | 14.5 (6.2) | 0.14 |
| Caffeine (mg/day); median (IQR) | 51.7 (16.4, 147.7) | 71.1 (23.3, 156.2) | 49.7 (16.7, 134.1) | 0.69 |
|
| ||||
| Menstrual Cycle Characteristics | ||||
| Menses Length (days); mean (SD) | 6.9 (1.5) | 7.0 (1.5) | 7.0 (1.9) | 0.94 |
| Blood loss (mL); median (IQR) | 55.0 (30.8 - 85.5) | 55.0 (27.5 - 78.0) | 53.5 (30.0 - 80.5) | 0.91 |
| Cycle length (days); mean (SD) | 28.5 (2.7) | 28.9 (3.0) | 28.9 (4.3) | 0.72 |
| Anovulatory (cycles); no. (%) | 6 (17) | 12 (34) | 17 (49) | 0.04 |
Abbreviations: IQR, interquartile range
Participants were asked to record their level of stress each day: 1 = not stressful; 2 = a little stressful; and 3 = very stressful. In Table 1, stress was averaged for each woman over the entire study period and categorized into tertiles (low, moderate, and high stress). All except baseline characteristics are averaged over the study period and compared between tertiles of daily stress. Analysis of variance for normally distributed continuous variables, Wilcoxon-Mann-Whitney test for non-normally distributed continuous variables; and Fisher's exact tests for categorical variables were used to test associations.
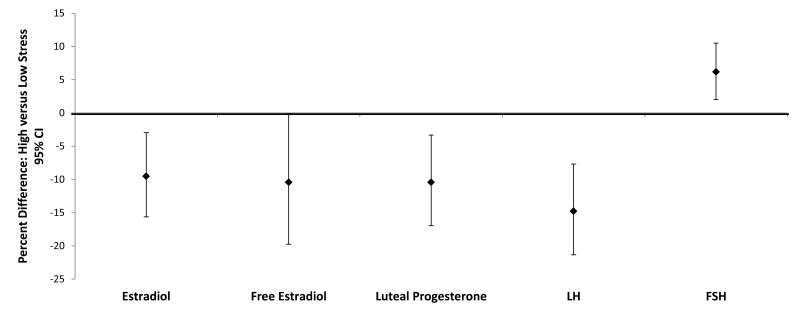
High recent daily stress was associated with lower estradiol (-9.5% [95% CI = -15.6% to -3.0%]), free estradiol (-10.4% [-16.5% to -3.9%]), luteal progesterone (-10.4% [-19.7% to -0.10%]), and LH (-14.8% [-21.3% to -7.7%]), and higher FSH (6.2% [2.0% to 10.5%]) concentrations after adjusting for age, race, percent body fat, depression score, and time-varying daily vigorous exercise and hormones compared with women with low recent daily stress (Figure). Continuous evaluation by cycle phase indicated that recent daily stress was associated with lower total and free E2 and LH during the follicular phase and with lower progesterone and higher FSH during the luteal phase. Specifically, for each one standard deviation increase in recent daily stress level, follicular total and free estradiol and follicular LH decreased by log -0.08 pg/mL (-0.12 to -0.04), -0.09 pg/mL (-0.12 to -0.05), and -0.07 ng/mL (-0.11, -0.03), respectively; luteal progesterone decreased by log -0.05 ng/mL (-0.11 to 0.01), and luteal FSH increased by log 0.04 mIU/mL (0.01 to 0.07) (Table 2). Similar, albeit attenuated, estimates were observed for the association between the PSS-4 and reproductive hormones with each standard deviation unit increase in PSS-4 associated with decreased log follicular free estradiol (β = -0.06 [95% CI = -0.09 to -0.001]) and LH (-0.06 [-0.12 to -0.03]); null findings were found for the baseline PSS-14. Our results were not appreciably altered by adjusting for other potential confounding factors individually, removing baseline depression score as a confounding factor, or assessing alternative exposure windows for daily stress prior to the clinic visit. No significant interactions between stress and age or race were identified (P>0.05).
Figure.
Analyses were conducted using weighted linear mixed models and adjusted for age, race, percent body fat, depression score, and time-varying daily vigorous exercise and concurrent reproductive hormones. FSH, follicle-stimulating hormone; LH, luteinizing hormone. Results are presented as average percent change in nontransformed reproductive hormone values for high versus low stress using the following formula: (exp (β) − 1) × 100%
Table 2. Mean difference in log serum concentrations of reproductive hormones according to one-standard-deviation continuous change in stress levela (n=507 cycles).
| Log Hormone | Daily Stress β (95% CI) | PSS-4 (cycle) β (95% CI) | PSS-14 (baseline) β (95% CI) |
|---|---|---|---|
| Estradiol (pg/mL)b | |||
| Follicular | -0.08 (-0.12 to -0.04) | -0.03 (-0.09 to 0.03) | 0.02 (-0.07 to 0.07) |
| Luteal | -0.01 (-0.04 to 0.03) | 0.03 (-0.03 to 0.06) | 0.05 (-0.01 to 0.14) |
| Free Estradiol (pg/mL)b | |||
| Follicular | -0.09 (-0.12 to -0.05) | -0.06 (-0.09 to -0.001) | 0.00 (-0.07 to 0.07) |
| Luteal | 0.00 (-0.08 to 0.07) | 0.01 (-0.03 to 0.03) | 0.03 (-0.02 to 0.14) |
| Progesterone (ng/mL)b | |||
| Luteal | -0.05 (-0.11 to 0.01) | 0.00 (-0.09 to 0.09) | 0.02 (-0.07 to 0.14) |
| FSH (mIU/mL)b | |||
| Follicular | 0.01 (-0.01 to 0.03) | 0.01 (-0.03 to 0.03) | 0.02 (-0.03 to 0.06) |
| Luteal | 0.04 (0.01 to 0.07) | 0.03 (-0.01 to 0.06) | 0.01 (-0.07 to 0.07) |
| LH (ng/mL)b | |||
| Follicular | -0.07 (-0.11 to -0.03) | -0.06 (-0.12 to -0.03) | -0.03 (-0.07 to 0.07) |
| Luteal | 0.03 (-0.01 to 0.07) | 0.01 (-0.06 to 0.06) | -0.06 (-0.14 to 0.01) |
Analyses conducted using linear mixed models adjusting for age, race, percent body fat, depression score, and daily vigorous exercise. Overall, there were 3891 (95.9%) measurements of each reproductive hormone collected from up to 16 clinic visits for the 259 women.
International System of Units (SI) conversion, estradiol: 1 pg/mL = 3.67 pmol/L; progesterone: 1 ng/mL = 3.18 nmol/L; LH: 1 ng/mL = 4.34 mIU/L; FSH 1 mIU/ml = 1 IU/L.
Sporadic anovulation was observed in 40 (7.9%) of the 507 cycles in this analysis. Women with high daily stress up until ovulation had greater odds of anovulation than women with low stress (adjusted OR = 2.3 [95% CI = 1.0 to 4.7]) after controlling for age, race, percent body fat, daily vigorous exercise, and depression score (Table 3). We found near-null relationships between the dichotomized 4- and 14-item PSS and anovulation. When assessed continuously, we found that for each increase in daily stress level, on average women had a 70% increased adjusted odds of an anovulatory episode (OR=1.7 [1.1 to 2.4]). Near-null associations were found for the 4- and 14-item PSS continuous assessment and anovulation. Similar but attenuated estimates were found when assessing (1) daily and PSS stress and (2) odds of anovulation based on the more conservative criterion for defining ovulation (data not shown). Women included in the case-crossover analysis (n=21) had a higher, increased odds of sporadic ovulation (OR = 3.0; P=0.24) for cycles with high versus low daily stress.
Table 3.
Adjusted odds of anovulation according to categorical and one standard deviation continuous change in stress level (n=507 cycles)*
| Stress | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Daily | PSS-4 (cycle) | PSS-14 (baseline) | |||||||
| Categorical | |||||||||
|
| |||||||||
| No. anovulatory/Total No. | (%) | OR (95% CI) | No. anovulatory/Total No. | (%) | OR (95% CI) | No. anovulatory/Total No. | (%) | OR (95% CI) | |
| Low Stress | 16/279 | (5.7) | 1.0 | 17/258 | (6.6) | 1.0 | 23/260 | (8.8) | 1.0 |
| High Stress | 23/227 | (10.1) | 2.2 (1.0 – 4.7) | 22/248 | (8.9) | 1.3 (0.6 – 2.7) | 16/244 | (6.6) | 0.6 (0.3 – 1.4) |
|
| |||||||||
| Continuous | |||||||||
|
| |||||||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||||
|
| |||||||||
| 1.7 (1.1 – 2.4) | 1.1 (0.8 – 1.8) | 1.1 (0.7 – 1.8) | |||||||
Anovulation was any cycle with peak progesterone concentration ≤ 5 ng/mL and no observed serum LH peak on the mid or late luteal phase visits (n=40/507 cycles; 7.9%). Daily stress was assessed via a daily diary (not stressful [1], a little stressful [2], very stressful [3]); PSS-4 was assessed at each clinic visit capturing the previous week's stress corresponding to menstruation, the mid-follicular phase, expected ovulation, and mid-luteal phases for each cycle; and PSS-14 was assessed at baseline to capture the previous month's stress. Daily and PSS-4 stress levels up until ovulation were averaged over each cycle. Categorical analyses: Average daily, PSS-4, and PSS-14 stress levels were categorized dichotomously (high stress: > median and low stress: ≤ median). Continuous analyses: For better comparison between instruments with different scales, results are presented as a one standard deviation change in stress level for each instrument. Analyses were performed using generalized linear mixed models adjusted for age, race, percent body fat, daily vigorous exercise, and depression score.
Discussion
We found that high daily stress was associated with lower total and free E2, LH, luteal progesterone, and higher FSH concentrations, and increased odds of sporadic anovulation, as compared with low stress. While it has long been demonstrated that severe stress under experimental conditions or among individuals sharing specific stressors can adversely affect female reproductive function,6 this is the first study to demonstrate that everyday stress among healthy women with no known reproductive disorders is linked to altered reproductive function. Our findings for daily, but not baseline, perceived stress indicate that it is recent stress that has the greatest impact on menstrual cycle parameters, including ovulatory function.
Our findings support the hypothesis that stress activates the HPA axis, leading to an inhibition of the requisite preovulatory LH surge, and consequent suppression of ovulation as evidenced by higher FSH levels.30–32 However, alternative pathways may affect reproductive function, such as activation of the sympathetic-adrenal medullary axis leading to altered blood flow and interrupted gamete transport.4 Given previous research showing a reduction in fecundability mediated via the sympathetic-adrenal medullary pathway, further research in this evolving area is warranted.4
We are aware of only two previous epidemiologic studies assessing the effects of stress on reproductive hormones and menstrual cycle function among premenopausal women.7,8 Similar to our findings of increased stress associated with decreased luteal progesterone levels, a study among 24 parous Mayan women not on hormonal contraception found that increased physiologic stress, as measured via cortisol, was associated with decreased urinary prenandiol during the luteal phase.7 Contrary to our findings pertaining to daily stress and anovulation, another study among 276 healthy, premenopausal U.S. women found that psychological stress in the workplace was not associated with ovulatory function.8 However, this prior study only assessed job-related stress measured at baseline.8 When using the baseline PSS-14 assessment, we, too, found no association between perceived stress and ovulatory function. The assessment of everyday psychosocial stress captured daily over the course of the entire menstrual cycle may more accurately capture how a woman reacts physically to daily stressors compared with a single baseline measurement found to have poor correlation with fecundity.33 Indeed, we found only slight to fair agreement34 between baseline PSS-14 and average daily stress tertiles (κ=0.21 [95% CI = 0.12 – 0.29]) and relatively poor correlation (r=0.31). Furthermore, our similar but attenuated estimates with the PSS-4 and reproductive hormones compared with average prior 5-day daily perceived stress (with only moderate agreement between tools, κ=0.46 [95% CI = 0.38 to 0.53],34 and moderate correlation, r=0.53) suggests that it is not only the timing of the stress measurement tool that matters but also the tool used. While the PSS has been validated in many populations for chronic disease, its use to measure reproductive function, particularly among healthy women, has not been validated. While previous research highlights the strengths of capturing stress through diary designs,10, 11 future validation work is needed.
An association of perceived stress with reproductive hormones and ovulatory function has substantial implications for women's health, if established by further research. Alterations in gonadotropins and gonadal steroids are known to affect a woman's chance of conceiving.3, 35 However, studies assessing the effect of psychosocial stress on female fecundity via the HPA axis, among women not undergoing in vitro fertilization, have been inconsistent to date.33, 36 Mixed findings could be attributable to both variation in assessment time points and differences in psychosocial stress measurement tools and/or threshold scores.37 Future studies looking to investigate the effects of perceived stress on female fecundity would be advised to measure recent stress and make certain that assessment tools are validated with physiologic measures in the population of interest.33
The BioCycle Study had several strengths including a prospective study design; large sample size (both in number of women and number of cycles) for assessing the effects of repeated measurements of perceived stress on reproductive hormones and ovulatory function; various tools for psychosocial stress assessment; and well-measured confounding factors associated with stress and reproduction. Nevertheless, this study was confined in its ability to take advantage of the powerful case-crossover analyses due to the limited number of women with both an ovulatory and anovulatory episode, as well as due to the relatively few women reporting high levels of stress compared with the national average.1 While we were limited in power to detect a difference in our case-crossover analyses, our prospective assessment of daily stress helped reduce any misclassification bias for all of our analyses. A larger epidemiologic study following women for more than two cycles should be considered. We do acknowledge that women with higher perceived stress levels may be less likely to complete their daily diaries; however, given the associations we found between daily perceived stress and suppression of estradiol, progesterone, LH, and ovulation, we expect that we may be underestimating, versus overestimating, the effect of stress on menstrual cycle function. A larger epidemiologic study assessing women's daily perceived stress levels and menstrual cycle function, following women for more than two cycles, should be considered.
In regard to the generalizability of our findings, although the BioCycle Study's racial/ethnic makeup was fairly representative of the US population with the exception of Hispanics,38 women in our study were of higher socioeconomic status as reflected in 87% having post-secondary education or above compared with 59% for US adult females.38 Although previous large population-based studies have corroborated our findings regarding the inverse relationship between education level and perceived stress,39 future work looking at the effects of stress on women's reproductive health should be expanded to include women of less privileged backgrounds who may experience more severe or alternative forms of psychosocial stress.
In summary, higher perceived daily stress levels among this cohort of healthy premenopausal women were associated with lower concentrations of total and free E2, LH, and luteal progesterone, higher concentrations of FSH, and increased odds for sporadic anovulation, after taking into account important baseline and time-varying confounding factors. While our study was not designed to assess the mechanism by which stress might lead to reproductive dysfunction, we were able to demonstrate that daily perceived stress does interfere with normal menstrual cycle function, warranting further research to explore potential population-level impacts and causal biologic mechanisms.
Acknowledgments
We are indebted to all the investigators and staff at the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the University at Buffalo for their respective roles in the study. We also recognize the BioCycle participants for their extraordinary commitment to the study.
Funding: Funded by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contract Number: HHSN275200403394C). KS, SM, KA, NP, LS, KK, AP, and ES received support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health for the submitted work and CV received support from grant T32-HL007055, National Heart, Lung, and Blood Institute, National Institutes of Health.
References
- 1.American Psychological Association. Stress in America Findings 2012: Stress by Gender. [Accessed 26 June 2014]; Available at: http://www.apa.org/news/press/releases/stress.
- 2.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 3.Ferin M. Clinical review 105: Stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 4.Louis GM, Lum KJ, Sundaram R, et al. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril. 2011;95:2184–2189. doi: 10.1016/j.fertnstert.2010.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor KA, Brindle E, Shofer J, et al. The effects of a long-term psychosocial stress on reproductive indicators in the baboon. Am J Phys Anthropol. 2011;145:629–638. doi: 10.1002/ajpa.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berga SL, Loucks TL. The diagnosis and treatment of stress-induced anovulation. Minerva Ginecol. 2005;57:45–54. [PubMed] [Google Scholar]
- 7.Nepomnaschy PA, Welch K, McConnell D, Strassmann BI, England BG. Stress and female reproductive function: a study of daily variations in cortisol, gonadotrophins, and gonadal steroids in a rural Mayan population. Am J Hum Biol. 2004;16:523–532. doi: 10.1002/ajhb.20057. [DOI] [PubMed] [Google Scholar]
- 8.Fenster L, Waller K, Chen J, et al. Psychological stress in the workplace and menstrual function. Am J Epidemiol. 1999;149:127–134. doi: 10.1093/oxfordjournals.aje.a009777. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 10.Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci. 2010 Sep;65:513–25. doi: 10.1093/geronb/gbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolger N1, Davis A, Rafaeli E. Diary methods: capturing life as it is lived. Annu Rev Psychol. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- 12.Wang L1, Wang X, Wang W, Chen C, Ronnennberg AG, Guang W, Huang A, Fang Z, Zang T, Wang L, Xu X. Stress and dysmenorrhoea: a population based prospective study. Occup Environ Med. 2004;61:1021–6. doi: 10.1136/oem.2003.012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjollund NH, Jensen TK, Bonde JP, et al. Distress and reduced fertility: a follow-up study of first-pregnancy planners. Fertil Steril. 1999;72:47–53. doi: 10.1016/s0015-0282(99)00186-7. [DOI] [PubMed] [Google Scholar]
- 14.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 17.Lynch KE, Mumford SL, Schliep KC, et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil Steril. 2014;102:511–518. doi: 10.1016/j.fertnstert.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasharathy SS, Mumford SL, Pollack AZ, et al. Menstrual bleeding patterns among regularly menstruating women. Am J Epidemiol. 2012;175:536–545. doi: 10.1093/aje/kwr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt KM, Dimmock PW, Walker TJ, O'Brien PM. Determination of total menstrual blood loss. Fertil Steril. 2001;76:125–131. doi: 10.1016/s0015-0282(01)01847-7. [DOI] [PubMed] [Google Scholar]
- 20.Gaskins AJ, Rovner AJ, Mumford SL, et al. Adherence to a Mediterranean diet and plasma concentrations of lipid peroxidation in premenopausal women. Am J Clin Nutr. 2010;92:1461–7. doi: 10.3945/ajcn.110.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schliep KC, Schisterman EF, Mumford SL, et al. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle Study. Am J Clin Nutr. 2012;95:488–497. doi: 10.3945/ajcn.111.021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Giurgescu C, Zenk SN, Dancy BL, Park CG, Dieber W, Block R. Relationships among neighborhood environment, racial discrimination, psychological distress, and preterm birth in African American women. J Obstet Gynecol Neonatal Nurs. 2012;41:E51–61. doi: 10.1111/j.1552-6909.2012.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–37. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]
- 25.Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Bäckström T, Fernández G. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35:47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 29.Toh S, Mitchell AA, Anderka M, de Jong-van den Berg LT, Hernández-Díaz S, Study NBDP. Antibiotics and oral contraceptive failure - a case-crossover study. Contraception. 2011;83:418–425. doi: 10.1016/j.contraception.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrekoussis T, Kalantaridou SN, Mastorakos G, et al. The role of stress in female reproduction and pregnancy: an update. Ann N Y Acad Sci. 2010;1205:69–75. doi: 10.1111/j.1749-6632.2010.05686.x. [DOI] [PubMed] [Google Scholar]
- 31.Fritz M, Sperhoff L. Clinical Gynecology Endocrinology and Infertility. Eighth. Philadelphia, PA: Lippincott, Williams & Wilkins; 2011. [Google Scholar]
- 32.De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG. Lasley BL High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–32. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- 33.Lynch CD, Sundaram R, Buck Louis GM, Lum KJ, Pyper C. Are increased levels of self-reported psychosocial stress, anxiety, and depression associated with fecundity? Fertil Steril. 2012;98:453–458. doi: 10.1016/j.fertnstert.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 35.Baird DD, Weinberg CR, Zhou H, et al. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril. 1999;71:40–49. doi: 10.1016/s0015-0282(98)00419-1. [DOI] [PubMed] [Google Scholar]
- 36.Sanders KA, Bruce NW. A prospective study of psychosocial stress and fertility in women. Hum Reprod. 1997;12:2324–29. doi: 10.1093/humrep/12.10.2324. [DOI] [PubMed] [Google Scholar]
- 37.Williams KE, Marsh WK, Rasgon NL. Mood disorders and fertility in women: a critical review of the literature and implications for future research. Hum Reprod Update. 2007;13:607–16. doi: 10.1093/humupd/dmm019. [DOI] [PubMed] [Google Scholar]
- 38.United States Census Bureau. 2010 Census. [Accessed on August 29, 2014]; Available at https://www.census.gov/
- 39.Redmond N, Richman J, Gamboa CM, Albert MA, Sims M, Durant RW, Glasser SP, Safford MM. Perceived stress is associated with incident coronary heart disease and all-cause mortality in low- but not high-income participants in the Reasons for Geographic And Racial Differences in Stroke study. J Am Heart Assoc. 2013;2:e000447. doi: 10.1161/JAHA.113.000447. [DOI] [PMC free article] [PubMed] [Google Scholar]