Abstract
Stiffness of large arteries has been long recognized as a significant determinant of pulse pressure. However, it is only in recent decades, with the accumulation of longitudinal data from large and varied epidemiological studies of morbidity and mortality associated with cardiovascular disease, that it has emerged as an independent predictor of cardiovascular risk. This has generated substantial interest in investigations related to intrinsic causative and associated factors responsible for the alteration of mechanical properties of the arterial wall, with the aim to uncover specific pathways that could be interrogated to prevent or reverse arterial stiffening. Much has been written on the haemodynamic relevance of arterial stiffness in terms of the quantification of pulsatile relationships of blood pressure and flow in conduit arteries. Indeed, much of this early work regarded blood vessels as passive elastic conduits, with the endothelial layer considered as an inactive lining of the lumen and as an interface to flowing blood. However, recent advances in molecular biology and increased technological sophistication for the detection of low concentrations of biochemical compounds have elucidated the highly important regulatory role of the endothelial cell affecting vascular function. These techniques have enabled research into the interaction of the underlying passive mechanical properties of the arterial wall with the active cellular and molecular processes that regulate the local environment of the load-bearing components. This review addresses these emerging concepts.
Key Words: Pulse wave velocity, Arterial haemodynamics, Endothelial function, Conduit arteries, Pulse pressure, Vascular ageing
Introduction
The concept of ‘hardening of arteries’ arose from the early autopsy observations showing predominantly atheromatous calcified plaques and obstructive lesions in the intima of blood vessels [1]. The more recent description of ‘arterial stiffening’ relates to alterations of medial properties leading to reduced distensibility of the arterial wall, and so decreasing the buffering capacity of arteries to pulsatile cardiac ejection [2,3]. The obstructive condition is generally considered to be due to atherosclerosis, usually defined as accumulation of lipid and calcium deposits, whereas medial degeneration is due to alterations of load-bearing components, a condition often referred to as arteriosclerosis [4,5]. Both conditions are inherent in the fundamental determinants of cardiovascular risk: vascular obstruction causing limb or organ ischaemia and arterial stiffness, particularly of the aorta, leading to increased pulse pressure, conditions that increase in severity with age [6]. The intrinsic importance of vascular function has been recognized for the past centuries, as exemplified by the famous dictum of Thomas Sydenham (1624-1688) that ‘a man is as old as his arteries ...’. Recent theories of ageing suggest that it is the changes which occur in the vasculature that essentially determine the fate of the entire organism [6,7,8,9]. Notwithstanding the overwhelming research effort that has taken place in the fields of vascular biology and hypertension, they still remain the most significant factors for cardiovascular disease, and although much has been learned, many of the underlying mechanisms and effective strategies for arresting or preventing the development of vascular degeneration still remain elusive.
Because of the intermittent ejection of blood from the ventricles into the aorta and pulmonary artery and the metabolic requirement of a steady flow in the microcirculation for efficient tissue perfusion, the distensibility of large arteries is an important and fundamental determinant of the relationship between pulsatile pressure and flow [2]. The loss of elasticity of the artery wall leads to stiffening of the conduit vessels, reducing arterial storage capacity as well as increasing the speed of the propagating pulse along the vessel wall. That is, for a given ventricular stroke volume, arterial stiffness is a major determinant of pulse pressure due to the combined influence on the capacitive effects of the artery wall to absorb the pulsatile energy and the wave propagation effects that influence peripheral wave reflection. These factors form the underlying mechanisms of the gradual increase in systolic pressure with age, especially after the 5th decade [10], leading to the development of isolated systolic hypertension in the elderly and to an increased cardiovascular risk [10,11,12]. These mechanisms also have a dominant role in the significance of pulse pressure [13] and the emergence of arterial pulse wave velocity (PWV) as an increasingly powerful independent predictor of cardiovascular morbidity and mortality [14,15,16] and significant reclassifier of cardiovascular risk [17]. Studies in subjects with diabetes and glucose intolerance suggest that aortic PWV, as an index of global arterial stiffness, may indeed be an integrated index of vascular function [18]. The early work by many investigators of the past six decades in quantifying the relationship between pulsatile pressure and flow in arteries has laid the basic biophysical foundations of the functional haemodynamics [2]. However, the mechanisms of what alter physical properties of the vessel wall leading to arterial stiffening are still not as well established.
The emerging field of molecular biology over the past two decades, in combination with the biophysical principles in arterial haemodynamics, is enabling investigations into the underlying factors that translate structural changes and modifications of artery wall constituents to functional correlates. These are seen as increased PWV and arterial pulse pressure, both highly significant factors of cardiovascular risk and end-organ damage. The artery wall constituents can be altered by passive stimuli, such as increased mechanical stress due to distending pressure. These lead to structural disorganization, fatiguing effects, and fragmentation of elastic fibres [3,8,19]. Alterations can also result from active changes mediated through a cascade of biochemical cellular signalling processes affecting the integrity of the extracellular matrix (ECM), translating to altered arterial functional properties. Molecular probes are making it possible to uncover pathways involved in the interaction between cellular processes and the ECM in the artery wall [20] through biochemical and mechanotransduction signalling [21,22], thus opening up avenues for active interrogation of these pathways for direct regulation of arterial stiffness. This review addresses the fundamental definition of the material stiffness of the artery wall in terms of physical mechanical quantities and describes the related underlying biological factors associated with the alteration of wall properties leading to arterial stiffening.
Definition of Arterial Stiffness
The functional effects of arterial stiffness involve alteration of fundamental mechanical behaviour of the material properties of the artery wall as well as the effect of wall properties on changes of geometry and wall tension. The material properties are defined in terms of the fractional deformation (strain) due to an applied force per unit area (stress). The ratio of uniaxial stress to uniaxial strain is defined as the elastic (Young's) modulus (E), describing the stiffness of the material. For an isotropic material, E is constant in all directions. However, since arteries are essentially non-isotropic, E does not have the same value for circumferential or axial deformation. Conventionally, due to tethering, the deformation produced by the intra-arterial pressure (P) is considered to be mainly in the circumferential direction with a change in diameter (D). Due to the cylindrical structure, the stress and strain can be represented by P and the fractional change in diameter (ΔD/D), respectively. Hence, for a constant length, the volume elastic modulus (or Peterson's elastic modulus, Ep) is defined as Ep = P/(ΔD/D). The important aspect of Ep as a stiffness parameter is that pressure and diameter are measurable quantities and can be obtained non-invasively. For a linear elastic material, the relation of stress and strain is constant, and the material has a single value of the elastic modulus. However, the material properties of the artery wall change with applied force, hence the value of the elastic modulus depends on pressure and consequently the state of distension. This is described as the incremental elastic modulus (Einc), which is the tangent of the stress-strain curve at any specific point: Einc = ΔP/(ΔD/D).
For any circular elastic structure, the circumferential wall tension (T) is related to the internal pressure by the law of Laplace (T = P · D/2), assuming the wall thickness (h) is much smaller than D. This allows the computation of the material stiffness property since the circumferential stress (S) is T · h, hence ΔS = ΔP · D/2h. The incremental stress ΔS will cause an incremental circumferential strain equivalent to ΔD/D. Thus, Einc = ΔP · D2/(2hΔD). From the relationship between PWV and square root of bulk elastic modulus (ΔP/(ΔD/D) and blood density (ρ) [2], the Moens-Korteweg relation is obtained, which relates PWV to the wall stiffness and geometry of the arterial cylindrical structure: PWV = (Einc · h/Dρ)1/2. This suggests that for a uniform arterial segment, PWV can be used as a surrogate of arterial stiffness, with the assumption that the relative wall thickness (h/D) remains constant.
The basic concepts outlined above have been developed and treated in early fundamental studies of arterial mechanics using isolated arterial segments assessing static and dynamic properties [23], pressure dependency of elastic properties [24,25] and effect of smooth muscle activation [26]. Recent studies and reviews have addressed the various indices of arterial stiffness that can be derived from measured quantities, essentially pressure and diameter [27,28,29]. These studies provide extensive tabulated formulas and definitions.
Surrogate Measures of Arterial Stiffness
Arterial stiffness is explicitly defined in terms of mechanical parameters of arterial properties (stress/strain relationships). However, these are not readily measured in vivo, and so various surrogate parameters are employed. These have been extensively treated in previous studies and reviews, including the inherent assumptions, confounding factors and limitations [27,28,29,30,31,32,33].
Load-Bearing Components
The main load-bearing components in large conduit arteries are elastin and collagen, with a much lower contribution by smooth muscle in the muscular arteries. Due to the anatomical arrangement of the elastin and collagen fibres, elastin engages at low distension (hence at low pressure) and collagen at higher distension (and pressure) [34]. However, although the lamella unit is proposed as being the fundamental structural element of elastin in the media of the artery wall [35], there is significant variation in human arteries compared to other species [36]. In addition, there is substantial variation in the isotropic properties [25] and the contribution to wall stiffness of elastin and collagen along the aortic trunk [37,38]. The adaptation seen with a change in function is evident as the design of load-bearing components is optimized to minimize the amount of collagen recruitment, and thus stiffness, as it is a necessary function in diving mammals [39].
Arterial Stiffness Dependence on Distending Pressure
An inherent feature of the mechanical properties of arteries is that the wall becomes stiffer with distending pressure [24]. This is due to the increased amount of recruitment of stiffer collagen fibres with increasing distension. That is, the relationship of stress (pressure) and strain (diameter) is non-linear, with concavity toward the distension axis, such that there is diminishing distension with increasing force. This property is essential for the efficient mechanical operation of arteries as conduits for blood, such that, with the maintaining of residual stress, the vessels do not collapse and so always ensure patency for blood flow. That is, the wall tension (T) as balanced by the transmural pressure (P) and radius (r) (T = P · r, as determined by Laplace's law) has a single operating point on the pressure-diameter curve. Indeed, the non-linear elastic behaviour of arteries has been described as a fundamental evolutionary property of the arterial design for all vertebrates and invertebrates with closed circulatory systems [40].
Haemodynamic Effects of Arterial Stiffness
Arterial stiffness is a major determinant of vascular impedance, hence affecting the relationship between arterial pressure and flow [2]. Since flow is determined by the local spatial (x) pressure (p) gradient (dp/dx) and the relationship between time (t) derivative (dp/dt) and dp/dx is dx/dt (that is, wave velocity), the effect is that local wave velocity becomes a determinant of the instantaneous relationship between pressure and flow. For elastic conduits, the wave velocity is related to the stiffness of the wall, so changes in stiffness will modulate the pressure-flow relationships. This is then expressed as changes in the frequency spectrum of arterial impedance [2].
Effects on Blood Storage (Compliance): Determinant of Pulse Pressure
In a closed circulatory system, blood is stored in distensible compartments, with the venous system being responsible for buffering slow and relatively large changes in volume. However, the arteries, with the residual wall stress and elastic walls, are also able to buffer rapid changes in blood volume, such as occur during a single cardiac cycle. Hence, the value of the elastic modulus of the artery wall is such that there is sufficient recoil so that the volume taken up during systole is returned during diastole, hence buffering the pressure due to pulsatile ejection. Thus, increases in arterial stiffness will generate higher pulse pressure (PP) for similar stroke volumes (SV). Since the SV is the volume taken up by arterial distension and that flowing through the peripheral resistance (R), the ratio SV/PP is related to the total arterial compliance (C). In terms of arterial design, arterial stiffness is matched to obtain a value of C so as to optimize blood volume in the arterial compartment. For example, for maximal damping of PP, a large value of C would be required for a given SV, that is, a highly distensible system. However, this would store large volumes with a slow time constant (RC) for recoil, and so would result in an inefficient circulation because of high inertia due to the large blood mass to be displaced. These concepts are quantified in terms of the lumped parameter Windkessel (RC) model of the arterial system and extended to a three-element model by the addition of the characteristic impedance (Zc) [41]. The model has been recently used to compute the intrinsic reservoir pressure due to the increase in aortic volume associated with cardiac ejection [42].
Effects of Wave Propagation: Pulse Wave Velocity – Surrogate Measure of Arterial Stiffness
Although the Windkessel model is adequate for lumped parameter estimation, it does not account for the finite time travel of the arterial pulse [41]. This requires a spatially distributed system which is described in terms of wave propagation characteristics. Arterial stiffness affects PWV through the constitutive relation of wall stiffness, vessel geometry and blood density (Moens-Korteweg equation). In the large conduit arteries, the small ratio of wall thickness in relation to diameter (h/D) makes changes in the material stiffness of the artery highly correlated with measured PWV. This is manifest by the similar non-linear dependency of PWV on distending pressure. Hence, the measurement of pulse propagation time over a known distance to compute PWV has been found to be a robust surrogate of arterial stiffness, in the absence of any confounding arterial malformation such as significant stenosis [29].
Arterial Stiffness as Manifestation of Vascular Ageing
The most significant parameters that alter stiffness of conduit arteries are age [43] and associated processes [8]. This has a complex association with the overall burden of vascular disease [6], in different populations [44] and with associated cardiovascular risk [9]. Indeed, recent reviews on the subject have focused on the association between vascular ageing and the broad spectrum of co-existing conditions associated with cardiovascular disease, such as hypertension, diabetes and metabolic syndrome and the management strategies of vascular ageing [45].
Mechanisms of Arterial Stiffness
Stiffening of arteries is generally associated with changes in mechanical properties of the arterial wall. That is, alterations of stress/strain characteristics due to modification of properties of load-bearing structural components. The underlying mechanisms responsible for such modifications involve a complex interaction between the material properties of connective tissue and cell signalling pathways that alter the intrinsic and combined function of elastin, collagen, proteoglycans and glycoproteins of the ECM of the artery wall. A number of reviews have addressed these issues [3,32,46], with the suggestion that the specific mechanisms can interact by way of positive or negative feedback pathways, depending on the extent of the stimulus [47].
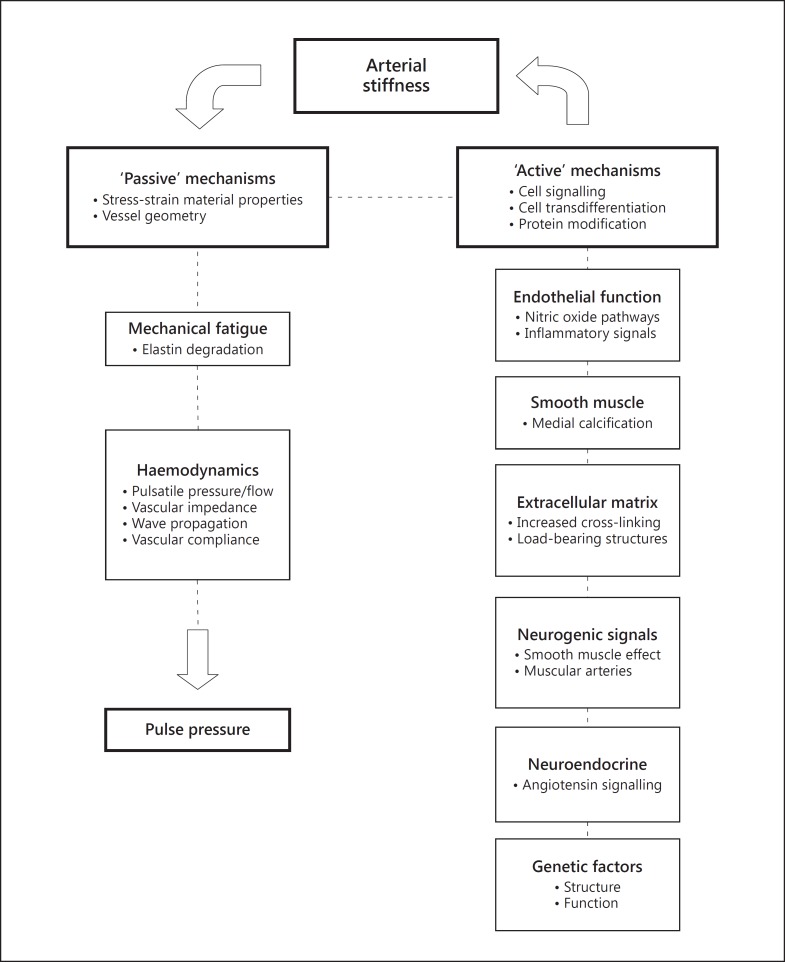
Essentially, the underlying mechanisms can be considered as those related to elementary material properties, that is, ‘passive’ mechanisms, and those that are regulated by cellular and molecular signalling where pathways can be interrogated, that is, ‘active’ mechanisms (fig. 1).
Fig. 1.
Components of passive and active mechanisms related to arterial stiffness as described in the text and that combine to affect pulse pressure, assuming a regulated stroke volume. The active mechanisms are mainly causative for the development of arterial stiffness and the passive properties are the result of arterial stiffening. There are complex feedback relationships between the components, some of which can be interrogated by altering the cellular signalling pathways. Passive pathways can be affected by alteration of haemodynamic parameters such as wave reflection.
Passive Mechanisms
Mechanical Properties Related to Intra-Arterial Distending
The interaction of loading function of the wall components that bear the wall tension due to the distending pressure produces non-linear wall mechanical properties such that the wall becomes stiffer with increased distension. That is, the stiffness becomes pressure dependent [24]. This is an important and intrinsic property of arterial design [40,48]. Since an increase in distending pressure leads to an increase in stiffness, which then can potentiate a further increase in pulse pressure, this property constitutes a potential positive feedback mechanism in relation to the relevance of arterial stiffness to cardiovascular risk, given the importance of systolic pressure, especially in age-related isolated systolic hypertension.
Effects of Mechanical Fatigue and Fracture of Elastin Structures
All structural proteins in biology have elastic characteristics, with some rubber-like proteins (e.g. elastin, resilin) functioning with high resilience, large deformability (strains) and low stiffness, resulting in the ability to store elastic strain energy [49]. In arteries, this is a characteristic of both elastin and collagen, although elastin is much more extensible at lower strains than collagen. However, just as the efficiency of resilin determines the performance of insect wings during their lifetime [50], the efficiency of elastin is also a significant determinant of the overall stiffness of the arterial wall throughout life. From evolutionary considerations, it is reasonable to assume that the range of properties of elastic proteins will predispose elastic structures that are subjected to repetitive strains to a high resistance to fracture.
Due to the pulsatile nature of the circulatory design, arteries are subjected to continuous and repetitive strain throughout life. In human tissue, radiocarbon prevalence data show a range of half-life of 40-174 years (mean 74 years) [51], making elastin the protein in the human body with the longest longevity. Having such a stable form with minimal turnover, the question is whether it can be subjected to the mechanical degradation effects of fatigue due to repetitive and unceasing strain throughout life. Such concepts are advanced as a mechanism of arterial stiffness due to elastin degradation, given the 30 million cycles per year to which the arteries are exposed [3], and so passive elastin degradation occurs with age, as distinct from active enzymatic processes [due to matrix metalloproteinase (MMP) activity] [8]. Evidence of an increased degree of disorganization and fracture of aortic elastin associated with the total number of cardiac cycles throughout life was found in a cross-sectional study of several species with a wide range of body size, heart rate and life span [19]. This is complemented by structural alterations due to embryonic abnormalities affecting the structure of elastin throughout life, with an increased predisposition to elevated arterial stiffness and associated cardiovascular risk [52]. This finding has been recently confirmed in the aorta of mice with elastin haploinsufficiency, where increased aortic stiffness precedes blood pressure elevation during postnatal development [53]. Other evidence of possible effects of fatigue on aortic elastin is obtained from the association of fragmentation and reduction of interlamellar fibres and the formation of aortic dissecting aneurysms [54]. Recent investigations in the role of elastin in arterial stiffness of large arteries have suggested means of reversing alterations to elastic fibres as a therapeutic treatment for hypertension [55].
Effects of Heart Rate
The cardiovascular risk associated with elevated heart rate has been shown to be comparable to that associated with increased arterial pressure, where a 20% increase in cardiac death is associated with both a rise in heart rate of 10 beats/min and an increase in systolic pressure of 10 mm Hg [56]. Although the underlying causes are mainly related to increased sympathetic activity, there is also evidence that elevated heart rate is independently associated with an increased progression of arterial stiffness as measured by aortic PWV [57]. Underlying mechanisms for this association have been investigated in experimental conditions in paced human subjects [30,58] as well as in rat models [59,60] and where interventions were controlled for changes in arterial pressure [31]. The effect has been suggested to be due to the viscoelastic properties of the arterial wall [61,62].
Active Mechanisms
Mechanisms of arterial stiffness associated with cellular and molecular processes have the potential for pharmacological interrogation of biochemical pathways. However, whereas the mechanical, structural and haemodynamic correlates of arterial stiffness that constitute the passive mechanisms are well established [2], the biochemical pathways that constitute possible active mechanisms and that lead to increased functional stiffness of the artery wall are not as well defined, although there is increasing interest across a range of fields in elucidating specific molecules that may play a significant role [63]. There is evidence that similar mechanisms are involved in vascular ageing [6,8] and inflammation [64,65]. Although specific proof in humans is yet to be fully established, there is increasing evidence in experimental animals, comprising mainly rats and mice models, of the modification of the ECM through cellular, molecular, neurogenic and neuroendocrine pathways, some of which may be potentiated by genetic mechanisms.
Cellular Mechanisms
In the artery wall, the cellular mechanisms related to arterial stiffness are mediated by endothelial cells and smooth muscle cells. The description below does not relate to the effect of the cells per se on the wall stiffness, but rather to the pathways associated with the modification of the structural integrity of the arterial media leading to modifications of functional stiffness of the arterial conduit.
Role of the Endothelial Cell in Arterial Stiffness
The interface of the endothelial cell layer with flowing blood predisposes the function of the endothelial cell to haemodynamic forces which have been shown to potentiate gene expression at the level of transcription [66]. Haemodynamic forces are associated with modification of the artery wall through phenotypic alterations of endothelial and smooth muscle cells through complex mechanotransduction receptor mechanisms [22]. Genetic expression has also been shown to be affected by the amount of pulsatility contributing to oscillatory shear. In cultured bovine aortic endothelial cells, the mRNA expression of endothelin-1 (ET-1) and endothelial nitric oxide synthase (eNOS) has been shown to depend on both time and amplitude of mechanical force [67]. These studies showed that, compared to unidirectional sheer, oscillatory shear stress combined with pressure upregulates transient expression of ET-1 while at the same time downregulating eNOS. Since arterial stiffness is associated with pulse pressure, this mechanism could constitute a potential positive feedback mechanism where downregulation of eNOS and upregulation of ET-1 could further increase wall stiffness, leading to an increase in oscillatory sheer stress and so amplifying the effect of wall stiffness. Although limited, there is in vitro experimental evidence of this from cells cultured in tubes of different compliance, where it was demonstrated that increased wall stiffness is associated with reduced endothelial Akt-dependent eNOS phosphorylation [68].
The association between endothelial-dependent reduction of nitric oxide (NO) and increased arterial stiffness has been demonstrated in vivo in the iliac artery of sheep [69] and humans [70]. These experiments were conducted in a segment of vessel where the local effects of altered endothelial function could be quantified independent of effects of intra-arterial distending pressure. Similar experiments demonstrated the effects of ET-1 in potentiating elevation of large artery stiffness which can be reversed by blockade of the ET(A) receptor [71]. Natriuretic peptides have also been shown to affect local iliac artery stiffness in the sheep via the NPRA receptor [72].
Role of the Vascular Smooth Muscle Cell in Arterial Stiffness
In addition to the important role of vascular smooth muscle in the regulation of vascular tone affecting peripheral resistance, the contractile properties of the vascular smooth muscle cells have a measurable effect on mechanics of the arterial wall of large conduit arteries [62,73,74] with suggestions of regulation of energetics of viscous damping [75]. There is a large body of literature spanning some five decades on the biology of smooth muscle cell phenotypic modulation, where the cell exits in a number of phenotypic states which depend on specific adaptive functional demands [76].
In relation to arterial stiffness, an important phenotypic change is the functional transdifferentiation leading to osteogenesis causing deposition of calcium in the media of the arterial wall [77]. The increase in arterial calcium deposition has been related to decreased bone mineral density [78]. Recent evidence from the Baltimore Longitudinal Study of Aging shows that in women there is an inverse relationship between arterial stiffness and cortical bone area, independent of age and blood pressure [79]. The compounding effect of calcification is that fracture of elastin fibres is associated with the signalling pathway for smooth muscle cell transdifferentiation [78] and that the downstream effect is elastocalcinosis resulting in increased wall stiffness [80,81].
In experimental investigations, an increase in arterial stiffness mediated through calcification is associated with administration of vitamin D and nicotine [80,82]. Vitamin D has also been found to be an independent correlate of arterial stiffness in patients with peripheral arterial disease [83]. Elevated calcification is also a hallmark of chronic kidney disease in Lewis polycystic kidney (LPK) rat models. LPK rats showed a 6- to 8-fold increase in aortic calcification with a 33% increase in aortic PWV and a 20% reduction in elastin density [84]. Although vascular calcification is potentiated by phenotypic changes in the vascular smooth muscle cell, tissue transglutaminase 2 (TG2) has been shown to be necessary for programming of chrono-osseous smooth muscle cell differentiation in response to increased bone morphogenic protein [85]. Recent studies have also identified other pathways affecting smooth muscle cells to potentiate vascular calcification. Calpain-1 has been shown to regulate MMP-2 activity affecting age-related calcification and fibrosis [86]. A mineralocorticoid receptor, usually thought to be present in the kidney, has recently been identified in the vascular smooth muscle, suggesting a possible regulatory role of smooth muscle function [87].
Molecular Mechanisms
Extracellular Matrix
Molecular mechanisms that alter the stiffness of the ECM of the artery wall are connected with ageing, involving changes in the structural proteins, elastin and collagen that are manifest as protein side chain modification and intermolecular cross-linking [7]. Whereas cross-linking involves enzymatic changes during developmental phases, ageing involves non-enzymatic processes with glucose, leading to advanced glycation end product (AGE) formation. In arteries, AGE formation in the ECM leads to increased stiffness, and it has been shown that non-enzymatic breaking of AGE cross-links can improve arterial compliance and reduce pulse pressure in the elderly as well as cardiac function [88,89]. Recent studies have questioned whether existing cross-links are actually cleaved by AGE breaking agents such as alagebrium (ALT-711), although these agents can act as inhibitors of metal-catalysed AGEs [90]. ECM remodelling is also modulated by the expression of MMPs due to effects of haemodynamics, oxidative stress and inflammation [91]. The role of cardiotropin-1, a member of interleukin-6, in promoting fibrosis in the ECM leading to increased arterial stiffness has also recently been described [92].
Protein Post-Translational Modification: S-Nitrosylation
The process of S-nitrosylation involves post-translational modification mediated by NO through cyclic GMP-independent pathways, where the protein cysteine thiol undergoes covalent modification by an NO group and generates an S-nitrosothiol [93]. The S-nitrosylation process of the tissue TG2 protein has been shown to be involved in the calcium-dependent TG2-mediated modification of the vascular ECM through formation of collagen cross-linking, affecting wall stiffness [94]. The endothelial production of NO produces acyclic redox-dependent S-nitrosylation and denitrosylation of TG2 [93]. The reduced S-nitrosylation (and therefore increased denitrosylation) of TG2 that takes place with reduced production or bioavailability of NO (e.g. due to endothelial dysfunction) causes exteriorization of the protein to the extracellular space. Increased activity of matrix TG2 has been shown to be associated with increased aortic PWV in TG2 knockout mouse models [20]. Studies in ageing rats and TG2 and eNOS knockout mice models have shown that a reduction in the bioavailability of NO as occurs with ageing, inflammation and endothelial dysfunction in general is associated with cellular mechanisms contributing to arterial stiffness [95].
Neurogenic Mechanisms
Investigations addressing the neurogenic influence of stiffness of large arteries through the effect of smooth muscle tone have been varied and have produced inconsistent results in terms of quantifying the intrinsic neurogenic effect on smooth muscle as separate from the passive mechanical stretch effect due to concomitant pressure changes. Studies simulating the neurogenic effect by administration of neurotransmitter substances have demonstrated increased aortic PWV in anaesthetized dogs [96] and wall stiffness changes measured by pressure/diameter relations in conscious dogs [97] and vagotomized cats [98]. Studies in rats have also been confined to measurement at specific sites (carotid and femoral arteries) and have not explicitly addressed the effect on the aortic trunk [99,100] in terms of functional stiffness determining pulse pressure. Recent studies in humans have shown an independent association between aortic PWV and muscle sympathetic nerve activity [101,102].
Neuroendocrine Mechanisms
Early studies on cardiovascular effects of angiotensin-converting enzyme (ACE) inhibition suggested a role of angiotensin II in cardiac and vascular remodelling independent of the passive effects of arterial pressure [103]. The remodelling of the ECM affecting arterial stiffness involves ACE inhibition preventing medial accumulation of collagen mediated by inhibition of angiotensin II through the AT1 receptor [104]. Recent studies have associated the age-related changes in the arterial wall with angiotensin II signalling in complex path-ways involving calpain-1, transforming growth factor-beta1, MMP-2 and MMP-9, monocyte chemoattractant protein-1, NADPH-oxidase and reactive oxygen species. Increased angiotensin II signalling has also been shown to induce the accumulation of collagen and AGEs as well as elastin degradation [105].
In models of chronic kidney disease (LPK rat), it was shown that the increase in aortic stiffness was associated with a 6-fold increase in aortic calcium content [84]. ACE inhibition by perindopril in the LKP rats reduced the accumulation of aortic calcium during development as well as the degree of elastin degradation and collagen content. In spontaneously hypertensive rats, early ACE inhibition for a brief period of only 4 weeks was associated with persistent reduction of isobaric wall stiffness [106].
Direct angiotensin receptor blockade (ARB) is associated with haemodynamic effects consistent with reduced arterial stiffness and peripheral wave reflection [107]. ARB also potentiates the reduction of arterial stiffness in combination with ACE inhibition in chronic disease [108] and is associated with blockade of the angiotensin II type 1 receptor. However, recent studies which have addressed the type 2 receptor have shown that chronic stimulation was associated with reduced aortic stiffening and lower collagen accumulation. This occurred without preventing hypertension in rats in which NO synthase was inhibited. The effects of type 2 receptor stimulation were additive to angiotensin II type 1 receptor blockade [109].
Genetic Associations
The use of high-density array single nucleotide polymorphism technology is enabling the identification of gene variants associated with markers of vascular function. Perusal of genome-wide association studies is uncovering groups of genes affecting NO pathways, MMPs, matrix elastin structure, endothelin receptors and inflammatory molecules [110]. Specific associations with carotid-femoral PWV have been found in a gene locus associated with gene enhancers related to increased stiffness as measured by PWV [111]. Studies in specific populations, such as African-Americans, have not yet yielded specific genes, although it is estimated that some 20% of the variance in arterial stiffness is inherited. Although genome-wide association study techniques would seem to identify relatively week genetic associations with arterial stiffness, studies of congenic strains of rats for identification of quantitative trait loci for arterial stiffness and blood pressure have shown that, although not finding specific associations, the female blood pressure quantitative trait locus has been narrowed to a range of less than 7 Mbp in chromosome 5 [112].
Conclusions
The relevance of arterial stiffness as a fundamental property of the relationship of pulsatile blood pressure and flow and wave propagation phenomena has been firmly established from investigations in the physical sciences. Clinical and epidemiological evidence suggests the emergence of arterial stiffness as a powerful factor in cardiovascular risk. It is therefore of importance to elucidate the underlying mechanical and biological mechanisms that lead to the degeneration of arterial properties resulting in an increase in pulse pressure, manifest predominantly as elevated systolic pressure, a significant risk factor for cardiovascular morbidity and mortality. In a similar way that early interdisciplinary research produced coherent biophysical explanations of pulsatile blood flow in arteries, the improved understanding of the cellular, molecular and potentially neurogenic mechanisms will emerge from similar interaction of investigators from the broad field of physical sciences and molecular biology. However, due to the complex and numerous interacting cellular and molecular processes inherent in evolutionary survival, this will present a greater challenge to uncover the relevant parameters and signalling pathways that translate to functional correlates of arterial stiffness, such as alterations in PWV, vascular storage capacity and dynamic changes in arterial pressure. Data to date suggest the existence of signalling pathways that form dynamic systems involving positive and negative feedback mechanisms that act on arterial stiffness. This systems approach has the potential to lend itself to uncovering integrated mechanisms and parameters, making use of the emerging fields of extensive databases and bioinformatics techniques of database mining in genomics and proteomics, combined with physical measurements such as arterial blood pressure and PWV.
References
- 1.Mitchell JR, Schwartz CJ. Relationship between arterial disease in different sites. A study of the aorta and coronary, carotid, and iliac arteries. Br Med J. 1962;1:1293–1301. doi: 10.1136/bmj.1.5288.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols WW, O'Rourke MF, Vlachopoulos C. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. ed 6. London: Hodder Arnold; 2011. [Google Scholar]
- 3.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 4.Pickering G. Arteriosclerosis and atherosclerosis. The need for clear thinking. Am J Med. 1963;34:7–18. doi: 10.1016/0002-9343(63)90035-4. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson IB, McEniery CM, Cockcroft JR. Arteriosclerosis and atherosclerosis: guilty by association. Hypertension. 2009;54:1213–1215. doi: 10.1161/HYPERTENSIONAHA.109.142612. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 7.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 8.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 9.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 10.Franklin SS, Jacobs MJ, Wong ND, L'Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 11.Amery A, Fagard R, Guo C, Staessen J, Thijs L. Isolated systolic hypertension in the elderly: an epidemiologic review. Am J Med. 1991;90:64S–70S. doi: 10.1016/0002-9343(91)90441-y. [DOI] [PubMed] [Google Scholar]
- 12.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 13.Franklin SS. Pulse pressure as a risk factor. Clin Exp Hypertens. 2004;26:645–652. doi: 10.1081/ceh-200031962. [DOI] [PubMed] [Google Scholar]
- 14.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol. 1994;140:669–682. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 15.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551–565. doi: 10.1161/CIRCULATIONAHA.109.912568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 19.Avolio A, Jones D, Tafazzoli-Shadpour M. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension. 1998;32:170–175. doi: 10.1161/01.hyp.32.1.170. [DOI] [PubMed] [Google Scholar]
- 20.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, et al. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- 21.Intaglietta M, Frangos JA. Introduction: mechanotransduction and translational medicine workshop. J Intern Med. 2006;259:336–338. doi: 10.1111/j.1365-2796.2006.01627.x. [DOI] [PubMed] [Google Scholar]
- 22.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 23.Bergel DH. The static elastic properties of the arterial wall. J Physiol. 1961;156:445–457. doi: 10.1113/jphysiol.1961.sp006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox RH. Pressure dependence of the mechanical properties of arteries in vivo. Am J Physiol. 1975;229:1371–1375. doi: 10.1152/ajplegacy.1975.229.5.1371. [DOI] [PubMed] [Google Scholar]
- 25.Dobrin PB, Mrkvicka R. Estimating the elastic modulus of non-atherosclerotic elastic arteries. J Hypertens Suppl. 1992;10:S7–S10. [PubMed] [Google Scholar]
- 26.Bauer RD, Busse R, Schabert A. Mechanical properties of arteries. Biorheology. 1982;19:409–424. doi: 10.3233/bir-1982-19303. [DOI] [PubMed] [Google Scholar]
- 27.O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 28.Gosling RG, Budge MM. Terminology for describing the elastic behavior of arteries. Hypertension. 2003;41:1180–1182. doi: 10.1161/01.HYP.0000072271.36866.2A. [DOI] [PubMed] [Google Scholar]
- 29.Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255. doi: 10.1007/s12265-012-9359-6. [DOI] [PubMed] [Google Scholar]
- 30.Albaladejo P, Copie X, Boutouyrie P, Laloux B, Declere AD, Smulyan H, et al. Heart rate, arterial stiffness, and wave reflections in paced patients. Hypertension. 2001;38:949–952. doi: 10.1161/hy1001.096210. [DOI] [PubMed] [Google Scholar]
- 31.Tan I, Butlin M, Liu YY, Ng K, Avolio AP. Heart rate dependence of aortic pulse wave velocity at different arterial pressures in rats. Hypertension. 2012;60:528–533. doi: 10.1161/HYPERTENSIONAHA.112.194225. [DOI] [PubMed] [Google Scholar]
- 32.Wang YX, Fitch RM. Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol. 2004;2:379–384. doi: 10.2174/1570161043385448. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolinsky H, Glagov S. Structural basis for the static mechanical properties of the aortic media. Circ Res. 1964;14:400–413. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- 35.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 36.Wolinsky H, Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circ Res. 1969;25:677–686. doi: 10.1161/01.res.25.6.677. [DOI] [PubMed] [Google Scholar]
- 37.Lillie MA, Armstrong TE, Gerard SG, Shadwick RE, Gosline JM. Contribution of elastin and collagen to the inflation response of the pig thoracic aorta: assessing elastin's role in mechanical homeostasis. J Biomech. 2012;45:2133–2141. doi: 10.1016/j.jbiomech.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Lillie MA, Shadwick RE, Gosline JM. Mechanical anisotropy of inflated elastic tissue from the pig aorta. J Biomech. 2010;43:2070–2078. doi: 10.1016/j.jbiomech.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Gosline JM, Shadwick RE. The mechanical properties of fin whale arteries are explained by novel connective tissue designs. J Exp Biol. 1996;199:985–997. doi: 10.1242/jeb.199.4.985. [DOI] [PubMed] [Google Scholar]
- 40.Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202:3305–3313. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 41.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 42.Wang JJ, O'Brien AB, Shrive NG, Parker KH, Tyberg JV. Time-domain representation of ventricular-arterial coupling as a windkessel and wave system. Am J Physiol Heart Circ Physiol. 2003;284:H1358–H1368. doi: 10.1152/ajpheart.00175.2002. [DOI] [PubMed] [Google Scholar]
- 43.Learoyd BM, Taylor MG. Alterations with age in the viscoelastic properties of human arterial walls. Circ Res. 1966;18:278–292. doi: 10.1161/01.res.18.3.278. [DOI] [PubMed] [Google Scholar]
- 44.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 45.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 46.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 47.Avolio A, Butlin M, Liu YY, Viegas K, Avadhanam B, Lindesay G. Regulation of arterial stiffness: cellular, molecular and neurogenic mechanisms. Artery Res. 2011;5:122–127. [Google Scholar]
- 48.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Elastic proteins: biological roles and mechanical properties. Philos Trans R Soc Lond B Biol Sci. 2002;357:121–132. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, et al. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 51.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martyn CN, Greenwald SE. A hypothesis about a mechanism for the programming of blood pressure and vascular disease in early life. Clin Exp Pharmacol Physiol. 2001;28:948–951. doi: 10.1046/j.1440-1681.2001.03555.x. [DOI] [PubMed] [Google Scholar]
- 53.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301:H221–H229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakashima Y, Shiokawa Y, Sueishi K. Alterations of elastic architecture in human aortic dissecting aneurysm. Lab Invest. 1990;62:751–760. [PubMed] [Google Scholar]
- 55.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012;5:264–273. doi: 10.1007/s12265-012-9349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perret-Guillaume C, Joly L, Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog Cardiovasc Dis. 2009;52:6–10. doi: 10.1016/j.pcad.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 58.Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. doi: 10.1161/01.hyp.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- 59.Mangoni AA, Mircoli L, Giannattasio C, Ferrari AU, Mancia G. Heart rate-dependence of arterial distensibility in vivo. J Hypertens. 1996;14:897–901. doi: 10.1097/00004872-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Mircoli L, Mangoni AA, Giannattasio C, Mancia G, Ferrari AU. Heart rate-dependent stiffening of large arteries in intact and sympathectomized rats. Hypertension. 1999;34:598–602. doi: 10.1161/01.hyp.34.4.598. [DOI] [PubMed] [Google Scholar]
- 61.Bergel DH. The dynamic elastic properties of the arterial wall. J Physiol. 1961;156:458–469. doi: 10.1113/jphysiol.1961.sp006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salvucci FP, Schiavone J, Craiem D, Barra JG. Arterial wall mechanics as a function of heart rate: role of vascular smooth muscle. J Phys Conf Ser. 2007;90:1–7. [Google Scholar]
- 63.Luft FC. Molecular mechanisms of arterial stiffness: new insights. J Am Soc Hypertens. 2012;6:436–438. doi: 10.1016/j.jash.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53:258–261. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC, et al. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126:2473–2480. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 66.Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 67.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- 68.Peng X, Haldar S, Deshpande S, Irani K, Kass DA. Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension. 2003;41:378–381. doi: 10.1161/01.hyp.0000049624.99844.3d. [DOI] [PubMed] [Google Scholar]
- 69.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 70.Schmitt M, Avolio A, Qasem A, McEniery CM, Butlin M, Wilkinson IB, et al. Basal NO locally modulates human iliac artery function in vivo. Hypertension. 2005;46:227–231. doi: 10.1161/01.HYP.0000164581.39811.bd. [DOI] [PubMed] [Google Scholar]
- 71.McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42:1975–1981. doi: 10.1016/j.jacc.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 72.Schmitt M, Qasem A, McEniery C, Wilkinson IB, Tatarinoff V, Noble K, et al. Role of natriuretic peptides in regulation of conduit artery distensibility. Am J Physiol Heart Circ Physiol. 2004;287:H1167–H1171. doi: 10.1152/ajpheart.00101.2004. [DOI] [PubMed] [Google Scholar]
- 73.Cox RH. Comparison of arterial wall mechanics in normotensive and spontaneously hypertensive rats. Am J Physiol. 1979;237:H159–H167. doi: 10.1152/ajpheart.1979.237.2.H159. [DOI] [PubMed] [Google Scholar]
- 74.Dobrin PB, Rovick AA. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am J Physiol. 1969;217:1644–1651. doi: 10.1152/ajplegacy.1969.217.6.1644. [DOI] [PubMed] [Google Scholar]
- 75.Armentano RL, Barra JG, Santana DB, Pessana FM, Graf S, Craiem D, et al. Smart damping modulation of carotid wall energetics in human hypertension: effects of angiotensin-converting enzyme inhibition. Hypertension. 2006;47:384–390. doi: 10.1161/01.HYP.0000205915.15940.15. [DOI] [PubMed] [Google Scholar]
- 76.Campbell JH, Campbell GR. Smooth muscle phenotypic modulation – a personal experience. Arterioscler Thromb Vasc Biol. 2012;32:1784–1789. doi: 10.1161/ATVBAHA.111.243212. [DOI] [PubMed] [Google Scholar]
- 77.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 78.Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Giallauria F, Ling SM, Schreiber C, Maggio M, Shetty V, Muller D, et al. Arterial stiffness and bone demineralization: the Baltimore longitudinal study of aging. Am J Hypertens. 2011;24:970–975. doi: 10.1038/ajh.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Atkinson J. Age-related medial elastocalcinosis in arteries: mechanisms, animal models, and physiological consequences. J Appl Physiol. 2008;105:1643–1651. doi: 10.1152/japplphysiol.90476.2008. [DOI] [PubMed] [Google Scholar]
- 81.Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc Res. 2005;66:307–317. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J. Calcification of medial elastic fibers and aortic elasticity. Hypertension. 1997;29:999–1006. doi: 10.1161/01.hyp.29.4.999. [DOI] [PubMed] [Google Scholar]
- 83.Zagura M, Serg M, Kampus P, Zilmer M, Eha J, Unt E, et al. Aortic stiffness and vitamin D are independent markers of aortic calcification in patients with peripheral arterial disease and in healthy subjects. Eur J Vasc Endovasc Surg. 2011;42:689–695. doi: 10.1016/j.ejvs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 84.Ng K, Hildreth CM, Phillips JK, Avolio AP. Aortic stiffness is associated with vascular calcification and remodeling in a chronic kidney disease rat model. Am J Physiol Renal Physiol. 2011;300:F1431–F1436. doi: 10.1152/ajprenal.00079.2011. [DOI] [PubMed] [Google Scholar]
- 85.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, et al. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012;60:1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens. 2004;17:23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 89.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 90.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation – a mini-review. Gerontology. 2012;58:227–237. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- 91.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 92.Lopez-Andres N, Calvier L, Labat C, Fay R, Diez J, Benetos A, et al. Absence of cardiotrophin 1 is associated with decreased age-dependent arterial stiffness and increased longevity in mice. Hypertension. 2013;61:120–129. doi: 10.1161/HYPERTENSIONAHA.112.201699. [DOI] [PubMed] [Google Scholar]
- 93.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry. 2001;40:4904–4910. doi: 10.1021/bi002321t. [DOI] [PubMed] [Google Scholar]
- 95.Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nichols WW, McDonald DA. Wave-velocity in the proximal aorta. Med Biol Eng. 1972;10:327–335. doi: 10.1007/BF02474213. [DOI] [PubMed] [Google Scholar]
- 97.Barra JG, Armentano RL, Levenson J, Fischer EI, Pichel RH, Simon A. Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res. 1993;73:1040–1050. doi: 10.1161/01.res.73.6.1040. [DOI] [PubMed] [Google Scholar]
- 98.Pagani M, Schwartz PJ, Bishop VS, Malliani A. Reflex sympathetic changes in aortic diastolic pressure-diameter relationship. Am J Physiol. 1975;229:286–290. doi: 10.1152/ajplegacy.1975.229.2.286. [DOI] [PubMed] [Google Scholar]
- 99.Lacolley P, Glaser E, Challande P, Boutouyrie P, Mignot JP, Duriez M, et al. Structural changes and in situ aortic pressure-diameter relationship in long-term chemical-sympathectomized rats. Am J Physiol. 1995;269:H407–H416. doi: 10.1152/ajpheart.1995.269.2.H407. [DOI] [PubMed] [Google Scholar]
- 100.Mangoni AA, Mircoli L, Giannattasio C, Mancia G, Ferrari AU. Effect of sympathectomy on mechanical properties of common carotid and femoral arteries. Hypertension. 1997;30:1085–1088. doi: 10.1161/01.hyp.30.5.1085. [DOI] [PubMed] [Google Scholar]
- 101.Nakao M, Nomura K, Karita K, Nishikitani M, Yano E. Relationship between brachial-ankle pulse wave velocity and heart rate variability in young Japanese men. Hypertens Res. 2004;27:925–931. doi: 10.1291/hypres.27.925. [DOI] [PubMed] [Google Scholar]
- 102.Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, et al. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010;28:979–984. doi: 10.1097/hjh.0b013e328336ed9a. [DOI] [PubMed] [Google Scholar]
- 103.Levy BI, Michel JB, Salzmann JL, Devissaguet M, Safar ME. Remodeling of heart and arteries by chronic converting enzyme inhibition in spontaneously hypertensive rats. Am J Hypertens. 1991;4:240S–245S. doi: 10.1093/ajh/4.3.240s. [DOI] [PubMed] [Google Scholar]
- 104.Benetos A, Levy BI, Lacolley P, Taillard F, Duriez M, Safar ME. Role of angiotensin II and bradykinin on aortic collagen following converting enzyme inhibition in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1997;17:3196–3201. doi: 10.1161/01.atv.17.11.3196. [DOI] [PubMed] [Google Scholar]
- 105.Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev. 2010;6:266–281. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ng K, Butlin M, Avolio AP. Persistent effect of early, brief angiotensin-converting enzyme inhibition on segmental pressure dependency of aortic stiffness in spontaneously hypertensive rats. J Hypertens. 2012;30:1782–1790. doi: 10.1097/HJH.0b013e3283562e35. [DOI] [PubMed] [Google Scholar]
- 107.Mahmud A, Feely J. Effect of angiotensin ii receptor blockade on arterial stiffness: beyond blood pressure reduction. Am J Hypertens. 2002;15:1092–1095. doi: 10.1016/s0895-7061(02)02982-5. [DOI] [PubMed] [Google Scholar]
- 108.Frimodt-Moller M, Kamper AL, Strandgaard S, Kreiner S, Nielsen AH. Beneficial effects on arterial stiffness and pulse-wave reflection of combined enalapril and candesartan in chronic kidney disease – a randomized trial. PLoS One. 2012;7:e41757. doi: 10.1371/journal.pone.0041757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paulis L, Becker ST, Lucht K, Schwengel K, Slavic S, Kaschina E, et al. Direct angiotensin II type 2 receptor stimulation in Nomega-nitro-L-arginine-methyl ester-induced hypertension: the effect on pulse wave velocity and aortic remodeling. Hypertension. 2012;59:485–492. doi: 10.1161/HYPERTENSIONAHA.111.185496. [DOI] [PubMed] [Google Scholar]
- 110.Lacolley P, Challande P, Osborne-Pellegrin M, Regnault V. Genetics and pathophysiology of arterial stiffness. Cardiovasc Res. 2009;81:637–648. doi: 10.1093/cvr/cvn353. [DOI] [PubMed] [Google Scholar]
- 111.Mitchell GF, Verwoert GC, Tarasov KV, Isaacs A, Smith AV. Yasmin, et al Common genetic variation in the 3′-BCL11B gene desert is associated with carotid-femoral pulse wave velocity and excess cardiovascular disease risk: the AortaGen Consortium. Circ Cardiovasc Genet 2012;5:81-90. [DOI] [PMC free article] [PubMed]
- 112.Herrera VL, Pasion KA, Moran AM, Ruiz-Opazo N. Dahl (S×R) congenic strain analysis confirms and defines a chromosome 5 female-specific blood pressure quantitative trait locus to <7 Mbp. PLoS One. 2012;7:e42214. doi: 10.1371/journal.pone.0042214. [DOI] [PMC free article] [PubMed] [Google Scholar]