Abstract
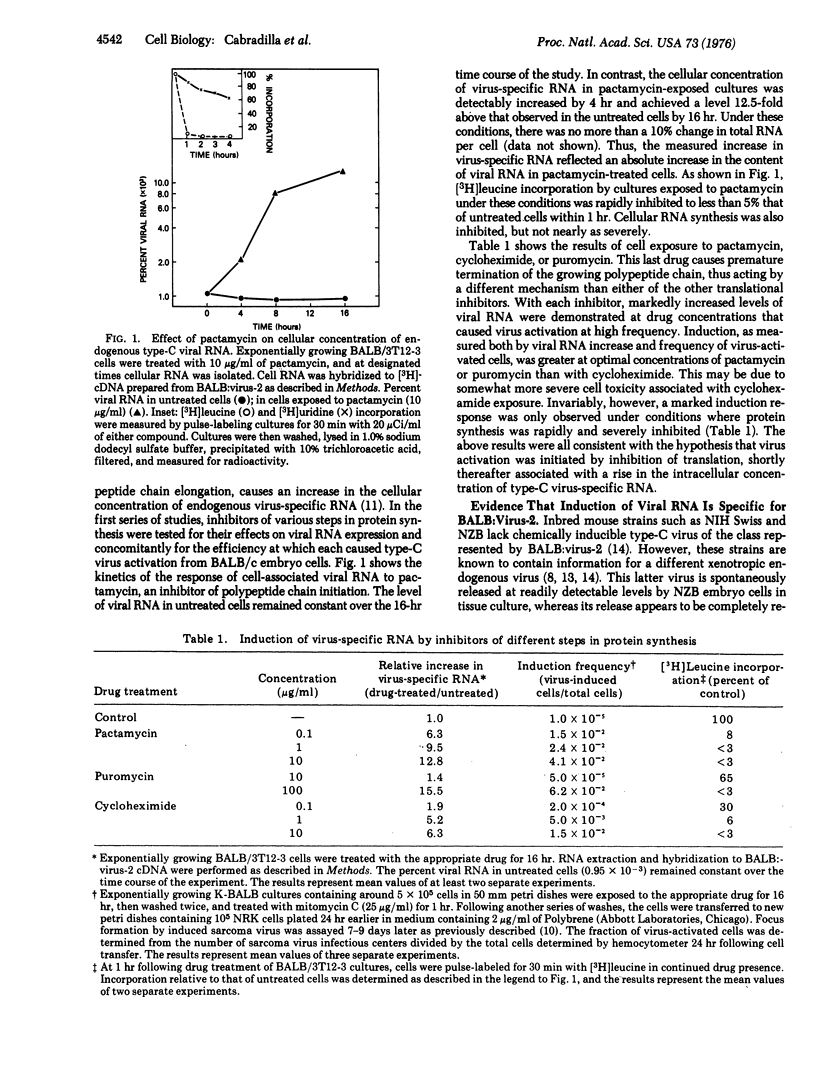
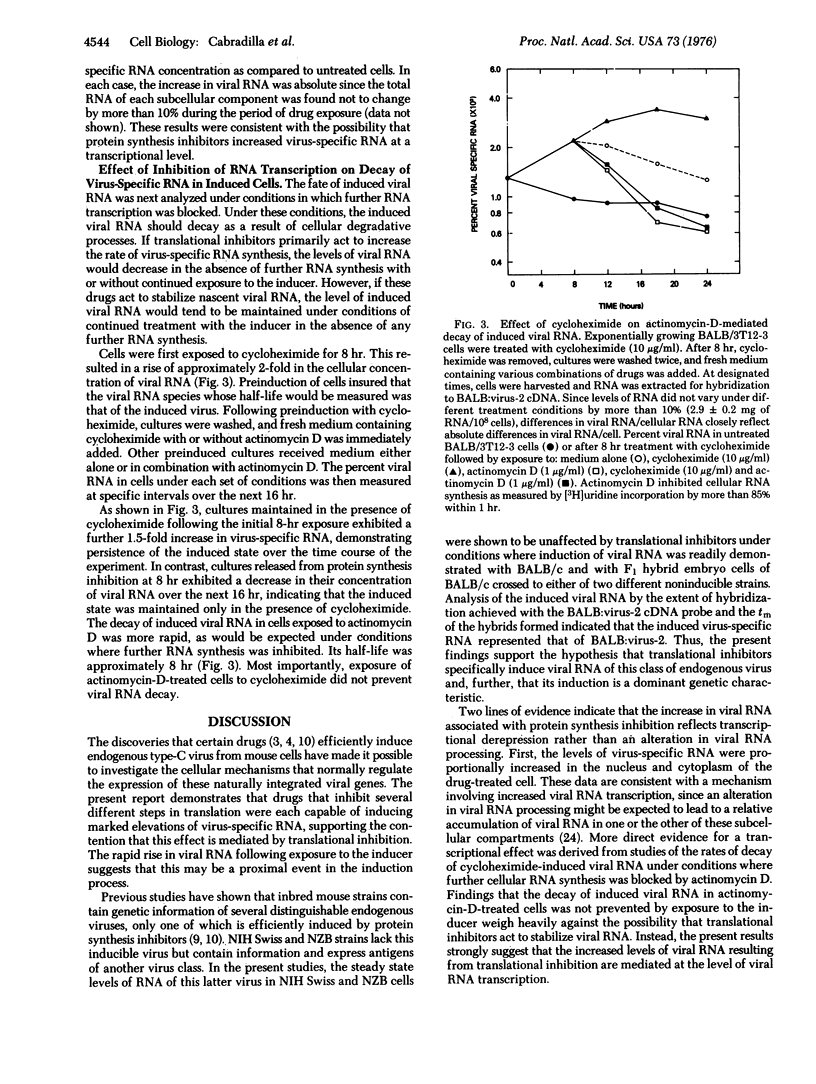
Several biologically distinguishable type-C RNA viruses are genetically transmitted in mouse cells. In the present report, chemicals that inhibit several different steps in protein synthesis are shown to cause marked increases in the cellular concentration of virus-specific RNA and the subsequent induction of virus. Analysis of the effect of translational inhibitors on mouse embryo cells of different genotypes indicates that activation of viral RNA is specific for one endogenous virus class and is a dominant genetic characteristic. Two lines of evidence favor the hypothesis that the induction of viral RNA involves transcriptional derepression rather than an alteration in its post-transcriptional processing. First, nuclear and cytoplasmic fractions of induced cells are shown to demonstrate similar increases in their concentrations of virus-specific RNA. Second, the decay of induced viral RNA following inhibition of further RNA synthesis by actinomycin D is not prevented by continued exposure to the inducer. These findings weigh heavily against the possibility that translational inhibitors act to stabilize viral RNA post-transcriptionally. The results are consistent with a model in which the expression of one class of endogenous virus is regulated by a labile repressor protein acting at a transcriptional level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Anderson G. R., Dunn C. Y., Robbins K. C. Induction of type-C RNA virus by cycloheximide: increased expression of virus-specific RNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3941–3945. doi: 10.1073/pnas.71.10.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Dunn C. Y. High-frequency C-type virus induction by inhibitors of protein synthesis. Science. 1974 Feb 1;183(4123):422–424. doi: 10.1126/science.183.4123.422. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Stephenson J. R. Differential cellular regulation of three distinct classes of type C RNA viruses endogenous to mouse cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1129–1137. doi: 10.1101/sqb.1974.039.01.129. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Besmer P., Smotkin D., Haseltine W., Fan H., Wilson A. T., Paskind M., Weinberg R., Baltimore D. Mechanism of induction of RNA tumor viruses by halogenated pyrimidines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1103–1107. doi: 10.1101/sqb.1974.039.01.125. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Evidence that the AKR murine-leukemia-virus genome is complete in DNA of the high-virus AKR mouse and incomplete in the DNA of the "virus-negative" NIH mouse. Proc Natl Acad Sci U S A. 1974 Jan;71(1):167–171. doi: 10.1073/pnas.71.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Radiommunoassays for the 70,000-molecular-weight glycoproteins of endogenous mouse type C viruses: viral antigen expression in normal mouse tissues and sera. J Virol. 1976 Jun;18(3):933–941. doi: 10.1128/jvi.18.3.933-941.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Wilbert S. M., Black P. H. Analysis of simian virus 40-induced transformation of hamster kidney tissue in vitro. 8. Induction of infectious simian virus 40 from virogenie transformed hamster cells by amino acid deprivation or cycloheximide treatment. J Virol. 1972 Mar;9(3):448–453. doi: 10.1128/jvi.9.3.448-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Rosenak M. J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. A genetic locus for inducibility of C-type in BALB-c cells: the effect of a nonlinked regulatory gene on detection of virus after chemical activation. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2798–2801. doi: 10.1073/pnas.69.10.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Demonstration of a genetic factor influencing spontaneous release of a xenotropic virus of mouse cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4925–4929. doi: 10.1073/pnas.71.12.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]


